Short-Term Effects of Primary and Secondary Particulate Matter on Ceramide Metabolism, Pro-Inflammatory Response, and Blood Coagulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Measurements
2.3. Health Outcomes and Biomarker Measurements
2.4. Statistical Analysis
2.4.1. Demographic Characterization
2.4.2. Linear Mixed-Effect Models
2.4.3. Mediation and Sensitivity Analyses
3. Results
4. Discussion
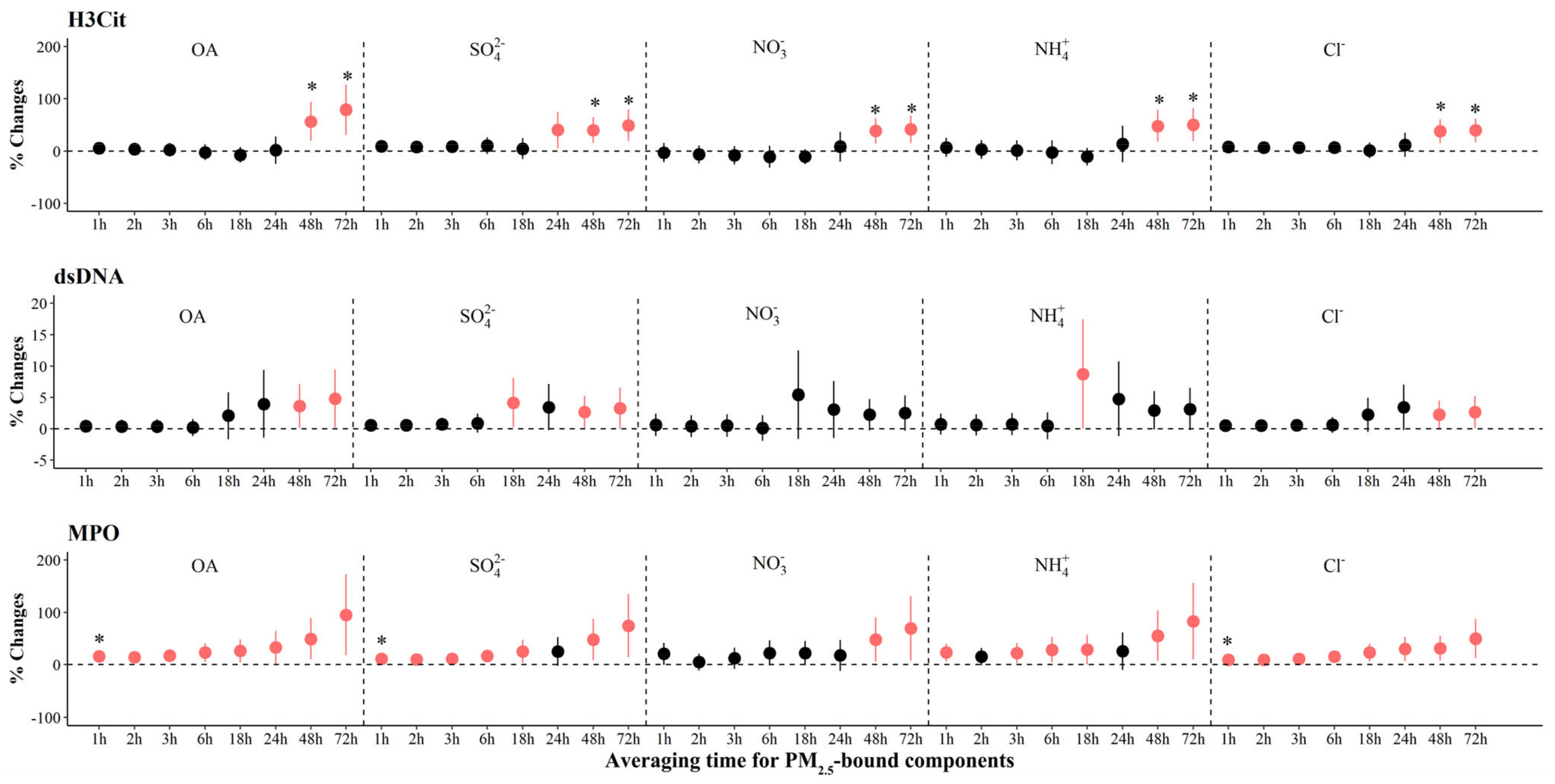
4.1. The Association between Ionic Aerosols and Biomarkers Relevant to Pathophysiology of Atherothrombosis
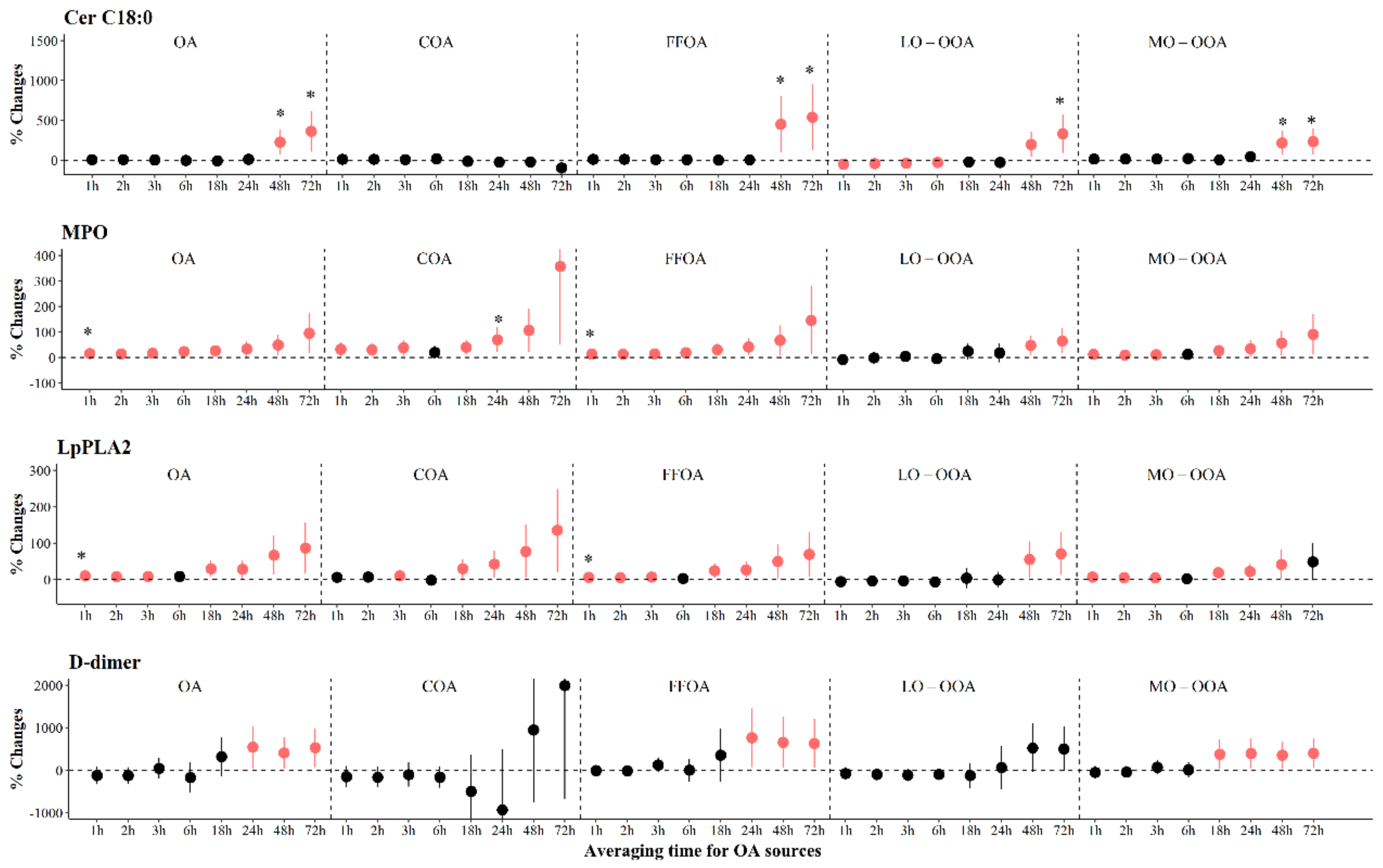
4.2. The Association between OA and Biomarkers Relevant to Pathophysiology of Atherothrombosis
4.3. The Association between Sources of OA and Biomarkers Relevant to Pathophysiology of Atherothrombosis
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Exposure & Health Impacts of Air Pollution. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/sectoral-interventions/ambient-air-pollution/health-risks (accessed on 13 February 2023).
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef]
- Rohr, A.C.; Wyzga, R.E. Attributing health effects to individual particulate matter constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Han, X.K.; Guo, Q.J.; Lang, Y.C.; Li, S.L.; Li, Y.; Guo, Z.B.; Hu, J.; Wei, R.F.; Tian, L.Y.; Wan, Y.X. Seasonal and long-term trends of sulfate, nitrate, and ammonium in PM in Beijing: Implication for air pollution control. Environ. Sci. Pollut. Res. 2020, 27, 23730–23741. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.D.; Feng, W.Y.; Broadwin, R.; Malig, B.J.; Green, R.S.; Lipsett, M.J. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup. Environ. Med. 2008, 65, 750–756. [Google Scholar] [CrossRef] [PubMed]
- So, R.; Chen, J.; Stafoggia, M.; de Hoogh, K.; Katsouyanni, K.; Vienneau, D.; Samoli, E.; Rodopoulou, S.; Loft, S.; Lim, Y.H.; et al. Long-term exposure to elemental components of fine particulate matter and all-natural and cause-specific mortality in a Danish nationwide administrative cohort study. Environ. Res. 2023, 224, 115552. [Google Scholar] [CrossRef] [PubMed]
- Pye, H.O.T.; Ward-Caviness, C.K.; Murphy, B.N.; Appel, K.W.; Seltzer, K.M. Secondary organic aerosol association with cardiorespiratory disease mortality in the United States. Nat. Commun. 2021, 12, 7215. [Google Scholar] [CrossRef] [PubMed]
- Levade, T.; Augé, N.; Veldman, R.J.; Cuvillier, O.; Nègre-Salvayre, A.; Salvayre, R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ. Res. 2001, 89, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.B.; Ross, J.S.; Cowart, L.A. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb. Exp. Pharmacol. 2013, 373–401. [Google Scholar]
- Zhao, C.; Zhu, L.; Li, R.J.; Wang, H.L.; Cai, Z.W. Omics approach reveals metabolic disorders associated with the cytotoxicity of airborne particulate matter in human lung carcinoma cells. Environ. Pollut. 2019, 246, 45–52. [Google Scholar] [CrossRef]
- Shaheen, H.M.; Onoda, A.; Shinkai, Y.; Nakamura, M.; El-Ghoneimy, A.A.; El-Sayed, Y.S.; Takeda, K.; Umezawa, M. The ceramide inhibitor fumonisin B1 mitigates the pulmonary effects of low-dose diesel exhaust inhalation in mice. Ecotoxicol. Environ. Saf. 2016, 132, 390–396. [Google Scholar] [CrossRef]
- Corriden, R.; Hollands, A.; Olson, J.; Derieux, J.; Lopez, J.; Chang, J.T.; Gonzalez, D.J.; Nizet, V. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat. Commun. 2015, 6, 8369. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Henriques-Normark, B. ETosis: A novel cell death pathway. Sci. Signal 2008, 1, pe25. [Google Scholar] [CrossRef]
- Gaggini, M.; Ndreu, R.; Michelucci, E.; Rocchiccioli, S.; Vassalle, C. Ceramides as Mediators of Oxidative Stress and Inflammation in Cardiometabolic Disease. Int. J. Mol. Sci. 2022, 23, 2719. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Lu, W.J.; Changou, C.A.; Hsiung, Y.C.; Trang, N.T.T.; Lee, C.Y.; Chang, T.H.; Jayakumar, T.; Hsieh, C.Y.; Yang, C.H.; et al. Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism. Autophagy 2021, 17, 4141–4158. [Google Scholar] [CrossRef]
- Münzer, P.; Borst, O.; Walker, B.; Schmid, E.; Feijge, M.A.H.; Cosemans, J.M.E.M.; Chatterjee, M.; Schmidt, E.M.; Schmidt, S.; Towhid, S.T.; et al. Acid Sphingomyelinase Regulates Platelet Cell Membrane Scrambling, Secretion, and Thrombus Formation. Arter. Thromb. Vacs. Biol. 2014, 34, 61–71. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Boronat, A.; Serreli, G.; Enriquez, L.; Gomez-Gomez, A.; Pozo, O.J.; Fito, M.; de la Torre, R. Effects of Wine and Tyrosol on the Lipid Metabolic Profile of Subjects at Risk of Cardiovascular Disease: Potential Cardioprotective Role of Ceramides. Antioxidants 2021, 10, 1679. [Google Scholar] [CrossRef]
- Xu, Y.F.; Han, Y.Q.; Wang, Y.W.; Gong, J.C.; Li, H.N.; Wang, T.; Chen, X.; Chen, W.; Fan, Y.F.; Qiu, X.H.; et al. Ambient Air Pollution and Atherosclerosis: A Potential Mediating Role of Sphingolipids. Arter. Thromb. Vasc. Biol. 2022, 42, 906–918. [Google Scholar] [CrossRef]
- Huang, W.; Cao, J.; Tao, Y.; Dai, L.; Lu, S.E.; Hou, B.; Wang, Z.; Zhu, T. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi’an, a Central City in China. Am. J. Epidemiol. 2012, 175, 556–566. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive Matrix Factorization—A Nonnegative Factor Model with Optimal Utilization of Error-Estimates of Data Values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Ulbrich, I.M.; Canagaratna, M.R.; Zhang, Q.; Worsnop, D.R.; Jimenez, J.L. Interpretation of organic components from Positive Matrix Factorization of aerosol mass spectrometric data. Atmos. Chem. Phys. 2009, 9, 2891–2918. [Google Scholar] [CrossRef]
- Li, Y.; Lei, L.; Sun, J.; Gao, Y.; Wang, P.; Wang, S.; Zhang, Z.; Du, A.; Li, Z.; Wang, Z.; et al. Significant Reductions in Secondary Aerosols after the Three-Year Action Plan in Beijing Summer. Environ. Sci. Technol. 2023, 57, 15945–15955. [Google Scholar] [CrossRef]
- Sun, Y.L.; Du, W.; Fu, P.Q.; Wang, Q.Q.; Li, J.; Ge, X.L.; Zhang, Q.; Zhu, C.M.; Ren, L.J.; Xu, W.Q.; et al. Primary and secondary aerosols in Beijing in winter: Sources, variations and processes. Atmos. Chem. Phys. 2016, 16, 8309–8329. [Google Scholar] [CrossRef]
- Hu, J.L.; Wu, L.; Zheng, B.; Zhang, Q.; He, K.B.; Chang, Q.; Li, X.H.; Yang, F.M.; Ying, Q.; Zhang, H.L. Source contributions and regional transport of primary particulate matter in China. Environ. Pollut. 2015, 207, 31–42. [Google Scholar] [CrossRef]
- Fröhlich, R.; Cubison, M.J.; Slowik, J.G.; Bukowiecki, N.; Prévôt, A.S.H.; Baltensperger, U.; Schneider, J.; Kimmel, J.R.; Gonin, M.; Rohner, U.; et al. The ToF-ACSM: A portable aerosol chemical speciation monitor with TOFMS detection. Atmos. Meas. Tech. 2013, 6, 3225–3241. [Google Scholar] [CrossRef]
- Carroll, T.A.; Pinnick, H.A.; Carroll, W.E. Probability and the Westgard Rules. Ann. Clin. Lab. Sci. 2003, 33, 113–114. [Google Scholar] [PubMed]
- Levey, S.; Jennings, E.R. The Use of Control Charts in the Clinical Laboratory. Arch. Pathol. Lab. Med. 1992, 116, 791–798. [Google Scholar] [CrossRef]
- Westgard, J.O.; Groth, T.; Aronsson, T.; Falk, H.; de Verdier, C.H. Performance characteristics of rules for internal quality control: Probabilities for false rejection and error detection. Clin. Chem. 1977, 23, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.L.; Xu, J.; Peng, X.; Xiao, Z.M.; Chen, K.; Tian, Y.Z.; Guan, X.B.; Feng, Y.C.; Yu, H.F.; Nenes, A.; et al. pH of Aerosols in a Polluted Atmosphere: Source Contributions to Highly Acidic Aerosol. Environ. Sci. Technol. 2017, 51, 4289–4296. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.M.; Cao, J.J.; Zhang, R.J. A review of current knowledge concerning PM chemical composition, aerosol optical properties and their relationships across China. Atmos. Chem. Phys. 2017, 17, 9485–9518. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Du, W.; Sun, Y.L.; Wang, Z.F.; Tang, G.Q.; Zhu, J. Submicron-scale aerosol above the city canopy in Beijing in spring based on in-situ meteorological tower measurements. Atmos. Res. 2022, 271, 106128. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Liu, H.; Lv, W.; Aikawa, M.; Liu, B.; Wang, J. Pollution sources of atmospheric fine particles and secondary aerosol characteristics in Beijing. J. Environ. Sci. 2020, 95, 91–98. [Google Scholar] [CrossRef]
- Basagaña, X.; Jacquemin, B.; Karanasiou, A.; Ostro, B.; Querol, X.; Agis, D.; Alessandrini, E.; Alguacil, J.; Artiñano, B.; Catrambone, M.; et al. Short-term effects of particulate matter constituents on daily hospitalizations and mortality in five South-European cities: Results from the MED-PARTICLES project. Environ. Int. 2015, 75, 151–158. [Google Scholar] [CrossRef]
- Rodins, V.; Lucht, S.; Ohlwein, S.; Hennig, F.; Soppa, V.; Erbel, R.; Jöckel, K.H.; Weimar, C.; Hermann, D.M.; Schramm, S.; et al. Long-term exposure to ambient source-specific particulate matter and its components and incidence of cardiovascular events-The Heinz Nixdorf Recall study. Environ. Int. 2020, 142, 105854. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhu, Y.T.; Li, L.J.; Liu, S.C.; Song, X.M.; Yi, T.C.; Wang, Y.; Wang, T.; Zhao, Q.; Liu, L.Y.; et al. Combustion-derived particulate organic matter associated with hemodynamic abnormality and metabolic dysfunction in healthy adults. J. Hazard. Mater. 2021, 418, 126261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Xu, H.B.; Wang, T.; Xie, Y.F.; Liu, L.Y.; He, X.H.; Liu, C.J.; Zhao, Q.; Song, X.M.; Zheng, L.M.; et al. Pro-inflammation and pro-atherosclerotic responses to short-term air pollution exposure associated with alterations in sphingolipid ceramides and neutrophil extracellular traps. Environ. Pollut. 2023, 335, 122301. [Google Scholar] [CrossRef]
- Xu, Y.F.; Chen, X.; Han, Y.Q.; Chen, W.; Wang, T.; Gong, J.C.; Fan, Y.F.; Zhang, H.X.Y.; Zhang, L.N.; Li, H.N.; et al. Ceramide metabolism mediates the impaired glucose homeostasis following short-term black carbon exposure: A targeted lipidomic analysis. Sci. Total Environ. 2022, 829, 154657. [Google Scholar] [CrossRef] [PubMed]
- Sadanaga, T.; Sadanaga, M.; Ogawa, S. Evidence That D-Dimer Levels Predict Subsequent Thromboembolic and Cardiovascular Events in Patients With Atrial Fibrillation During Oral Anticoagulant Therapy. J. Am. Coll. Cardiol. 2010, 55, 2226–2231. [Google Scholar] [CrossRef]
- Sako, E.Y. D-dimer and early coronary graft closure: Not so fast. J. Thorac. Cardiovasc. Surg. 2018, 155, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, J.; Qiao, L.; Wang, H.; Xu, W.; Li, H.; Zhao, Z.; Chen, R.; Kan, H. The Acute Effects of Fine Particulate Matter Constituents on Blood Inflammation and Coagulation. Environ. Sci. Technol. 2017, 51, 8128–8137. [Google Scholar] [CrossRef]
- Wiman, B. Plasminogen-Activator Inhibitor-1 (Pai-1) in Plasma—Its Role in Thrombotic Disease. Thromb. Haemost. 1995, 74, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Inflammation as A Precursor of Atherothrombosis, Diabetes and Early Vascular Aging. Int. J. Mol. Sci. 2022, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Sobel, B.E. Increased plasminogen activator inhibitor-1 and vasculopathy. A reconcilable paradox. Circulation 1999, 99, 2496–2498. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Soberanes, S.; Lux, E.; Shang, M.; Aillon, R.P.; Eren, M.; Budinger, G.R.S.; Miyata, T.; Vaughan, D.E. Pharmacological inhibition of PAI-1 alleviates cardiopulmonary pathologies induced by exposure to air pollutants PM2.5. Environ. Pollut. 2021, 287, 117283. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, M.G.; Li, M.M.; Yin, P.; Hu, J.L.; Zhang, C.L.; Wang, H.; Liu, Q.Y.; Wang, B.G. Fine particulate matter constituents and cause-specific mortality in China: A nationwide modelling study. Environ. Int. 2020, 143, 105927. [Google Scholar] [CrossRef]
- Du, X.H.; Zhang, Y.; Liu, C.; Fang, J.L.; Zhao, F.; Chen, C.; Du, P.; Wang, Q.; Wang, J.N.; Shi, W.Y.; et al. Fine particulate matter constituents and sub-clinical outcomes of cardiovascular diseases: A multi-center study in China. Sci. Total Environ. 2021, 759, 143555. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Polidori, A.; Arhami, M.; Kleinman, M.T.; Vaziri, N.D.; Longhurst, J.; Sioutas, C. Air Pollution Exposures and Circulating Biomarkers of Effect in a Susceptible Population: Clues to Potential Causal Component Mixtures and Mechanisms. Environ. Health Perspect. 2009, 117, 1232–1238. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.Y.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, M.; Yu, M.Y.; Cai, X.H.; Du, H.Y.; Li, J.; Zhou, T.; Yan, C.Q.; Wang, X.S.; Shi, Z.B.; et al. High-time-resolution source apportionment of PM in Beijing with multiple models. Atmos. Chem. Phys. 2019, 19, 6595–6609. [Google Scholar] [CrossRef]
- Shang, D.J.; Peng, J.F.; Guo, S.; Wu, Z.J.; Hu, M. Secondary aerosol formation in winter haze over the Beijing-Tianjin-Hebei Region, China. Front. Env. Sci. Eng. 2021, 15, 34. [Google Scholar] [CrossRef]
- Moller, P.; Christophersen, D.V.; Jacobsen, N.R.; Skovmand, A.; Gouveia, A.C.; Andersen, M.H.; Kermanizadeh, A.; Jensen, D.M.; Danielsen, P.H.; Roursgaard, M.; et al. Atherosclerosis and vasomotor dysfunction in arteries of animals after exposure to combustion-derived particulate matter or nanomaterials. Crit. Rev. Toxicol. 2016, 46, 437–476. [Google Scholar] [CrossRef]
- Miller, M.R.; McLean, S.G.; Duffin, R.; Lawal, A.O.; Araujo, J.A.; Shaw, C.A.; Mills, N.L.; Donaldson, K.; Newby, D.E.; Hadoke, P.W. Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice. Part. Fibre Toxicol. 2013, 10, 61. [Google Scholar] [CrossRef]
- Quan, C.; Sun, Q.; Lippmann, M.; Chen, L.C. Comparative effects of inhaled diesel exhaust and ambient fine particles on inflammation, atherosclerosis, and vascular dysfunction. Inhal. Toxicol. 2010, 22, 738–753. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, Y.; Cheng, W.; Wang, T.; Li, Y.; Li, X.; Zhang, J.; Xia, X.; Zheng, Y.; Zhang, R.; et al. Ex vivo coronary endothelial cell activation associated with indoor coal combustion initiated atherosclerosis. Sci. Total Environ. 2023, 858, 160174. [Google Scholar] [CrossRef]
- Jiang, X.; Han, Y.; Qiu, X.; Liu, J.; Cheng, Z.; Zhang, H.; Wang, Y.; Chen, X.; Fan, Y.; Shang, Y.; et al. Personal exposure to electrophilic compounds of fine particulate matter and the inflammatory response: The role of atmospheric transformation. J. Hazard. Mater. 2022, 432, 128559. [Google Scholar] [CrossRef]
- Arashiro, M.; Lin, Y.H.; Zhang, Z.; Sexton, K.G.; Gold, A.; Jaspers, I.; Fry, R.C.; Surratt, J.D. Effect of secondary organic aerosol from isoprene-derived hydroxyhydroperoxides on the expression of oxidative stress response genes in human bronchial epithelial cells. Environ. Sci. Process Impacts 2018, 20, 332–339. [Google Scholar] [CrossRef]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Munoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordonez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, C.; Yang, G.L.; Fang, J.; Sun, Y.; Tang, L.; Wang, H.; Xiang, W.; Zhang, H.; Croteau, P.L.; et al. Sources and processes of organic aerosol in non-refractory PM1 and PM2.5 during foggy and haze episodes in an urban environment of the Yangtze River Delta, China. Environ. Res. 2022, 212, 113557. [Google Scholar] [CrossRef] [PubMed]





| Variables | Total (N = 152) | Women (N = 102) | Men (N = 50) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Characteristics | ||||||
| Age, years | 23.9 (2.4) | 23.8 (2.2) | 23.6 (2.2) | 23.8 (2.8) | 24.4 (2.7) | 24.1 (2.3) |
| BMI, kg/m2 | 21.6 (2.7) | 21.4 (3.4) | 20.9 (2.6) | 20.6 (23.2) | 23.0 (2.4) | 22.6 (2.8) |
| WHR | 0.8 (0.1) | 0.8 (0.10) | 0.8 (0.05) | 0.76 (0.07) | 0.8 (0.04) | 0.8 (0.05) |
| MET, minutes/week | 1884 (1794) | 1386 (1962) | 1763 (1700) | 1224 (1889) | 2126 (1951) | 1551 (2330) |
| PSQI | 4.9 (2.3) | 5.0 (3.0) | 4.7 (2.4) | 5.0 (3.0) | 5.3 (2.9) | 5.0 (4.0) |
| Cortisol, ng/mg creatinine | 1.8 (1.2) | 1.5 (1.0) | 2.0 (1.3) | 1.7 (1.2) | 1.6 (0.8) | 1.3 (0.6) |
| Cotinine, ng/mg creatinine | 1.8 (1.2) | 1.5 (1.1) | 2.0 (1.3) | 1.6 (1.3) | 1.5 (0.8) | 1.2 (0.7) |
| Measured biomarkers | ||||||
| Ceramides, μM | ||||||
| Cer C16:0 | 22.9 (48.3) | 8.8 (22.0) | 25.5 (37.2) | 5.9 (24.3) | 14.4 (20.5) | 7.4 (17.3) |
| Cer C18:0 | 27.9 (58.0) | 10.3(28.6) | 29.6 (30.2) | 19.9 (40.6) | 21.5 (30.3) | 7.3 (21.5) |
| Cer C24:1 | 30.1 (49.5) | 14.6 (31.6) | 29.1 (50.6) | 14.5 (28.2) | 32.0 (47.5) | 15.7 (38.7) |
| NETs, pg/mL | ||||||
| H3Cit | 4.8 (6.5) | 3.2 (4.7) | 4.5 (4.6) | 3.3 (4.5) | 5.5 (9.1) | 3.0 (5.3) |
| dsDNA | 163.4 (21.6) | 162.4 (28.5) | 157.0 (22.8) | 154.4 (24.0) | 175.5 (20.9) | 173.9 (29.1) |
| MPO | 18.7 (9.2) | 17.0 (10.7) | 17.8 (8.1) | 16.6 (14.9) | 23.0 (12.8) | 19.4 (15.0) |
| Systemic inflammation, pg/mL | ||||||
| RANTES | 10.2 (3.8) | 9.7 (4.3) | 10.1 (3.8) | 9.7 (4.0) | 10.4 (3.7) | 9.7 (5.2) |
| MCP-1 | 146.7 (67.2) | 138.1 (46.2) | 144.2 (78.1) | 133.2 (43.6) | 151.5 (36.7) | 145.8 (46.3) |
| CD163 | 215.1 (87.1) | 203.7 (129.0) | 213.9 (82.6) | 207.1 (121.1) | 217.4 (95.6) | 193.7 (146.5) |
| LpPLA2 | 40.3 (11.8) | 39.6 (16.7) | 38.5 (11.0) | 36.7 (13.8) | 44.0 (12.5) | 44.7 (19.1) |
| Coagulation, pg/mL | ||||||
| PAI-1 | 38.5 (140.9) | 26.4 (17.6) | 28.3 (15.1) | 23.8 (15.5) | 58.9 (242.0) | 32.0 (21.6) |
| P-selectin | 23.0 (5.4) | 22.7 (7.7) | 20.2 (3.7) | 20.0 (5.0) | 24.6 (5.2) | 23.5 (8.5) |
| D-dimer, ng/mL | 17.6 (45.3) | 5.7 (13.7) | 19.7 (53.7) | 5.2 (15.6) | 13.4 (19.3) | 6.2 (11.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xu, H.; He, X.; Wang, T.; Li, M.; Shan, X.; Zhu, Y.; Liu, C.; Zhao, Q.; Song, X.; et al. Short-Term Effects of Primary and Secondary Particulate Matter on Ceramide Metabolism, Pro-Inflammatory Response, and Blood Coagulation. Toxics 2024, 12, 225. https://doi.org/10.3390/toxics12030225
Zhang B, Xu H, He X, Wang T, Li M, Shan X, Zhu Y, Liu C, Zhao Q, Song X, et al. Short-Term Effects of Primary and Secondary Particulate Matter on Ceramide Metabolism, Pro-Inflammatory Response, and Blood Coagulation. Toxics. 2024; 12(3):225. https://doi.org/10.3390/toxics12030225
Chicago/Turabian StyleZhang, Bin, Hongbing Xu, Xinghou He, Tong Wang, Mengyao Li, Xuyang Shan, Yutong Zhu, Changjie Liu, Qian Zhao, Xiaoming Song, and et al. 2024. "Short-Term Effects of Primary and Secondary Particulate Matter on Ceramide Metabolism, Pro-Inflammatory Response, and Blood Coagulation" Toxics 12, no. 3: 225. https://doi.org/10.3390/toxics12030225





