Restricted-Access Media Column Switching Online Solid-Phase Extraction UHPLC–MS/MS for the Determination of Seven Type B Trichothecenes in Whole-Grain Preprocessed Foods and Human Exposure Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Standard Solutions
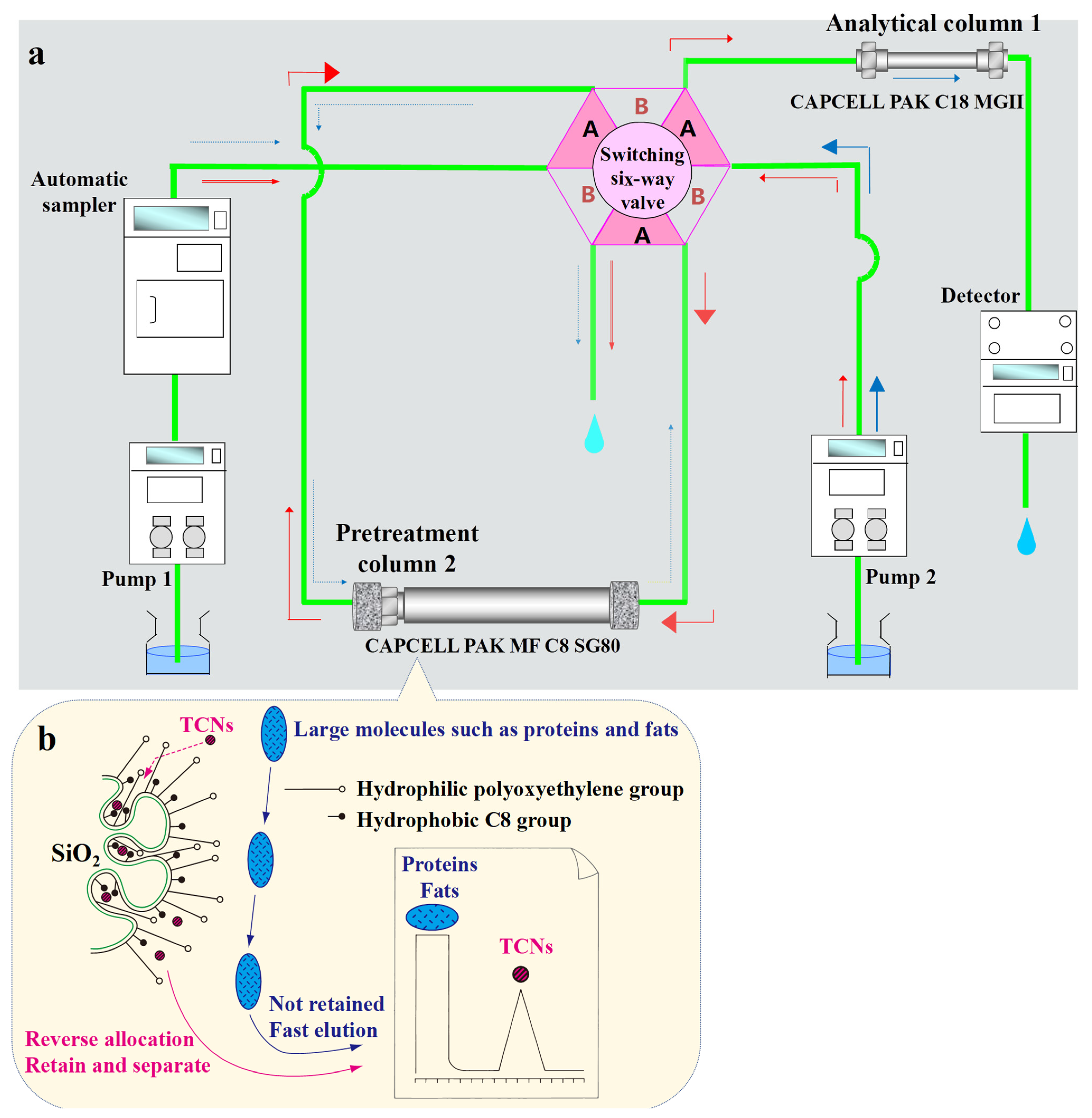
2.3. Online SPE–LC–MS/MS Method
2.3.1. Mass Spectrometry Conditions
2.3.2. Liquid Chromatography Analysis
2.4. Samples and Sample Preparation
Extraction Procedure
2.5. Method Validation
2.6. Risk Assessment
2.7. Data Analysis
3. Results and Discussion
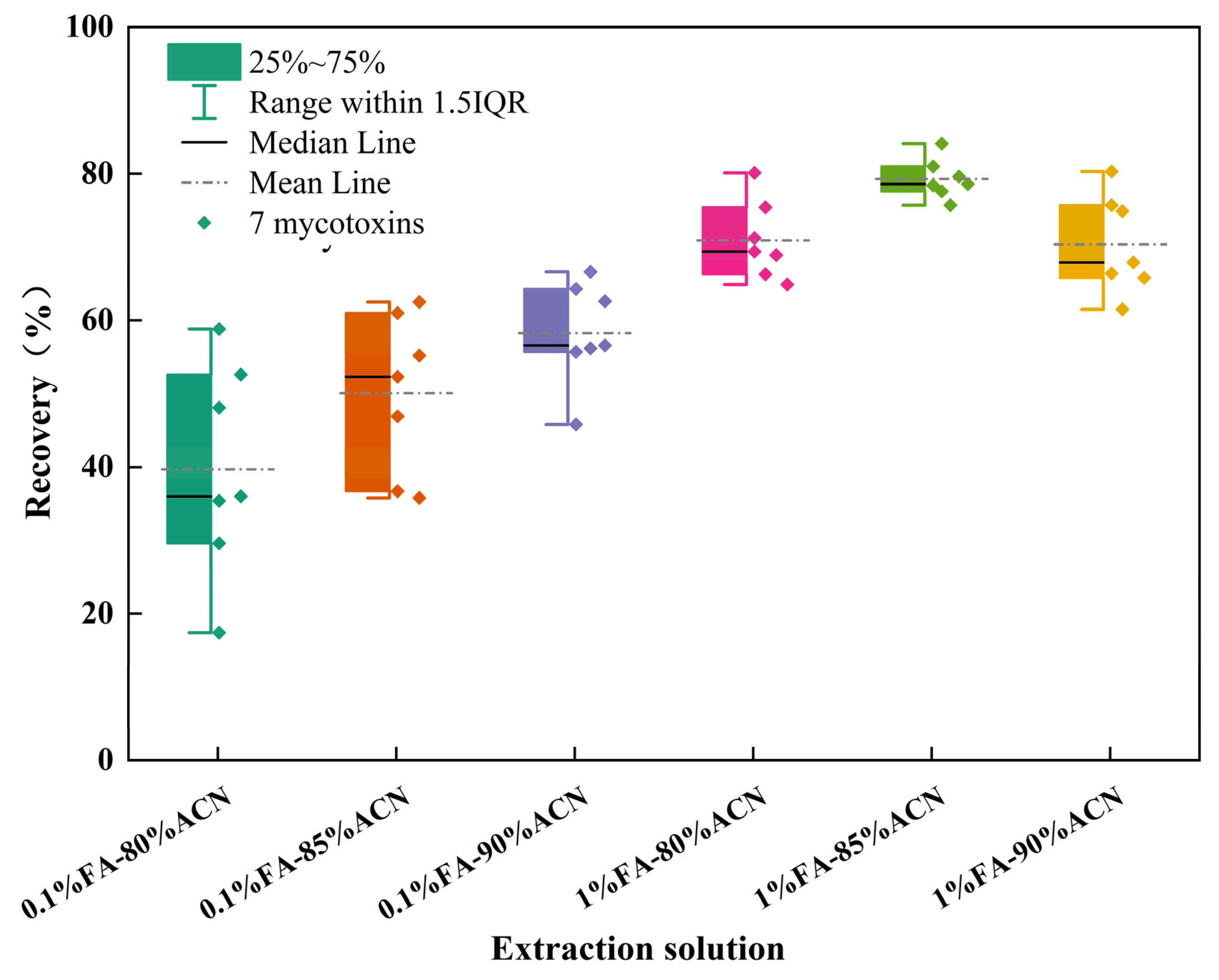
3.1. Selection of Sample Extraction
3.2. Optimization of UPLC–MS/MS
3.3. Time of Valve Switching
3.4. Method Validation
3.5. Application to Real Samples
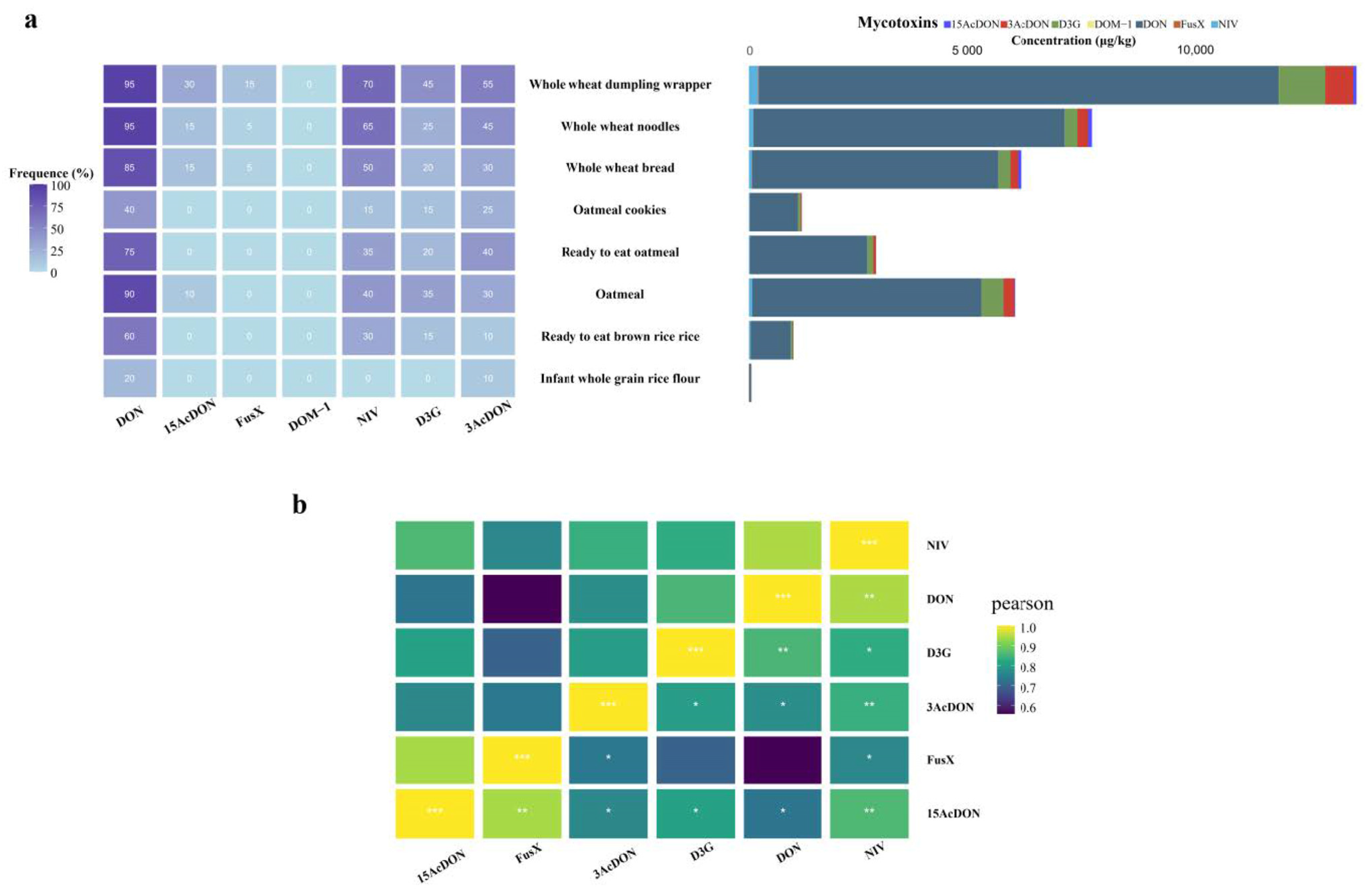
3.5.1. Occurrence of Mycotoxins in Samples
3.5.2. Contamination Characteristics of Type B Trichothecenes in Different Types of Samples
3.5.3. Co-Contamination Patterns
3.6. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- European Food Safety Authority. Risk to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2020. [Google Scholar] [CrossRef]
- World Health Organization. Deoxynivalenol (DON) in Feed and Food; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- You, Y.; Hu, Q.; Liu, N.; Xu, C.; Lu, S.; Xu, T.; Mao, X. Metabolite analysis of Alternaria mycotoxins by LC-MS/MS and multiple tools. Molecules 2023, 28, 3258. [Google Scholar] [CrossRef]
- Pavlenko, R.; Berzina, Z.; Reinholds, I.; Bartkiene, E.; Bartkevics, V. An occurrence study of mycotoxins in plant-based beverages using liquid chromatography-mass spectrometry. Toxins 2024, 16, 53. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New Advanced materials and sorbent-based microextraction techniques as strategies in sample preparation to improve the determination of natural toxins in food samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef]
- Meneely, J.P.; Ricci, F.; van Egmond, H.P.; Elliott, C.T. Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends Anal. Chem. 2011, 30, 192–203. [Google Scholar] [CrossRef]
- Svahn, O.; Björklund, E. High flow-rate sample loading in large volume whole water organic trace analysis using positive pressure and finely ground sand as a SPE-column in-line filter. Molecules 2019, 24, 1426. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Communities 2002, 50, 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002D0657 (accessed on 11 February 2024).
- Zhao, Z.; Rao, Q.; Song, S.; Liu, N.; Han, Z.; Hou, J.; Wu, A. Simultaneous determination of major type B trichothecenes and deoxynivalenol-3-glucoside in animal feed and raw materials using improved DSPE combined with LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 963, 75–82. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. The Chinese Dietary Guidelines 2022; People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- World Health Organization. Towards a harmonised total diet study approach: A guidance document. EFSA J. 2011, 9, 2450. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed. EFSA J. 2017, 15, 4718. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4718 (accessed on 11 February 2024).
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Risks for Animal and Public Health Related to the Presence of Nivalenol in Food and Feed. EFSA J. 2013, 11, 3262. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3262 (accessed on 12 February 2024). [CrossRef]
- Aupanun, S.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Poapolathep, A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J. Vet. Med. Sci. 2017, 79, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Nie, J.Y.; Yan, Z.; Li, Z.X.; Cheng, Y.; Saqib, F. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agri. 2023, 164, 112456. [Google Scholar] [CrossRef]
- Ojuri, O.T.; Ezekiel, C.N.; Eskola, M.K.; Šarkanj, B.; Babalola, A.D.; Sulyok, M.; Krska, R. Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice. Food Control 2019, 98, 312–322. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the processes of nixtamalization and tortilla production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Solanki, M.K.; Abdelfattah, A.; Sadhasivam, S.; Zakin, V.; Wisniewski, M.; Droby, S.; Sionov, E. Analysis of stored wheat grain-associated microbiota reveals biocontrol activity among microorganisms against mycotoxigenic Fungi. J. Fungi 2020, 6, 781. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2020, 9, 811971. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; De Mil, T.; Croubels, S. Development and validation of an LC-MS/MS method for the toxicokinetic study of deoxynivalenol and its acetylated derivatives in chicken and pig plasma. J. Chromatogr. B. 2014, 971, 43–51. [Google Scholar] [CrossRef]
- Tahoun, I.F.; Gab-Allah, M.A.; Yamani, R.N.; Shehata, A.B. Development and validation of a reliable LC-MS/MS method for simultaneous determination of deoxynivalenol and T-2 toxin in maize and oats. Microchem. J. 2021, 169, 106599. [Google Scholar] [CrossRef]
- Mohamed, A.; Gab-Allah, K.C.; Byungjoo, K. Accurate determination of type B trichothecenes and conjugated deoxynivalenol in grains by isotope dilution-liquid chromatography tandem mass spectrometry. Food Control 2021, 121, 107557. [Google Scholar]
- Woo, S.Y.; Lee, S.Y.; Park, S.B.; Chun, H.S. Simultaneous determination of 17 regulated and non-regulated Fusarium mycotoxins co-occurring in foodstuffs by UPLC-MS/MS with solid-phase extraction. Food Chem. 2024, 438, 137624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhao, M.J.; Liu, C.Y.; Ma, K.; Liu, T.Y.; Chen, F.; Wu, L.N.; Hu, D.J.; Lv, G.P. Comparison of two commercial methods with a UHPLC-MS/MS method for the determination of multiple mycotoxins in cereals. Food Chem. 2023, 406, 135056. [Google Scholar] [CrossRef] [PubMed]
- Kresse, M.; Drinda, H.; Romanotto, A.; Speer, K. Simultaneous determination of pesticides, mycotoxins, and metabolites as well as other contaminants in cereals by LC-LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2019, 1117, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S. A multi-analyte LC-MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chem. 2015, 117, 397–404. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Document No. SANTE/11813/2017. 2017. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11813_2017-fin.pdf (accessed on 8 March 2024).
- De Colli, L.; Elliott, C.; Finnan, J.; Grant, J.; Arendt, E.K.; McCormick, S.P.; Danaher, M. Determination of 42 mycotoxins in oats using a mechanically assisted QuEChERS sample preparation and UHPLC-MS/MS detection. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2020, 1150, 122187. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry. Harmonized Guidelines for Single-Laboratory Validation of Methods of Analysis. Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Food and Drug Administration. Analytical Procedures and Methods Validation for Drugs and Biologics. 2015. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/analytical-procedures-and-methods-validation-drugs-and-biologics (accessed on 8 March 2024).
- López-Serna, R.; Pérez, S.; Ginebreda, A.; Petrović, M.; Barceló, D. Fully automated determination of 74 pharmaceuticals in environmental and waste waters by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2010, 83, 410–424. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupolelinear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. General Standard for Contaminants and Toxins in Food and Feed (CODEX STAN 193–1995); Codex Alimentarius Commission: Rome, Italy, 2015. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- National Health and Family Planning Commission of the People’s Republic of China, Standardization Administration of China. GB 2761–2017 National Food Safety Standard-Maximum Levels of Mycotoxins in Foods; Standards Press of China: Beijing, China, 2017. [Google Scholar]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Simsek, S.; Brûlé-Babel, A.; Fernando, W.G. Analysis of deoxynivalenol and deoxynivalenol-3-glucosides content in Canadian spring wheat cultivars inoculated with Fusarium graminearum. Food Addit. Contam. Part A 2016, 33, 1254–1264. [Google Scholar] [CrossRef]
- Ueno, Y.; Hsieh, D.P.H. The toxicology of mycotoxins. Crit. Rev. Toxicol. 1985, 14, 99–132. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Khiaosa-Ard, R.; Nagl, V.; Faas, J.; Jenkins, T.; Sulyok, M.; Zebeli, Q. Mycotoxins, Phytoestrogens and other secondary metabolites in Austrian pastures: Occurrences, contamination levels and implications of geo-climatic factors. Toxins 2021, 13, 460. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, A.; Liu, M.; Yan, Z.; Qin, L.; Liu, H.; Wu, A.; Liu, N. Mycotoxins in wheat flours marketed in Shanghai, China: Occurrence and dietary risk assessment. Toxins 2022, 14, 748. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Roscoe, M.; Trelka, R.; Gräfenhan, T. Fusarium damage in small cereal grains from Western Canada. 2. Occurrence of fusarium toxins and their source organisms in durum wheat harvested in 2010. J. Agr. Food Chem. 2013, 61, 5438–5448. [Google Scholar] [CrossRef]
- Chen, X.; Dong, F.; Zhong, L.; Wu, D.; Wang, S.; Xu, J.; Ma, G.; Shi, J. Contamination Pattern of Fusarium Toxinsin Cerealsin Sichuan Province. J. Sichuan Agri. Univ. 2021, 39, 141–148. [Google Scholar]
- Broekaert, N.; Devreese, M.; De Mil, T.; Fraeyman, S.; Antonissen, G.; De Baere, S.; De Backer, P.; Vermeulen, A.; Croubels, S. Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol in broiler chickens and pigs. J. Agric. Food Chem. 2015, 63, 8734–8742. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during the processing of wheat for human consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.-B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef]
- Wu, Q.; Kuča, K.; Humpf, H.U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef]
- Brown, D.W.; McCormick, S.P.; Alexander, N.J.; Proctor, R.H.; Desjardins, A.E. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet. Biol. 2001, 32, 121–133. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef]
- Gao, X.; Mu, P.; Wen, J.; Sun, Y.; Chen, Q.; Deng, Y. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018, 112, 310–319. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, D.; Zhou, M.; Zhang, P.; Wang, D. Determination of deoxynivalenol in Chinese steamed bread by HPLC-UV method: A survey of the market in Hebei province, China. Food Control 2018, 92, 43–49. [Google Scholar]
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Ani. Feed Sci. Tech. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Li, C.; Gurusamy, S.; Sun, X.; Gong, Z.; Qian, H. Combined toxicity of prevalent mycotoxins studied in fish cell line and zebrafish larvae revealed that type of interactions is dose-dependent. Aquat. Toxicol. 2017, 193, 60–71. [Google Scholar] [CrossRef]
- Mann, K.D.; Pearce, M.S.; McKevith, B.; Thielecke, F.; Seal, C.J. Whole grain intake and its association with intakes of other foods, nutrients and markers of health in the National Diet and Nutrition Survey rolling programme 2008–2011. Br. J. Nutr. 2015, 13, 1595–1602. [Google Scholar] [CrossRef]

| Analytes | Adduct | Retention Time (min) | m/z | Precursor Ion | Collision Energy (V) | Product Ion | Collision Energy (V) |
|---|---|---|---|---|---|---|---|
| DON | [M+CH3COO]− | 7.43 | 355.1 | 295.1 | −14 | 59.2 | −50 |
| D3G | [M+CH3COO]− | 7.25 | 517.1 | 427.1 | −30 | 457.1 | −20 |
| DOM | [M+CH3COO]− | 8.34 | 339.1 | 249.1 | −15 | 59.1 | −50 |
| FusX | [M+CH3COO]− | 8.60 | 413.3 | 262.9 | −22 | 59.1 | −50 |
| NIV | [M+CH3COO]− | 6.40 | 371.1 | 281.1 | −30 | 59.0 | −46 |
| 3AcDON | [M+CH3COO]− | 10.46 | 397.3 | 337.1 | −13 | 307.2 | −40 |
| 15AcDON | [M+CH3COO]− | 10.33 | 397.3 | 59.0 | −40 | 337.1 | −9 |
| B-Type C-TCNs | Matrixs | Calibration Range (μg/kg) | Linear Equation | Correlation Coefficient (r) | LOD (μg/kg) | LOQ (μg/kg) | ME (%) |
|---|---|---|---|---|---|---|---|
| DON | Ready–to–eat brown rice | 1–1000 | y = 28,048x + 111,732 | 0.9991 | 0.4 | 1 | −6.4 |
| Oatmeal | y = 27,171x + 137,284 | 0.9991 | 0.4 | 1 | −10.6 | ||
| Whole wheat bread | y = 28,249x + 98,763 | 0.9998 | 0.3 | 0.9 | −8.1 | ||
| D3G | Ready–to–eat brown rice | 0.2–200 | y = 18,775x − 987 | 0.9996 | 0.06 | 0.2 | −14.0 |
| Oatmeal | y = 17,106x − 1211 | 0.9993 | 0.05 | 0.15 | −9.3 | ||
| Whole wheat bread | y = 17,614x − 373 | 0.9995 | 0.05 | 0.15 | −10.0 | ||
| 3AcDON | Ready–to–eat brown rice | 1–1000 | y = 25,092x + 52,203 | 0.9992 | 0.2 | 0.8 | −2.5 |
| Oatmeal | y = 24,956x + 34,251 | 0.9994 | 0.25 | 0.8 | −6.6 | ||
| Whole wheat bread | y = 24,278x + 19,342 | 0.9994 | 0.3 | 1 | −5.5 | ||
| 15AcDON | Ready–to–eat brown rice | 2–2000 | y= 12,969x + 10,982 | 0.9993 | 0.5 | 1.5 | 5.4 |
| Oatmeal | y= 13,311x + 41,537 | 0.9996 | 0.6 | 2 | 3.4 | ||
| Whole wheat bread | y= 12,510x + 38,762 | 0.9997 | 0.5 | 1.5 | 4.5 | ||
| DOM–1 | Ready–to–eat brown rice | 1–1000 | y = 22,102x + 12,324 | 0.9996 | 0.4 | 1 | 1.6 |
| Oatmeal | y = 24,397x + 10,981 | 0.9998 | 0.3 | 1 | 2.0 | ||
| Whole wheat bread | y = 22,274x + 8976 | 0.9993 | 0.4 | 1 | 2.6 | ||
| FusX | Ready–to–eat brown rice | 1–1000 | y = 7115x + 3791 | 0.9998 | 0.2 | 0.8 | −3.1 |
| Oatmeal | y = 6113x + 3429 | 0.9997 | 0.2 | 0.8 | −6.2 | ||
| Whole wheat bread | y = 6202x + 2167 | 0.9995 | 0.3 | 1 | −3.6 | ||
| NIV | Ready–to–eat brown rice | 1–1000 | y = 10,810x + 8035 | 0.9994 | 0.3 | 1 | −9.3 |
| Oatmeal | y = 9879x + 8035 | 1.0000 | 0.3 | 1 | −12.8 | ||
| Whole wheat bread | y = 9976x + 8035 | 0.9994 | 0.3 | 1 | −12.2 |
| Whole Grain Pre-Processed Foods | Type B Trichothecenes | C (μg/kg) | TDI (μg/kg·day·bw) | EDI (μg/kg·day·bw) | HQ (%) | HI (%) |
|---|---|---|---|---|---|---|
| Ready-to-eat brown rice | DON | 45.60 | 1 | 0.0760 | 7.600 | 9.510 |
| D3G | 2.24 | 0.3 | 0.0037 | 1.242 | ||
| NIV | 2.06 | 0.7 | 0.0034 | 0.490 | ||
| 3AcDON | 0.67 | 1 | 0.0011 | 0.112 | ||
| 15AcDON | 0.25 | 1 | 0.0004 | 0.042 | ||
| FusX | 0.10 | 0.7 | 0.0002 | 0.024 | ||
| Oatmeal | DON | 260.36 | 1 | 0.4339 | 43.393 | 60.419 |
| D3G | 24.95 | 0.3 | 0.0416 | 13.862 | ||
| NIV | 3.93 | 0.7 | 0.0066 | 0.936 | ||
| 3AcDON | 12.28 | 1 | 0.0205 | 2.046 | ||
| 15AcDON | 0.96 | 1 | 0.0016 | 0.159 | ||
| FusX | 0.10 | 0.7 | 0.0002 | 0.024 | ||
| Ready-to-eat oatmeal | DON | 133.93 | 1 | 0.2232 | 22.321 | 27.010 |
| D3G | 6.86 | 0.3 | 0.0114 | 3.808 | ||
| NIV | 1.27 | 0.7 | 0.0021 | 0.303 | ||
| 3AcDON | 3.03 | 1 | 0.0050 | 0.504 | ||
| 15AcDON | 0.30 | 1 | 0.0005 | 0.050 | ||
| FusX | 0.10 | 0.7 | 0.0002 | 0.024 | ||
| Oatmeal cookies | DON | 55.86 | 1 | 0.0931 | 9.310 | 11.490 |
| D3G | 2.89 | 0.3 | 0.0048 | 1.606 | ||
| NIV | 1.29 | 0.7 | 0.0022 | 0.308 | ||
| 3AcDON | 1.15 | 1 | 0.0019 | 0.192 | ||
| 15AcDON | 0.30 | 1 | 0.0005 | 0.050 | ||
| FusX | 0.10 | 0.7 | 0.0002 | 0.024 | ||
| Whole-wheat bread | DON | 279.51 | 1 | 0.4659 | 46.585 | 57.242 |
| D3G | 13.83 | 0.3 | 0.0230 | 7.681 | ||
| NIV | 3.59 | 0.7 | 0.0060 | 0.854 | ||
| 3AcDON | 8.88 | 1 | 0.0148 | 1.480 | ||
| 15AcDON | 3.43 | 1 | 0.0057 | 0.572 | ||
| FusX | 0.30 | 0.7 | 0.0005 | 0.071 | ||
| Whole-wheat noodles | DON | 354.05 | 1 | 0.5901 | 59.009 | 71.100 |
| D3G | 14.47 | 0.3 | 0.0241 | 8.041 | ||
| NIV | 5.21 | 0.7 | 0.0087 | 1.241 | ||
| 3AcDON | 12.13 | 1 | 0.0202 | 2.022 | ||
| 15AcDON | 4.42 | 1 | 0.0074 | 0.736 | ||
| FusX | 0.21 | 0.7 | 0.0004 | 0.051 | ||
| Whole-wheat dumpling wrapper | DON | 590.77 | 1 | 0.9846 | 98.462 | 136.408 |
| D3G | 52.70 | 0.3 | 0.0878 | 29.277 | ||
| NIV | 10.84 | 0.7 | 0.0181 | 2.580 | ||
| 3AcDON | 31.40 | 1 | 0.0523 | 5.234 | ||
| 15AcDON | 3.88 | 1 | 0.0065 | 0.647 | ||
| FusX | 0.88 | 0.7 | 0.0015 | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, X.; Ye, Y.; Ji, J.; Hui, Y.; Li, J.; Chen, P.; Jin, S.; Liu, T.; Zhang, Y.; Cao, J.; et al. Restricted-Access Media Column Switching Online Solid-Phase Extraction UHPLC–MS/MS for the Determination of Seven Type B Trichothecenes in Whole-Grain Preprocessed Foods and Human Exposure Risk Assessment. Toxics 2024, 12, 336. https://doi.org/10.3390/toxics12050336
Ning X, Ye Y, Ji J, Hui Y, Li J, Chen P, Jin S, Liu T, Zhang Y, Cao J, et al. Restricted-Access Media Column Switching Online Solid-Phase Extraction UHPLC–MS/MS for the Determination of Seven Type B Trichothecenes in Whole-Grain Preprocessed Foods and Human Exposure Risk Assessment. Toxics. 2024; 12(5):336. https://doi.org/10.3390/toxics12050336
Chicago/Turabian StyleNing, Xiao, Yongli Ye, Jian Ji, Yanchun Hui, Jingyun Li, Po Chen, Shaoming Jin, Tongtong Liu, Yinzhi Zhang, Jin Cao, and et al. 2024. "Restricted-Access Media Column Switching Online Solid-Phase Extraction UHPLC–MS/MS for the Determination of Seven Type B Trichothecenes in Whole-Grain Preprocessed Foods and Human Exposure Risk Assessment" Toxics 12, no. 5: 336. https://doi.org/10.3390/toxics12050336







