A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use
Abstract
:1. Introduction
2. The Research Trends and Knowledge Gaps
3. Stress-Induced Plastic Item Fragmentation Leading to the Production of MNPs
3.1. Environmental Stress
3.2. Mechanical Abrasion and Wear
4. The Characteristics of Secondary MNPs Released by Fragmentation
4.1. Geometrical Features
4.2. Surface Textures
5. Human Exposure to MNPs via Mechanical Fragmentation
5.1. The Pathways of Acute Exposure
5.2. Long-Term Exposure
| Production | Pathways | Pollution Matrix | Environmental Stress | Primary Mechanical Abrasion | Release Tendency | Reference |
|---|---|---|---|---|---|---|
| water bottle | waste recycling | waste water/sludge | natural conditions | shearing force | 967–24,798 MNPs/L; 773–1450 MNPs/kg | [86] |
| household plastic | waste recycling | waste water | natural conditions with UV | shearing force | 110–200,000 MNPs/L | [87] |
| household plastic | landfill | surface soil | natural conditions with UV | compression | 56–122 MNPs/5 cm2 samples | [88] |
| construction material | municipal activities | surface dust | liquid/heat | compression | 0.53 ± 0.15 g MNPs/m2 | [73] |
| plastic kettles | municipal activities | drinking water | liquid | compression | N.A. a | [89] |
6. Outlook
6.1. Toxicological Approach Based on the Full Life Cycle of Plastic Products
6.2. Integration of Aging Evaluation into Risk Assessment Frameworks
6.3. Establishing Early Warning Signals of MNPs Released from Plastic Items
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, 16. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Varg, J.E.; Svanbäck, R. Multi stress system: Microplastics in freshwater and their effects on host microbiota. Sci. Total. Environ. 2023, 856, 159106. [Google Scholar] [CrossRef] [PubMed]
- Seltenrich, N. New link in the food chain? marine plastic pollution and seafood safety. Environ. Health Perspect. 2015, 123, A34–A41. [Google Scholar] [CrossRef] [PubMed]
- Bergami, E.; Bocci, E.; Vannuccini, M.L.; Monopoli, M.; Salvati, A.; Dawson, K.A.; Corsi, I. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotox. Environ. Safe. 2016, 123, 18–25. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Li, B.W.; Liang, W.W.H.; Liu, Q.X.; Fu, S.J.; Ma, C.Z.; Chen, Q.Q.; Su, L.; Craig, N.J.; Shi, H.H. Fish ingest microplastics unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef]
- Kramm, J.; Völker, C.; Wagner, M. Superficial or substantial: Why care about microplastics in the anthropocene? Environ. Sci. Technol. 2018, 52, 3336–3337. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Collard, F.; Ask, A. Plastic ingestion by Arctic fauna: A review. Sci. Total. Environ. 2021, 786, 147462. [Google Scholar] [CrossRef]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of seafood intended for human consumption: A systematic review and meta–analysis. Environ. Health Perspect. 2020, 128, 32. [Google Scholar] [CrossRef]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Wright, S.; Borm, P.J.A. Applying Existing Particle Paradigms to Inhaled Microplastic Particles. Front. Public Health 2022, 10, 868822. [Google Scholar] [CrossRef]
- Roychand, R.; Pramanik, B.K. Identification of micro-plastics in Australian road dust. J. Environ. Chem. Eng. 2020, 8, 103647. [Google Scholar] [CrossRef]
- Su, L.; Nan, B.; Craig, N.J.; Pettigrove, V. Temporal and spatial variations of microplastics in roadside dust from rural and urban Victoria, Australia: Implications for diffuse pollution. Chemosphere 2020, 252, 126567. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Queiroz, L.G.; Pradd, C.C.A.; de Melo, E.C.; de Moraes, B.R.; Ando, R.A.; de Paiva, T.C.B.; Pompeo, M. Exposure of the amphipod Hyalella azteca to microplastics. A study on subtoxic responses and particle biofragmentation. Aquat. Toxicol. 2023, 258, 106516. [Google Scholar] [CrossRef] [PubMed]
- ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef]
- Zheng, Y.; Hamed, M.; De-la-Torre, G.E.; Frias, J.; Jong, M.-C.; Kolandhasamy, P.; Chavanich, S.; Su, L.; Deng, H.; Zhao, W.; et al. Holes on surfaces of the weathered plastic fragments from coastal beaches. Mar. Pollut. Bull. 2023, 193, 115180. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Coley, I.; Olivero-Verbel, J. Microplastic resin pellets on an urban tropical beach in Colombia. Environ. Monit. Assess. 2015, 187, 435. [Google Scholar] [CrossRef] [PubMed]
- Hays, H.; Cormons, G. Plastic particles found in tern pellets, on coastal beaches and at factory sites. Mar. Pollut. Bull. 1974, 5, 44–46. [Google Scholar] [CrossRef]
- Bour, A.; Sturve, J.; Hojesjo, J.; Almroth, B.C. Microplastic vector effects: Are fish at risk when exposed via the trophic chain? Front. Environ. Sci. 2020, 8, 10. [Google Scholar] [CrossRef]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef]
- ter Halle, A.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Garvey, C.J.; Impéror-Clerc, M.; Rouzière, S.; Gouadec, G.; Boyron, O.; Rowenczyk, L.; Mingotaud, A.F.; ter Halle, A. Molecular-scale understanding of the embrittlement in polyethylene ocean debris. Environ. Sci. Technol. 2020, 54, 11173–11181. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected microplastics pollution in global COVID-19: Disposable surgical masks. Sci. Total. Environ. 2021, 790, 148130. [Google Scholar] [CrossRef]
- Hussain, K.A.; Romanova, S.; Okur, I.; Zhang, D.; Kuebler, J.; Huang, X.; Wang, B.; Fernandez-Ballester, L.; Lu, Y.; Schubert, M.; et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health. Environ. Sci. Technol. 2023, 57, 9782–9792. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.M.; Akbor, M.A.; Siddique, M.A.B.; Uddin, M.K.; Malafaia, G. Is there tea complemented with the appealing flavor of microplastics? A pioneering study on plastic pollution in commercially available tea bags in Bangladesh. Sci. Total. Environ. 2022, 837, 155833. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Kühnel, D.; Rummel, C.; MacLeod, M.; Potthoff, A.; Reichelt, S.; Rojo-Nieto, E.; Schmitt-Jansen, M.; Sonnenberg, J.; Toorman, E.; et al. Weathering plastics as a planetary boundary threat: Exposure, fate, and hazards. Environ. Sci. Technol. 2021, 55, 7246–7255. [Google Scholar] [CrossRef]
- Bourne, W.R.P. Nylon netting as a hazard to birds. Mar. Pollut. Bull. 1977, 8, 75–76. [Google Scholar] [CrossRef]
- Satoto, R.; Subowo, W.S.; Yusiasih, R.; Takane, Y.; Watanabe, Y.; Hatakeyama, T. Weathering of high-density polyethylene in different latitudes. Polym. Degrad. Stabil. 1997, 56, 275–279. [Google Scholar] [CrossRef]
- Born, M.P.; Brüll, C. From model to nature—A review on the transferability of marine (micro-) plastic fragmentation studies. Sci. Total. Environ. 2022, 811, 151389. [Google Scholar] [CrossRef] [PubMed]
- Khosrovyan, A.; Kahru, A. Evaluation of the potential toxicity of UV-weathered virgin polyamide microplastics to non-biting midge Chironomus riparius. Environ. Pollut. 2021, 287, 117334. [Google Scholar] [CrossRef]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef]
- Alimi, O.S.; Claveau-Mallet, D.; Kurusu, R.S.; Lapointe, M.; Bayen, S.; Tufenkji, N. Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: What are we missing? J. Hazard. Mater. 2022, 423, 126955. [Google Scholar] [CrossRef]
- Hayes, M.D.; Edwards, D.B.; Shah, A.R. 1. Introduction. In Fractography in Failure Analysis of Polymers; Hayes, M.D., Edwards, D.B., Shah, A.R., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 1–5. [Google Scholar]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Fallahi, H.; Taheri-Behrooz, F.; Asadi, A. Nonlinear Mechanical Response of Polymer Matrix Composites: A Review. Polym. Rev. 2020, 60, 42–85. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Menzel, T.; Meides, N.; Mauel, A.; Mansfeld, U.; Kretschmer, W.; Kuhn, M.; Herzig, E.M.; Altstädt, V.; Strohriegl, P.; Senker, J.; et al. Degradation of low-density polyethylene to nanoplastic particles by accelerated weathering. Sci. Total. Environ. 2022, 826, 154035. [Google Scholar] [CrossRef] [PubMed]
- Julienne, F.; Lagarde, F.; Bardeau, J.-F.; Delorme, N. Thin polyethylene (LDPE) films with controlled crystalline morphology for studying plastic weathering and microplastic generation. Polym. Degrad. Stabil. 2022, 195, 109791. [Google Scholar] [CrossRef]
- Julienne, F.; Lagarde, F.; Delorme, N. Influence of the crystalline structure on the fragmentation of weathered polyolefines. Polym. Degrad. Stabil. 2019, 170, 8. [Google Scholar] [CrossRef]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Yang, J.; Xiong, Y.; Huang, Z.; Wang, J.; Cai, T.; Dang, Z.; Yang, C. Formation of environmentally persistent free radicals on microplastics under UV irradiations. J. Hazard. Mater. 2023, 453, 131277. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Andrady, A.L. Weathering and fragmentation of plastic debris in the ocean environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Shim, W.J. The fragmentation of nano- and microplastic particles from thermoplastics accelerated by simulated-sunlight-mediated photooxidation. Environ. Pollut. 2022, 311, 119847. [Google Scholar] [CrossRef]
- Hueper, W.C. Cancer Induction by polyurethan and polysilicone plastics. J. Natl. Cancer Inst. 1964, 33, 1005–1027. [Google Scholar]
- Thomson, J.G.; Kaschula, R.O.; Macdonald, R.R. Asbestos as a modern urban hazard. S. Afr. Med. J. 1963, 37, 77–81. [Google Scholar]
- Takahashi, Y.; Tanaka, K.; Kajiwara, T.; Suzuki, G.; Osako, M.; Kuramochi, H. Cross-sectional microstructural analysis to evaluate the crack growth pattern of weathered marine plastics. Chemosphere 2023, 331, 138794. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Su, L.; Zheng, Y.F.; Du, F.N.; Liu, Q.X.; Zheng, J.; Zhou, Z.W.; Shi, H.H. Crack Patterns of Environmental Plastic Fragments. Environ. Sci. Technol. 2022, 56, 6399–6414. [Google Scholar] [CrossRef] [PubMed]
- Forster, N.A.; Wilson, S.C.; Tighe, M.K. Trail running events contribute microplastic pollution to conservation and wilderness areas. J. Environ. Manag. 2023, 331, 117304. [Google Scholar] [CrossRef] [PubMed]
- Eisentraut, P.; Dumichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two birds with one stone—Fast and simultaneous analysis of microplastics: Microparticles derived from thermoplastics and tire wear. Environ. Sci. Technol. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- Parker, B.W.; Beckingham, B.A.; Ingram, B.C.; Ballenger, J.C.; Weinstein, J.E.; Sancho, G. Microplastic and tire wear particle occurrence in fishes from an urban estuary: Influence of feeding characteristics on exposure risk. Mar. Pollut. Bull. 2020, 160, 111539. [Google Scholar] [CrossRef]
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does mechanical stress cause microplastic release from plastic water bottles? Water Res. 2019, 166, 115082. [Google Scholar] [CrossRef]
- Tvisha, S. Generation of microplastics from the opening and closing of disposable plastic water bottles. J. Water Health 2021, 19, 488–498. [Google Scholar] [CrossRef]
- Su, L.; Du, F.; Sun, C.; Shi, H. Linking the physical and chemical characteristics of single small microplastics or nanoplastics via photolithographic silicon substrates. Anal. Methods 2022, 14, 1547–1552. [Google Scholar] [CrossRef]
- Deen, N.G.; Kriebitzsch, S.H.L.; van der Hoef, M.A.; Kuipers, J.A.M. Direct numerical simulation of flow and heat transfer in dense fluid-particle systems. Chem. Eng. Sci. 2012, 81, 329–344. [Google Scholar] [CrossRef]
- Forward, K.M.; Lacks, D.J.; Sankaran, R.M. Charge segregation depends on particle size in triboelectrically charged granular materials. Phys. Rev. Lett. 2009, 102, 028001. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; An, Y.-J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Al Amin, M.; Gibson, C.T.; Chuah, C.; Tang, Y.; Naidu, R.; Fang, C. Raman imaging of microplastics and nanoplastics generated by cutting PVC pipe. Environ. Pollut. 2022, 298, 118857. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, I.; Miroslavov, A. Post-effects of radioactive decay in magnetite nano-crystals labelled with Auger- and internal conversion electron-emitters, alpha- and beta decay radionuclides. Radiat. Phys. Chem. 2020, 177, 109160. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Qiu, Z.; Cui, Z.; Li, N.; Li, X.; Wang, Y.; Zhang, H.; Zhao, C. Effects of polyethylene microplastics on cell membranes: A combined study of experiments and molecular dynamics simulations. J. Hazard. Mater. 2022, 429, 128323. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total. Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, H.; Wang, Y.; Ren, R.; Qin, X.; Wang, S. Effects of microplastics and their adsorption of cadmium as vectors on the cladoceran Moina monogolica Daday: Implications for plastic-ingesting organisms. J. Hazard. Mater. 2020, 400, 123239. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef]
- Cella, C.; La Spina, R.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Valsesia, A.; Gilliland, D. Detecting Micro- and Nanoplastics Released from Food Packaging: Challenges and Analytical Strategies. Polymers 2022, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Myszka, R.; Giustozzi, F. Paving roads with recycled plastics: Microplastic pollution or eco-friendly solution? J. Hazard. Mater. 2022, 437, 129334. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kwon, M.; Park, M.-J.; Kim, J. Analysis of Microplastics Released from Plain Woven Classified by Yarn Types during Washing and Drying. Polymers 2021, 13, 2988. [Google Scholar] [CrossRef] [PubMed]
- Tingna, M.; Jiahua, W.; Xiaofeng, X.; Jingwen, L.; Qiaocong, L.; Huang, D.; Xiaodan, L.; Fuwei, P. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods 2022, 11, 2871. [Google Scholar] [CrossRef]
- Lant, N.J.; Hayward, A.S.; Peththawadu, M.M.D.; Sheridan, K.J.; Dean, J.R. Microfiber release from real soiled consumer laundry and the impact of fabric care products and washing conditions. PLoS ONE 2020, 15, e0233332. [Google Scholar] [CrossRef]
- Napper, I.E.; Wright, L.S.; Barrett, A.C.; Parker-Jurd, F.N.F.; Thompson, R.C. Potential microplastic release from the maritime industry: Abrasion of rope. Sci. Total. Environ. 2022, 804, 150155. [Google Scholar] [CrossRef]
- Jander, J.; Hummel, D.; Stuermer, S.; Monteleone, A.; Neumaier, T.; Broghammer, F.; Lewin-Kretzschmar, U.; Brock, T.; Knoll, M.; Fath, A.S. Release of Microplastics from Reusable Kitchen Plasticware and Generation of Thermal Potential Toxic Degradation Products in the Oven. Appl. Sci. 2022, 12, 2535. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Gibert, Y.; Basheer, F.; Kong, L.; Dumée, L.F. Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. Hazard. Mater. 2020, 384, 121393. [Google Scholar] [CrossRef]
- Joseph, A.; Parveen, N.; Ranjan, V.P.; Goel, S. Drinking hot beverages from paper cups: Lifetime intake of microplastics. Chemosphere 2023, 317, 137844. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Monira, S.; Roychand, R.; Bhuiyan, M.A.; Pramanik, B.K. Role of water shear force for microplastics fragmentation into nanoplastics. Environ. Res. 2023, 237, 116916. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Roychand, R.; Hai, F.I.; Bhuiyan, M.; Dhar, B.R.; Pramanik, B.K. Nano and microplastics occurrence in wastewater treatment plants: A comprehensive understanding of microplastics fragmentation and their removal. Chemosphere 2023, 334, 139011. [Google Scholar] [CrossRef] [PubMed]
- de Haan, W.P.; Quintana, R.; Vilas, C.; Cózar, A.; Canals, M.; Uviedo, O.; Sanchez-Vidal, A. The dark side of artificial greening: Plastic turfs as widespread pollutants of aquatic environments. Environ. Pollut. 2023, 334, 122094. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Yang, L.; Tang, Y.; Liang, Y.; Wang, Q.; Su, L.; Liu, J.; Zhuang, Y.; Li, D. Microplastics released from artificial turf applied as hedge walls: Their aging-induced properties and uptake by grass carp, mussels and earthworms. Process. Saf. Environ. 2023, 174, 53–62. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, X.; Ruan, J.; Wang, Y.; Zhang, J.; LeBlanc, G.A.; An, L. Ignored microplastic sources from plastic bottle recycling. Sci. Total. Environ. 2022, 838, 156038. [Google Scholar] [CrossRef]
- Suzuki, G.; Uchida, N.; Le Huu, T.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Pham Hung, V.; Kuramochi, H.; Osako, M. Mechanical recycling of plastic waste as a point source of microplastic pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef]
- Sholokhova, A.; Denafas, G.; Ceponkus, J.; Kriukiene, R. Microplastics Release from Conventional Plastics during Real Open Windrow Composting. Sustainability 2023, 15, 758. [Google Scholar] [CrossRef]
- Shi, Y.; Li, D.; Xiao, L.; Sheerin, E.D.; Mullarkey, D.; Yang, L.; Bai, X.; Shvets, I.V.; Boland, J.J.; Wang, J.J. The influence of drinking water constituents on the level of microplastic release from plastic kettles. J. Hazard. Mater. 2022, 425, 127997. [Google Scholar] [CrossRef]

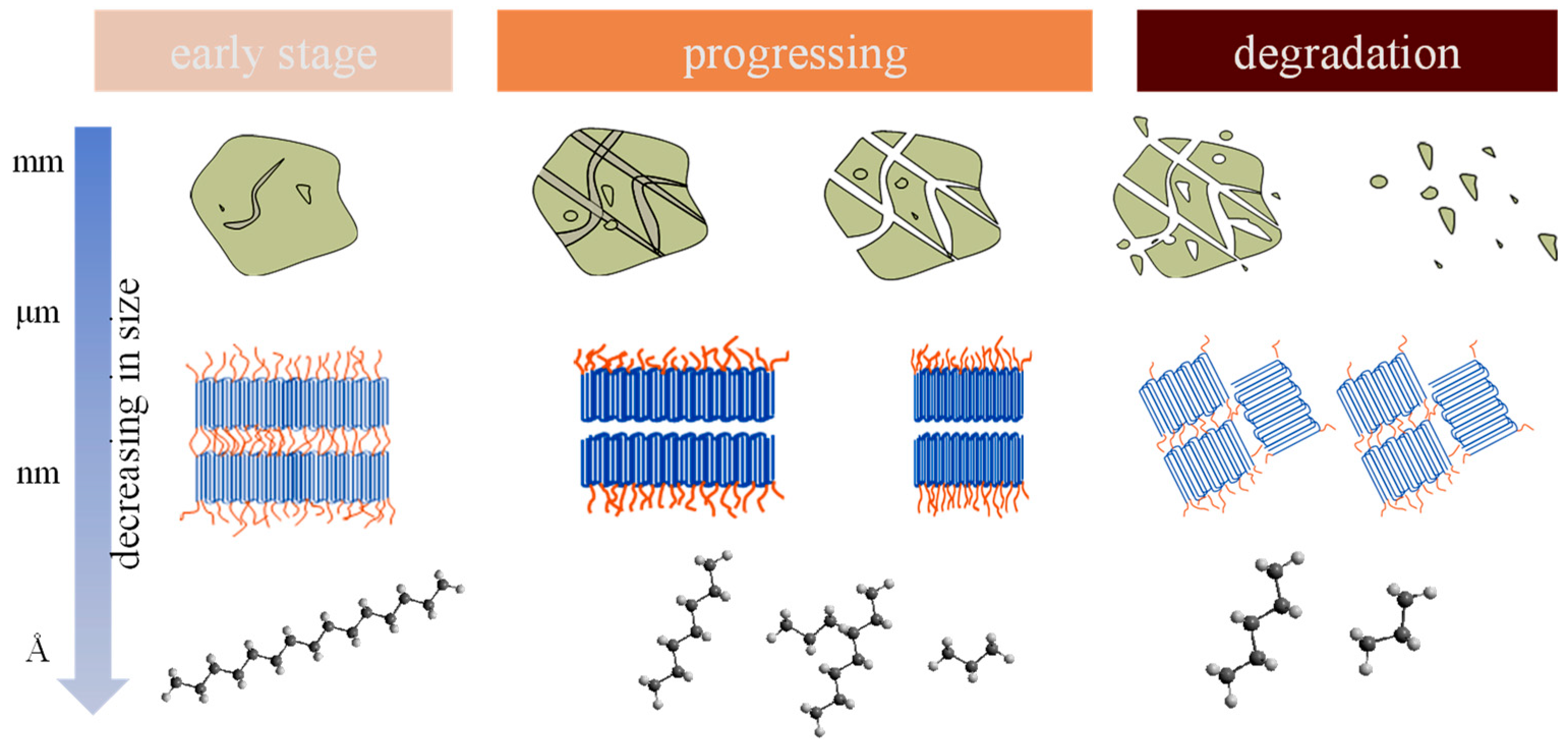
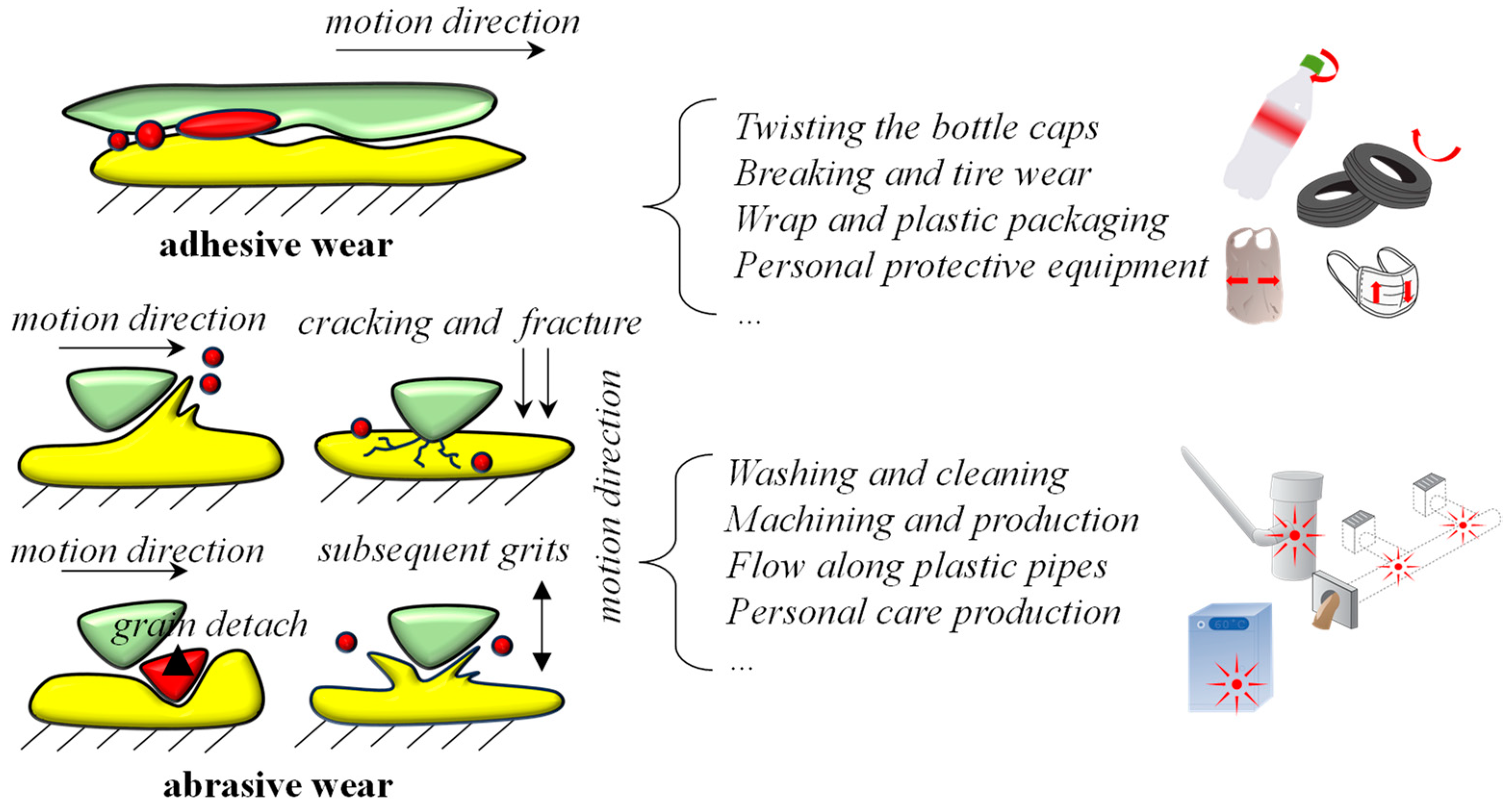

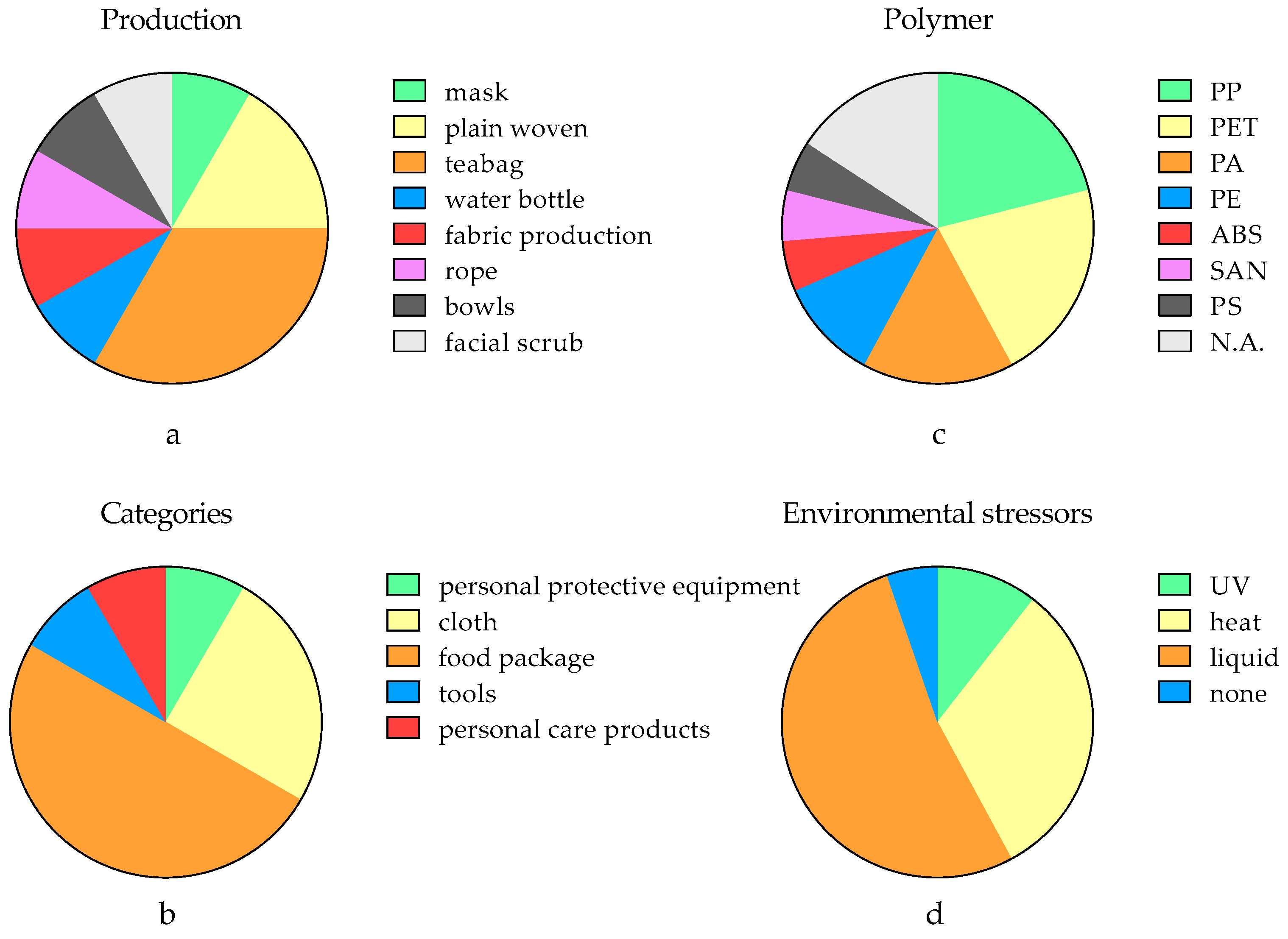
| Production | Primary Mechanical Abrasion (Force Analysis) | Secondary MNPs | Release Tendency | Reference |
|---|---|---|---|---|
| mask | adhesive wear (compression and twisting) | fiber (0.5 mm to 3.8 mm) | 24,300–55,900 MNPs/mask/d | [28] |
| plain woven | adhesive wear (compression and twisting) | fiber (0.1 mm to 1.5 mm) | 51.6–107.7 mg MNPs/kg cloth | [74] |
| plain woven | none | fiber (0.1 mm to 1.5 mm) | 20.6–59.9 mg MNPs/kg cloth | [74] |
| teabag | abrasive wear (particle collision) | fragments (N.A.) | 0.9–1.1 mg MNPs/teabag/filter | [72] |
| teabag | abrasive wear (particle collision) | fragments (N.A.) | 0.7–1.0 MNPs/teabag/filter | [72] |
| water bottle | adhesive wear (compression) | fragments (N.A.) | 112–553 MNPs/L/cycle | [59] |
| teabag | abrasive wear (particle collision) | fragments (N.A.) | 94% of samples released MNPs | [75] |
| teabag | abrasive wear (particle collision) | fiber (N.A.) | 53.4–80.1 MNPs/kg teabag | [30] |
| fabric production | adhesive wear (compression and twisting) | fiber (N.A.) | mean at 114 mg MNPs/kg fabric | [76] |
| rope | adhesive wear (compression and twisting) | fragments (N.A.) | 11–1052 μg MNPs/m rope | [77] |
| bowls | N.A. | N.A. | 331–898 MNPs/treatment | [78] |
| facial scrub | abrasive wear (shear stress) | fragments (nano-sized) | up to 1011 MNPs/4g samples | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Craig, N.; Su, L. A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use. Toxics 2023, 11, 774. https://doi.org/10.3390/toxics11090774
Yu Y, Craig N, Su L. A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use. Toxics. 2023; 11(9):774. https://doi.org/10.3390/toxics11090774
Chicago/Turabian StyleYu, Yang, Nicholas Craig, and Lei Su. 2023. "A Hidden Pathway for Human Exposure to Micro- and Nanoplastics—The Mechanical Fragmentation of Plastic Products during Daily Use" Toxics 11, no. 9: 774. https://doi.org/10.3390/toxics11090774





