Spatial Variability of Rare Earth Elements in Groundwater in the Vicinity of a Coal-Fired Power Plant and Associated Health Risk
Abstract
:1. Introduction
2. Materials and Methods
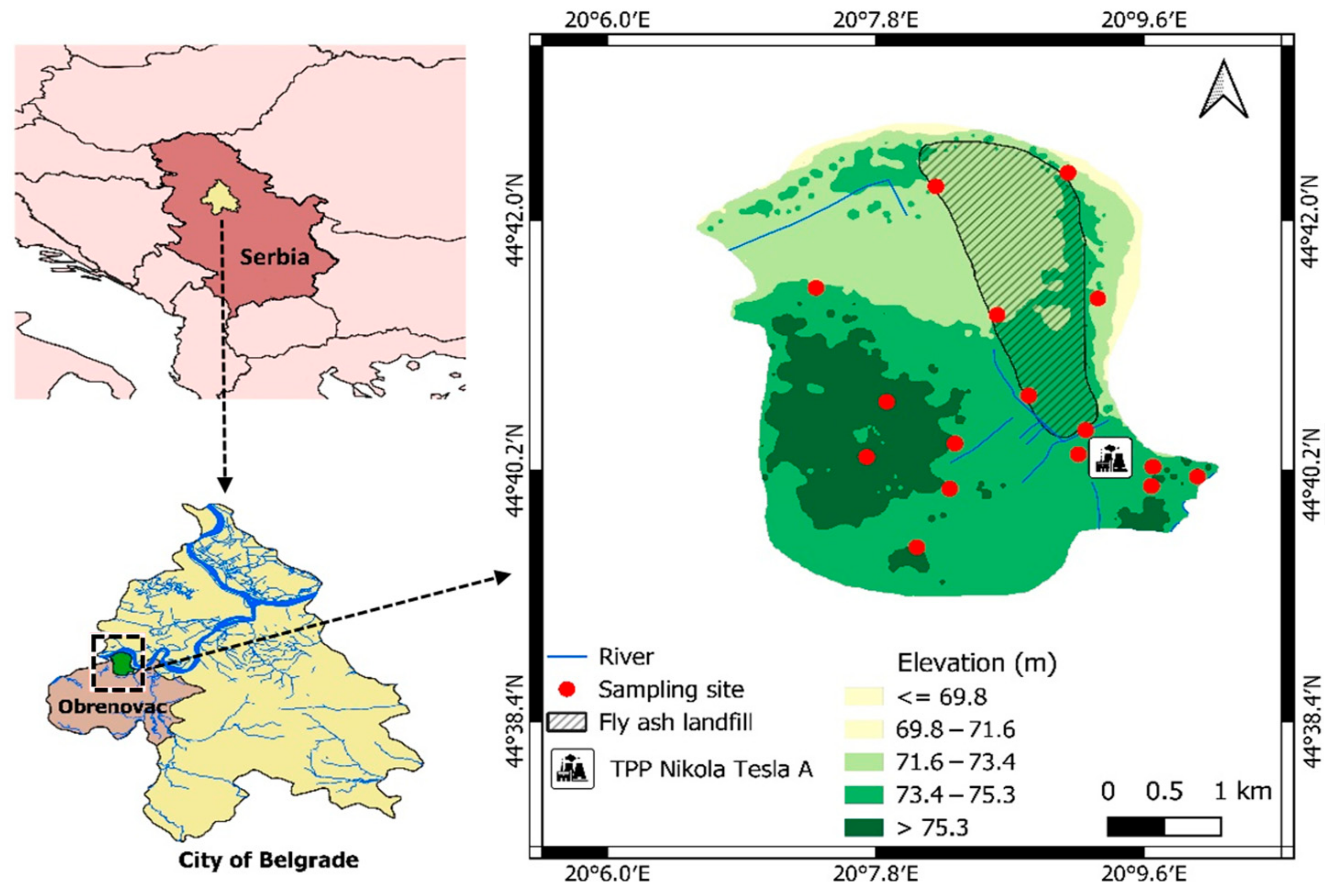
2.1. Study Area
2.2. Sampling and Laboratory Analysis
2.3. REE Distribution Pattern and Anomalies Calculation
2.4. Health Risk Assessment
2.5. Data Analysis
3. Results and Discussion
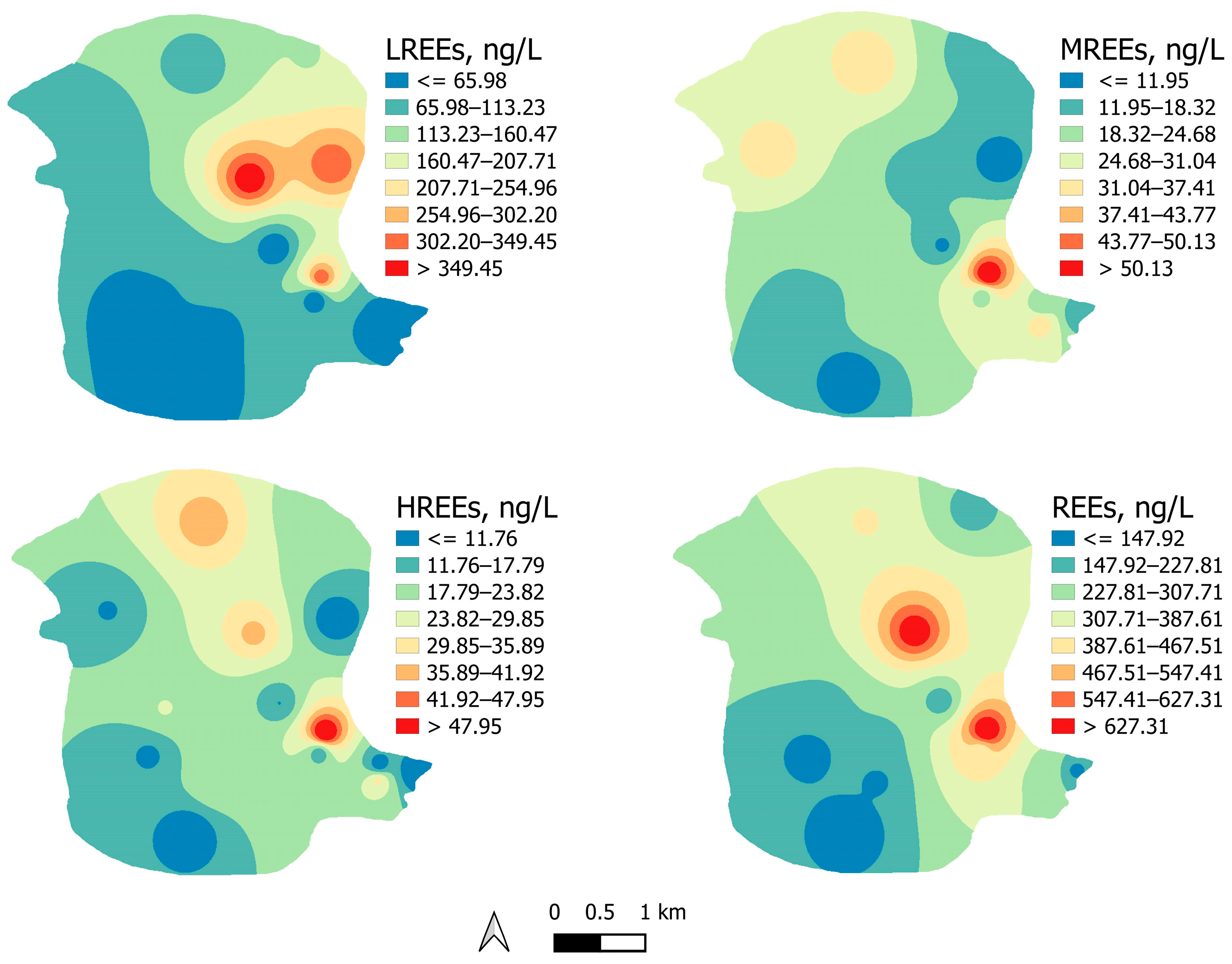
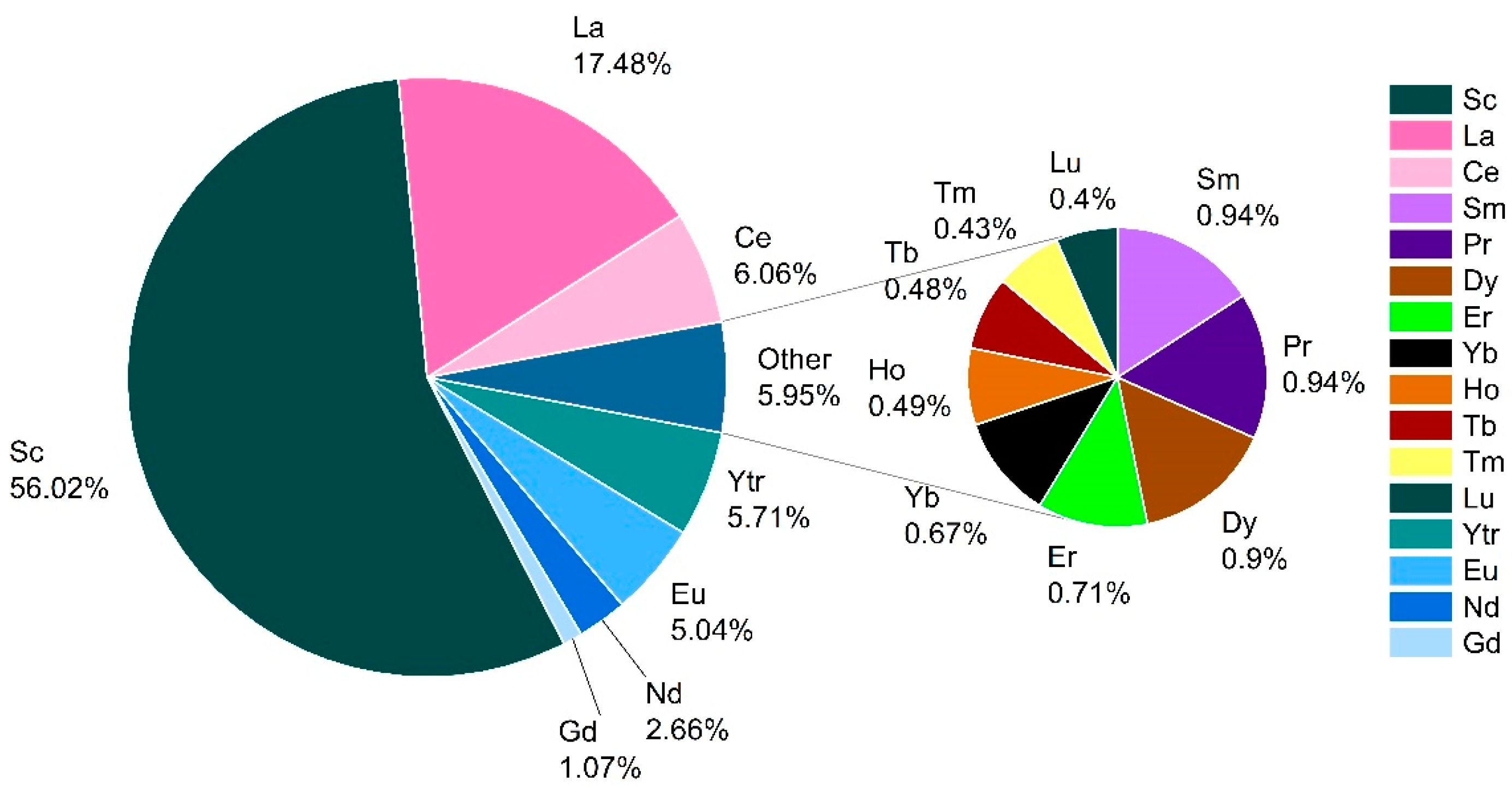
3.1. Occurrence and Geospatial Variation of REEs in Groundwater
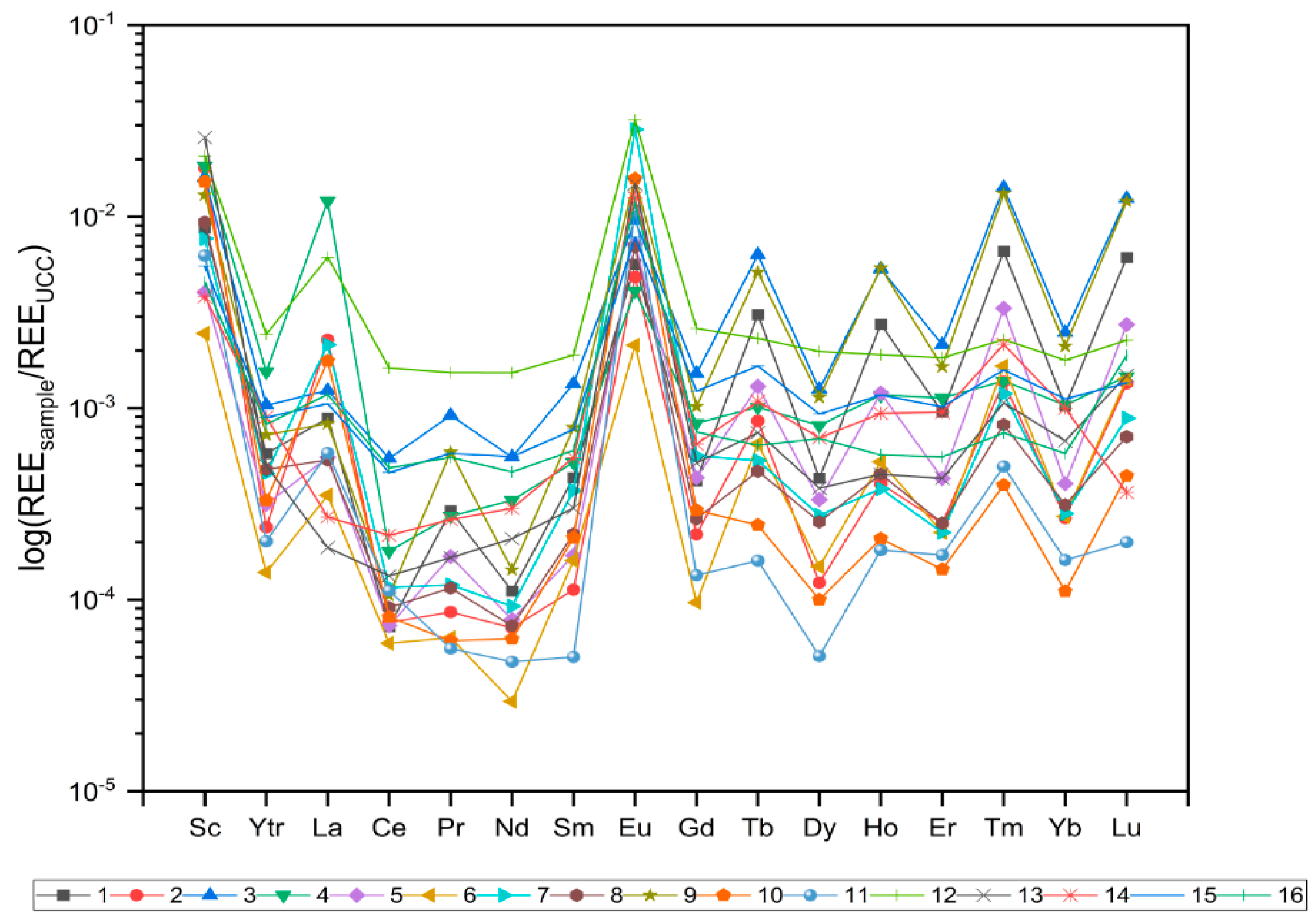
3.2. REE Distribution Pattern and Anomalies
3.3. Multivariate Statistics
3.4. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.D.S.; Fontes, M.P.F.; Lima, M.T.W.D.C.; Cordeiro, S.G.; Wyatt, N.L.P.; Lima, H.N.; Fendorf, S. Human Health Risk Assessment and Geochemical Mobility of Rare Earth Elements in Amazon Soils. Sci. Total Environ. 2022, 806, 151191. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, H.; An, Y.; Ma, J.; Zhao, W.; Qu, Y.; Chen, H.; Liu, L.; Wu, F. Source, Environmental Behavior and Potential Health Risk of Rare Earth Elements in Beijing Urban Park Soils. J. Hazard. Mater. 2023, 445, 130451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive Review of Rare Earth Elements Recovery from Coal-Related Materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Soroaga, L.V.; Amarandei, C.; Negru, A.G.; Olariu, R.I.; Arsene, C. Assessment of the Anthropogenic Impact and Distribution of Potentially Toxic and Rare Earth Elements in Lake Sediments from North-Eastern Romania. Toxics 2022, 10, 242. [Google Scholar] [CrossRef]
- Zhuang, M.; Zhao, J.; Li, S.; Liu, D.; Wang, K.; Xiao, P.; Yu, L.; Jiang, Y.; Song, J.; Zhou, J.; et al. Concentrations and Health Risk Assessment of Rare Earth Elements in Vegetables from Mining Area in Shandong, China. Chemosphere 2017, 168, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Wu, Q.; An, Y.; Jia, H.; Shen, Y. Anthropogenic Rare Earth Elements: Gadolinium in a Small Catchment in Guizhou Province, Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 4052. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Stopić, S.; Friedrich, B. Advances in Understanding of the Application of Unit Operations in Metallurgy of Rare Earth Elements. Metals 2021, 11, 978. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; Makarava, I.; Kraslawski, A.; Repo, E. Global Environmental Cost of Using Rare Earth Elements in Green Energy Technologies. Sci. Total Environ. 2022, 832, 155022. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Musgrove, M.; White, S.J.O. Outlining Potential Biomarkers of Exposure and Effect to Critical Minerals: Nutritionally Essential Trace Elements and the Rare Earth Elements. Toxics 2023, 11, 188. [Google Scholar] [CrossRef]
- Martín-León, V.; Rubio, C.; Rodríguez-Hernández, Á.; Zumbado, M.; Acosta-Dacal, A.; Henríquez-Hernández, L.A.; Boada, L.D.; Travieso-Aja, M.D.M.; Luzardo, O.P. Evaluation of Essential, Toxic and Potentially Toxic Elements in Leafy Vegetables Grown in the Canary Islands. Toxics 2023, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Krasavtseva, E.; Maksimova, V.; Slukovskaya, M.; Ivanova, T.; Mosendz, I.; Elizarova, I. Accumulation and Translocation of Rare Trace Elements in Plants near the Rare Metal Enterprise in the Subarctic. Toxics 2023, 11, 898. [Google Scholar] [CrossRef]
- Ayedun, H.; Arowolo, T.A.; Gbadebo, A.M.; Idowu, O.A. Evaluation of Rare Earth Elements in Groundwater of Lagos and Ogun States, Southwest Nigeria. Environ. Geochem. Health 2017, 39, 649–664. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Liu, T.; Chen, J.; Wu, T.; Feng, X. Rare Earth Elements in Street Dust and Associated Health Risk in a Municipal Industrial Base of Central China. Environ. Geochem. Health 2017, 39, 1469–1486. [Google Scholar] [CrossRef]
- Van Dung, N.; Thuan, D.D.; Nhan, D.D.; Carvalho, F.P.; Van Thang, D.; Quang, N.H. Radiation Exposure in a Region with Natural High Background Radiation Originated from Rare Earth Element Deposits at Bat Xat District, Vietnam. Radiat. Environ. Biophys. 2022, 61, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Thomas, P.J.; Di Nunzio, A.; Trifuoggi, M. Human Exposures to Rare Earth Elements: Present Knowledge and Research Prospects. Environ. Res. 2019, 171, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhong, B.; Liang, T.; Xing, B.; Zhu, Y. Atmospheric Thorium Pollution and Inhalation Exposure in the Largest Rare Earth Mining and Smelting Area in China. Sci. Total Environ. 2016, 572, 1–8. [Google Scholar] [CrossRef]
- Lecomte, J.-F.; Shaw, P.; Liland, A.; Markkanen, M.; Egidi, P.; Andresz, S.; Mrdakovic-Popic, J.; Liu, F.; Da Costa Lauria, D.; Okyar, H.B.; et al. ICRP Publication 142: Radiological Protection from Naturally Occurring Radioactive Material (NORM) in Industrial Processes. Ann. ICRP 2019, 48, 5–67. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kim, H.-O.; Rim, K.-T. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf. Health Work. 2019, 10, 409–419. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of Rare Earth Elements: An Overview on Human Health Impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Allajbeu, S.; Yushin, N.S.; Qarri, F.; Duliu, O.G.; Lazo, P.; Frontasyeva, M.V. Atmospheric Deposition of Rare Earth Elements in Albania Studied by the Moss Biomonitoring Technique, Neutron Activation Analysis and GIS Technology. Environ. Sci. Pollut. Res. 2016, 23, 14087–14101. [Google Scholar] [CrossRef] [PubMed]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Slavković-Beškoski, L.; Ignjatović, L.; Bolognesi, G.; Maksin, D.; Savić, A.; Vladisavljević, G.; Onjia, A. Dispersive Solid–Liquid Microextraction Based on the Poly(HDDA)/Graphene Sorbent Followed by ICP-MS for the Determination of Rare Earth Elements in Coal Fly Ash Leachate. Metals 2022, 12, 791. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Liu, C.; Tang, M.; Cao, S.; Hu, T.; Ji, W.; Luo, Y.; Wen, M.; Zhang, N. Modes of Occurrence of Rare Earth Elements in Coal Fly Ash: A Case Study. Energy Fuels 2018, 32, 9738–9743. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An Investigation into Six Coal Fly Ashes from the United Kingdom and Poland to Evaluate Rare Earth Element Content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Hsu-Kim, H.; Taggart, R.K. Distribution of Rare Earth Elements in Fly Ash Derived from the Combustion of Illinois Basin Coals. Fuel 2021, 289, 119990. [Google Scholar] [CrossRef]
- Zierold, K.M.; Odoh, C. A Review on Fly Ash from Coal-Fired Power Plants: Chemical Composition, Regulations, and Health Evidence. Rev. Environ. Health 2020, 35, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal Fly Ash as a Resource for Rare Earth Elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Graham, U.M.; Hood, M.M.; Joshi, P.; Preda, D.V. Ponded and Landfilled Fly Ash as a Source of Rare Earth Elements from a Kentucky Power Plant. Coal Combust. Gasif. Prod. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Lin, R.; Stuckman, M.; Howard, B.H.; Bank, T.L.; Roth, E.A.; Macala, M.K.; Lopano, C.; Soong, Y.; Granite, E.J. Application of Sequential Extraction and Hydrothermal Treatment for Characterization and Enrichment of Rare Earth Elements from Coal Fly Ash. Fuel 2018, 232, 124–133. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mupatsi, N.M.; Mtisi, M.; Mungazi, A.A. Sources and Health Risks of Rare Earth Elements in Waters. In Water Pollution and Remediation: Heavy Metals; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 53, pp. 1–36. ISBN 978-3-030-52420-3. [Google Scholar]
- Vukadinović, B.; Popović, I.; Dunjić, B.; Jovović, A.; Vlajić, M.; Stanković, D.; Bajić, Z.; Kijevčanin, M. Correlation between Eco-Efficiency Measures and Resource and Impact Decoupling for Thermal Power Plants in Serbia. J. Clean. Prod. 2016, 138, 264–274. [Google Scholar] [CrossRef]
- Stevanovic, V.D.; Ilic, M.; Djurovic, Z.; Wala, T.; Muszynski, S.; Gajic, I. Primary Control Reserve of Electric Power by Feedwater Flow Rate Change through an Additional Economizer—A Case Study of the Thermal Power Plant “Nikola Tesla B”. Energy 2018, 147, 782–798. [Google Scholar] [CrossRef]
- Dragović, S.; Ćujić, M.; Slavković-Beškoski, L.; Gajić, B.; Bajat, B.; Kilibarda, M.; Onjia, A. Trace Element Distribution in Surface Soils from a Coal Burning Power Production Area: A Case Study from the Largest Power Plant Site in Serbia. Catena 2013, 104, 288–296. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Geochemical Evolution of the Continental Crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Tarasenko, I.; Kholodov, A.; Zin’kov, A.; Chekryzhov, I. Chemical Composition of Groundwater in Abandoned Coal Mines: Evidence of Hydrogeochemical Evolution. Appl. Geochem. 2022, 137, 105210. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Jia, H.; Wang, Z.; Gao, S.; Zeng, J. Anthropogenic Rare Earth Elements in Urban Lakes: Their Spatial Distributions and Tracing Application. Chemosphere 2022, 300, 134534. [Google Scholar] [CrossRef]
- USEPA. User’s Guide: Human Health Risk Assessment; Office of Emergency and Remedial Response, US Environmental Protection Agency: Washington, DC, USA, 2008.
- Vesković, J.; Bulatović, S.; Miletić, A.; Tadić, T.; Marković, B.; Nastasović, A.; Onjia, A. Source-specific Probabilistic Health Risk Assessment of Potentially Toxic Elements in Groundwater of a Copper Mining and Smelter Area. Stoch. Environ. Res. Risk Assess. 2024. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, J.; Wang, Y.; Elumalai, V.; Subramani, T. Seasonal Variation of Drinking Water Quality and Human Health Risk Assessment in Hancheng City of Guanzhong Plain, China. Expo. Health 2020, 12, 469–485. [Google Scholar] [CrossRef]
- Tomašek, I.; Mouri, H.; Dille, A.; Bennett, G.; Bhattacharya, P.; Brion, N.; Elskens, M.; Fontijn, K.; Gao, Y.; Gevera, P.K.; et al. Naturally Occurring Potentially Toxic Elements in Groundwater from the Volcanic Landscape around Mount Meru, Arusha, Tanzania and Their Potential Health Hazard. Sci. Total Environ. 2022, 807, 150487. [Google Scholar] [CrossRef]
- Muhammad, S.; Usman, Q.A. Heavy Metal Contamination in Water of Indus River and Its Tributaries, Northern Pakistan: Evaluation for Potential Risk and Source Apportionment. Toxin Rev. 2022, 41, 380–388. [Google Scholar] [CrossRef]
- Pereira, W.V.D.S.; Ramos, S.J.; Melo, L.C.A.; Dias, Y.N.; Martins, G.C.; Ferreira, L.C.G.; Fernandes, A.R. Human and Environmental Exposure to Rare Earth Elements in Gold Mining Areas in the Northeastern Amazon. Chemosphere 2023, 340, 139824. [Google Scholar] [CrossRef] [PubMed]
- Lučić, M.; Miletić, A.; Savić, A.; Lević, S.; Ignjatović, I.S.; Onjia, A. Dietary Intake and Health Risk Assessment of Essential and Toxic Elements in Pepper (Capsicum annuum). J. Food Compos. Anal. 2022, 111, 104598. [Google Scholar] [CrossRef]
- Bangotra, P.; Jakhu, R.; Prasad, M.; Aswal, R.S.; Ashish, A.; Mushtaq, Z.; Mehra, R. Investigation of Heavy Metal Contamination and Associated Health Risks in Groundwater Sources of Southwestern Punjab, India. Environ. Monit. Assess. 2023, 195, 367. [Google Scholar] [CrossRef]
- Marković, J.; Jović, M.; Smičiklas, I.; Pezo, L.; Šljivić-Ivanović, M.; Onjia, A.; Popović, A. Chemical Speciation of Metals in Unpolluted Soils of Different Types: Correlation with Soil Characteristics and an ANN Modelling Approach. J. Geochem. Explor. 2016, 165, 71–80. [Google Scholar] [CrossRef]
- Tokatlı, C.; Varol, M.; Ustaoğlu, F.; Muhammad, S. Pollution Characteristics, Sources and Health Risks Assessment of Potentially Hazardous Elements in Sediments of Ten Ponds in the Saros Bay Region (Türkiye). Chemosphere 2023, 340, 139977. [Google Scholar] [CrossRef]
- Hao, X.; Wang, D.; Wang, P.; Wang, Y.; Zhou, D. Evaluation of Water Quality in Surface Water and Shallow Groundwater: A Case Study of a Rare Earth Mining Area in Southern Jiangxi Province, China. Environ. Monit. Assess. 2016, 188, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Liu, H.; Fang, B. Geochemistry of Rare Earth Elements in the Hot Springs in the Simao Basin in Southwestern China. Environ. Earth Sci. 2020, 79, 121. [Google Scholar] [CrossRef]
- Kaczor-Kurzawa, D.; Wysocka, I.; Porowski, A.; Drzewicz, P.; Vassileva, E. The Occurrence and Distribution of Rare Earth Elements in Mineral and Thermal Waters in the Polish Lowlands. J. Geochem. Explor. 2022, 237, 106984. [Google Scholar] [CrossRef]
- Biddau, R.; Bensimon, M.; Cidu, R.; Parriaux, A. Rare Earth Elements in Groundwater from Different Alpine Aquifers. Geochemistry 2009, 69, 327–339. [Google Scholar] [CrossRef]
- Pignotti, E.; Dinelli, E.; Birke, M. Geochemical Characterization and Rare Earth Elements Anomalies in Surface- and Groundwaters of the Romagna Area (Italy). Rend. Lince 2017, 28, 265–279. [Google Scholar] [CrossRef]
- Singh, L.P.; Kshetrimayum, K.S. Distribution of Rare Earth Elements and Stable Isotopic Constituents along the Groundwater Flow Paths in the Quaternary Deposits of Imphal Valley in North-east India. Geol. J. 2022, 57, 832–846. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic Footprints of Rare Earth Elements on Natural Resources and Living Organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Piarulli, S.; Hansen, B.H.; Ciesielski, T.; Zocher, A.-L.; Malzahn, A.; Olsvik, P.A.; Sonne, C.; Nordtug, T.; Jenssen, B.M.; Booth, A.M.; et al. Sources, Distribution and Effects of Rare Earth Elements in the Marine Environment: Current Knowledge and Research Gaps. Environ. Pollut. 2021, 291, 118230. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Xing, L.; Zhan, Y.; Li, F.; Shao, J.; Niu, H.; Liang, X.; Li, C. Geochemical Behaviors of Rare Earth Elements in Groundwater along a Flow Path in the North China Plain. J. Asian Earth Sci. 2016, 117, 33–51. [Google Scholar] [CrossRef]
- Sun, L.; Gui, H.; Chen, S. Rare Earth Element Geochemistry of Groundwaters from Coal Bearing Aquifer in Renlou Coal Mine, Northern Anhui Province, China. J. Rare Earths 2011, 29, 185–192. [Google Scholar] [CrossRef]
- Alakangas, L.J.; Mathurin, F.A.; Åström, M.E. Diverse Fractionation Patterns of Rare Earth Elements in Deep Fracture Groundwater in the Baltic Shield—Progress from Utilisation of Diffusive Gradients in Thin-Films (DGT) at the Äspö Hard Rock Laboratory. Geochim. Cosmochim. Acta 2020, 269, 15–38. [Google Scholar] [CrossRef]
- Kharitonova, N.A.; Vakh, E.A.; Chelnokov, G.A.; Chudaev, O.V.; Aleksandrov, I.A.; Bragin, I.V. REE Geochemistry in Groundwater of the Sikhote Alin Fold Region (Russian Far East). Russ. J. Pac. Geol. 2016, 10, 141–154. [Google Scholar] [CrossRef]
- Duvert, C.; Cendón, D.I.; Raiber, M.; Seidel, J.-L.; Cox, M.E. Seasonal and Spatial Variations in Rare Earth Elements to Identify Inter-Aquifer Linkages and Recharge Processes in an Australian Catchment. Chem. Geol. 2015, 396, 83–97. [Google Scholar] [CrossRef]
- Nigro, A.; Sappa, G.; Barbieri, M. Boron Isotopes and Rare Earth Elements in the Groundwater of a Landfill Site. J. Geochem. Explor. 2018, 190, 200–206. [Google Scholar] [CrossRef]
- Deluca, F.; Mongelli, G.; Paternoster, M.; Zhu, Y. Rare Earth Elements Distribution and Geochemical Behaviour in the Volcanic Groundwaters of Mount Vulture, Southern Italy. Chem. Geol. 2020, 539, 119503. [Google Scholar] [CrossRef]
- Iwatsuki, T.; Munemoto, T.; Kubota, M.; Hayashida, K.; Kato, T. Characterization of Rare Earth Elements (REEs) Associated with Suspended Particles in Deep Granitic Groundwater and Their Post-Closure Behavior from a Simulated Underground Facility. Appl. Geochem. 2017, 82, 134–145. [Google Scholar] [CrossRef]
- Vinnarasi, F.; Srinivasamoorthy, K.; Saravanan, K.; Gopinath, S.; Prakash, R.; Ponnumani, G.; Babu, C. Rare Earth Elements Geochemistry of Groundwater from Shanmuganadhi, Tamilnadu, India: Chemical Weathering Implications Using Geochemical Mass-Balance Calculations. Geochemistry 2020, 80, 125668. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, R.; Awasthi, N.; Das, R. Provenance and Fate of Trace and Rare Earth Elements in the Sediment-Aquifers Systems of Majuli River Island, India. Chemosphere 2019, 237, 124477. [Google Scholar] [CrossRef] [PubMed]
- Pazand, K.; Javanshir, A.R. Rare Earth Element Geochemistry of Spring Water, North Western Bam, NE Iran. Appl. Water Sci. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Lian, Z.; Han, Y.; Zhao, X.; Xue, Y.; Gu, X. Rare Earth Elements in the Upland Soils of Northern China: Spatial Variation, Relationships, and Risk Assessment. Chemosphere 2022, 307, 136062. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.H.; Zhang, Z.; Wang, W.; Oursel, B.; Juillot, F.; Dupouy, C.; Lemonnier, H.; Mounier, S. Tropical Mangrove Forests as a Source of Dissolved Rare Earth Elements and Yttrium to the Ocean. Chem. Geol. 2021, 576, 120278. [Google Scholar] [CrossRef]
- Yin, X.; Martineau, C.; Demers, I.; Basiliko, N.; Fenton, N.J. The Potential Environmental Risks Associated with the Development of Rare Earth Element Production in Canada. Environ. Rev. 2021, 29, 354–377. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Ukah, B.U.; Ubido, O.E.; Unigwe, C.O. A Chemometric Approach to Source Apportionment, Ecological and Health Risk Assessment of Heavy Metals in Industrial Soils from Southwestern Nigeria. Int. J. Environ. Anal. Chem. 2020, 102, 3399–3417. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater Quality Evaluation Using Water Quality Index (WQI) for Drinking Purposes and Human Health Risk (HHR) Assessment in an Agricultural Region of Nanganur, South India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; He, S.; Wang, L. Occurrence and Distribution of Groundwater Fluoride and Manganese in the Weining Plain (China) and Their Probabilistic Health Risk Quantification. Expo. Health 2022, 14, 263–279. [Google Scholar] [CrossRef]
- Haghnazar, H.; Johannesson, K.H.; González-Pinzón, R.; Pourakbar, M.; Aghayani, E.; Rajabi, A.; Hashemi, A.A. Groundwater Geochemistry, Quality, and Pollution of the Largest Lake Basin in the Middle East: Comparison of PMF and PCA-MLR Receptor Models and Application of the Source-Oriented HHRA Approach. Chemosphere 2022, 288, 132489. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, Behaviour, and Environmental and Human Health Risks of High-Technology Rare Earth Elements as Emerging Contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Royer-Lavallée, A.; Neculita, C.M.; Coudert, L. Removal and Potential Recovery of Rare Earth Elements from Mine Water. J. Ind. Eng. Chem. 2020, 89, 47–57. [Google Scholar] [CrossRef]




| Region | REE Conc. Range, ng/L | REE Mean Conc., ng/L | Reference |
|---|---|---|---|
| Polish Lowlands, Poland | 0.6–10,103 | 559.3 | [51] |
| Jiangxi Province, China | 90.0–540.0 | 300.0 | [49] |
| Simao Basin, China | 58.0–783.0 | 220.0 | [50] |
| North China Plain, China | 81.2–163.3 | 109.0 | [57] |
| Anhui Province, China | 21.8–315.8 | 103.5 | [58] |
| Alpine aquifers, Switzerland | 2.60–67.00 | 23.90 | [52] |
| Aspo Hard Rock Laboratory, Sweden | 90.0–880 | 362.9 | [59] |
| Sikhote Alin, Russia | 40.0–920.0 | 450.0 | [60] |
| Teviot Brook catchment, Australia | 18.0–447 | 61.0 | [61] |
| Romagna area, Italy | 28.0–87.0 | 42.0 | [53] |
| Abbruzo region, Italy | 53.0–814 | 330.3 | [62] |
| Mount Vulture Basin, Italy | 19.8–947.1 | 203.2 | [63] |
| Mizunami URL, Japan | 9.3–119.1 | 48.7 | [64] |
| Dindigul District, India | 23.0–16,000 | 849.0 | [65] |
| Imphal Valley, India | 54,000–63,000 | 57,000 | [54] |
| Majuli Island, India | 1270–5710 | 3094 | [66] |
| Bam Plain, Iran | 180.0–8360 | 1250 | [67] |
| Ogun State, Nigeria | 1140–232,000 | 22,600 | [13] |
| Obrenovac, Serbia | 59.9–758 | 290 | this study |
| Sample | Eu/Eu* | Ce/Ce* | Tm/Tm* | Tb/Tb* | (La/Sm)N | (La/Yb)N | (Gd/Yb)N |
|---|---|---|---|---|---|---|---|
| 1 | 13.3 | 0.12 | 6.65 | 7.27 | 2.05 | 0.86 | 0.41 |
| 2 | 29.1 | 0.06 | 5.31 | 5.02 | 20.2 | 8.54 | 0.82 |
| 3 | 6.70 | 0.51 | 6.14 | 4.55 | 0.92 | 0.50 | 0.61 |
| 4 | 6.05 | 0.03 | 1.28 | 1.23 | 23.2 | 11.6 | 0.80 |
| 5 | 23.8 | 0.20 | 7.98 | 3.40 | 3.25 | 1.37 | 1.07 |
| 6 | 16.6 | 0.29 | 6.69 | 5.28 | 2.18 | 1.29 | 0.36 |
| 7 | 61.1 | 0.10 | 4.69 | 1.27 | 5.78 | 7.60 | 2.00 |
| 8 | 28.7 | 0.28 | 2.91 | 1.80 | 2.43 | 1.72 | 0.85 |
| 9 | 17.5 | 0.15 | 7.04 | 4.73 | 1.05 | 0.39 | 0.48 |
| 10 | 62.8 | 0.09 | 3.11 | 1.25 | 8.42 | 16.0 | 2.65 |
| 11 | 117 | 0.35 | 2.99 | 1.73 | 11.6 | 3.62 | 0.83 |
| 12 | 14.2 | 0.42 | 1.26 | 1.01 | 3.24 | 3.44 | 1.46 |
| 13 | 36.3 | 0.76 | 1.93 | 1.66 | 0.62 | 0.28 | 0.77 |
| 14 | 21.0 | 0.82 | 2.21 | 1.61 | 0.49 | 0.27 | 0.65 |
| 15 | 7.82 | 0.56 | 1.49 | 1.54 | 1.37 | 0.94 | 1.10 |
| 16 | 17.5 | 0.56 | 1.31 | 0.88 | 1.96 | 2.04 | 1.30 |
| Min | 6.05 | 0.03 | 1.26 | 0.88 | 0.49 | 0.27 | 0.36 |
| Max | 118 | 0.82 | 7.98 | 7.27 | 23.2 | 16.0 | 2.65 |
| Average | 30.0 | 0.33 | 3.94 | 2.76 | 5.55 | 3.78 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesković, J.; Lučić, M.; Ristić, M.; Perić-Grujić, A.; Onjia, A. Spatial Variability of Rare Earth Elements in Groundwater in the Vicinity of a Coal-Fired Power Plant and Associated Health Risk. Toxics 2024, 12, 62. https://doi.org/10.3390/toxics12010062
Vesković J, Lučić M, Ristić M, Perić-Grujić A, Onjia A. Spatial Variability of Rare Earth Elements in Groundwater in the Vicinity of a Coal-Fired Power Plant and Associated Health Risk. Toxics. 2024; 12(1):62. https://doi.org/10.3390/toxics12010062
Chicago/Turabian StyleVesković, Jelena, Milica Lučić, Mirjana Ristić, Aleksandra Perić-Grujić, and Antonije Onjia. 2024. "Spatial Variability of Rare Earth Elements in Groundwater in the Vicinity of a Coal-Fired Power Plant and Associated Health Risk" Toxics 12, no. 1: 62. https://doi.org/10.3390/toxics12010062






