The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Nanoplastics Synthesis and Characterization
2.3. Experimental Design
2.4. Burrowing Assay
2.5. Biochemical Analyses
2.5.1. Neurotransmission
2.5.2. Energy-Related Parameters
2.5.3. Antioxidant Enzyme Response
2.5.4. Oxidative Damage
2.6. Statistical Analyses
3. Results
3.1. Nanoplastics Synthesis and Characterization
3.2. Mortality
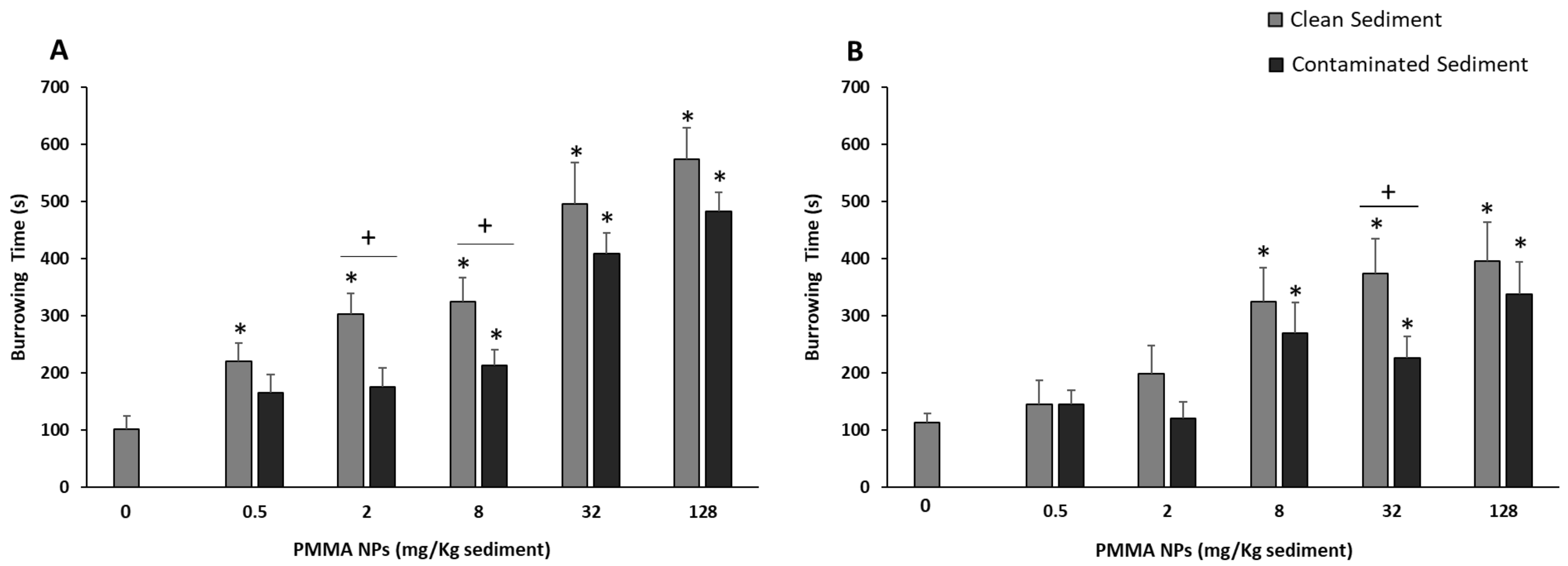
3.3. Behavioral Responses
3.4. Biochemical Responses
3.4.1. Neurotransmission
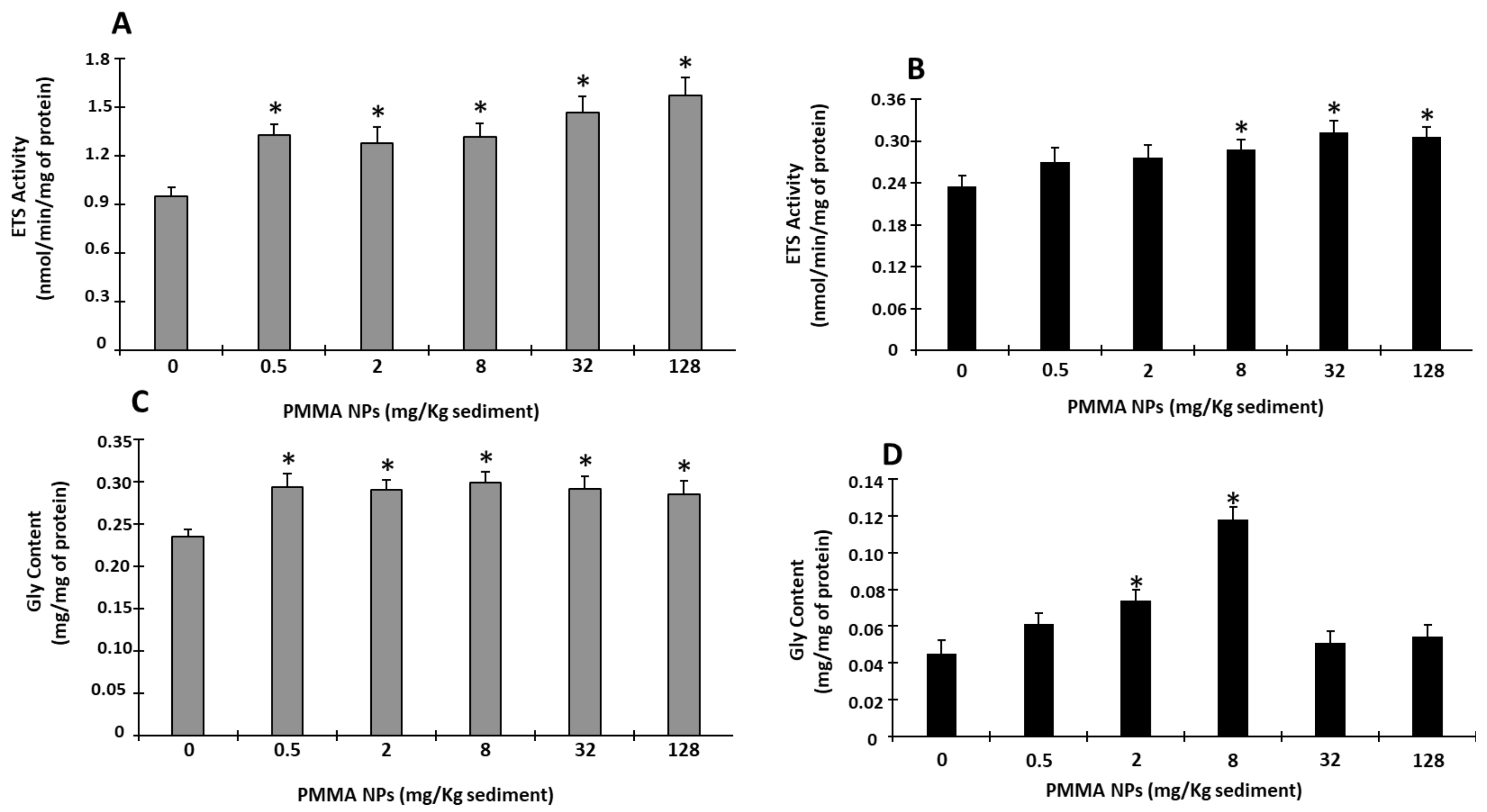
3.4.2. Energy-Related Parameters
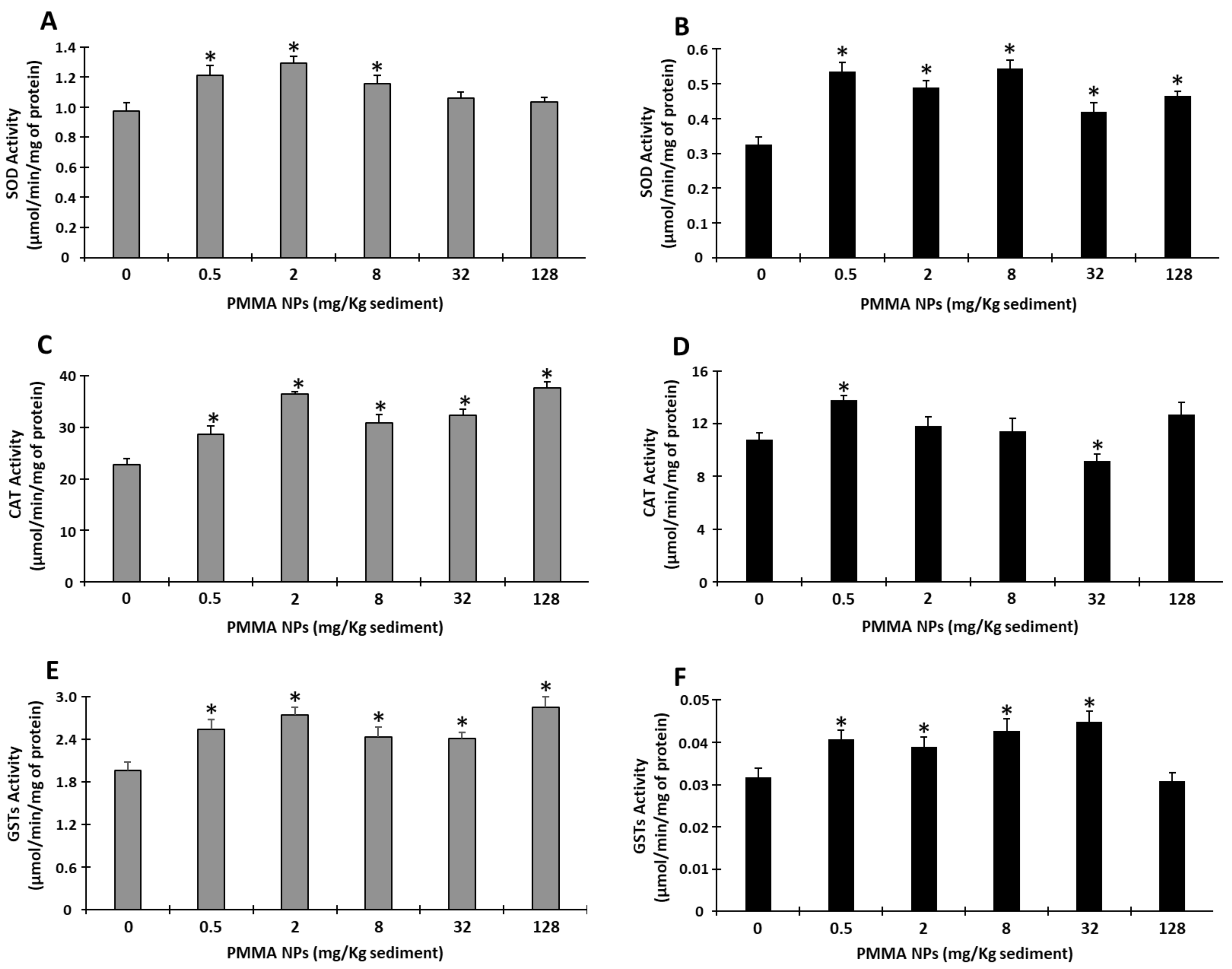
3.4.3. Antioxidant Enzyme Responses
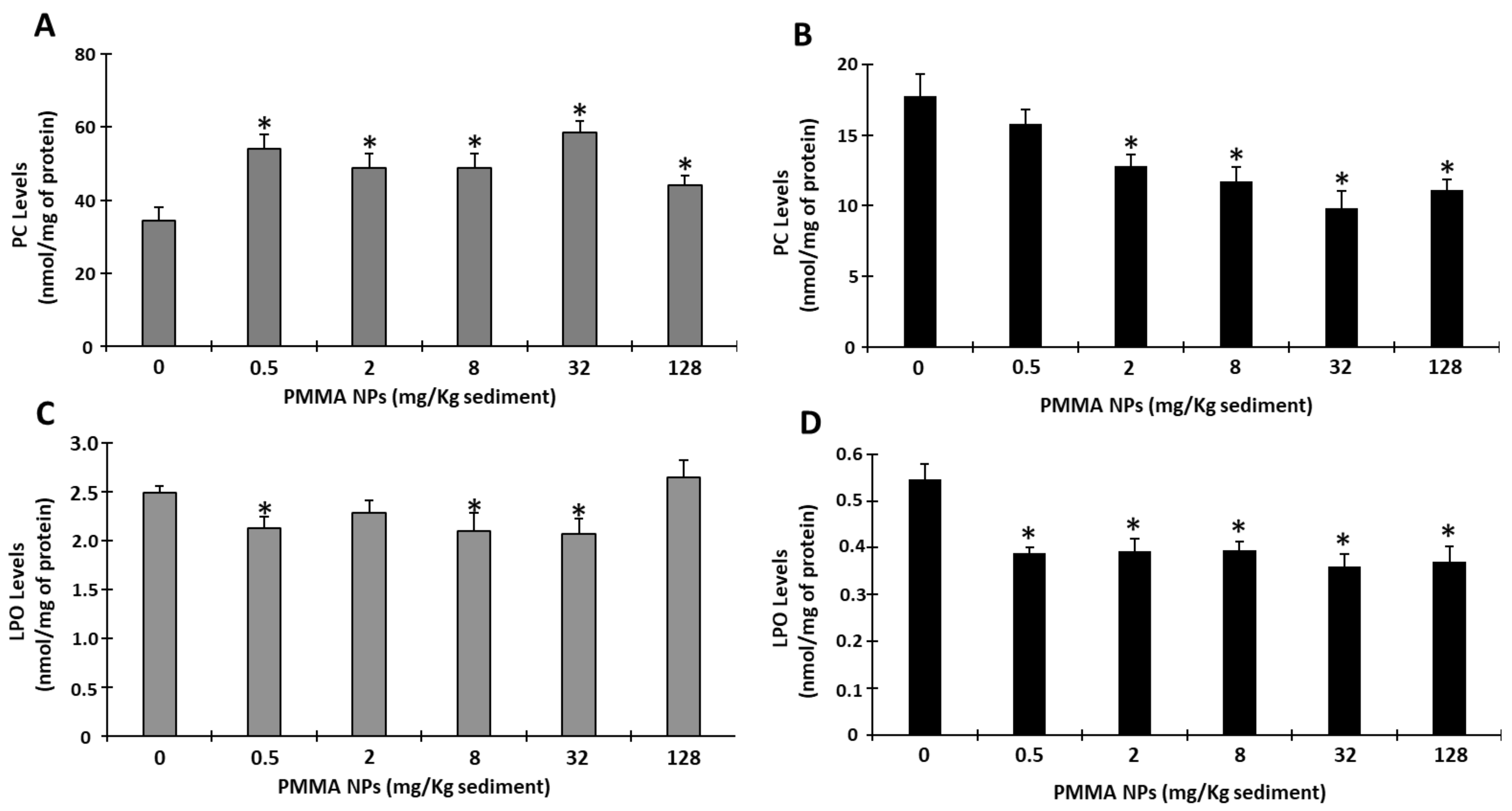
3.4.4. Oxidative Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics the Fast Facts 2023; Plastics Europe: Brussels, Belgium, 2023. [Google Scholar]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Colton, J.B.; Knapp, F.D.; Burns, B.R. Plastic Particles in Surface Waters of the Northwestern Atlantic The abundance, distribution, source, and significance of various types of plastics are discussed. Science 1974, 185, 491–497. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of plastic litter in the oceans. In Marine Anthropogenic Litter; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 29–56. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, F.; Yang, L.; Deng, L.; Shi, Z. Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation. Water 2023, 15, 1920. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Physiochemical properties and degradation. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–100. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Matsunobe, T.; Imai, T. Infrared analysis of depth profiles in UV-photochemical degradation of polymers. Polym. Degrad. Stab. 2005, 88, 224–233. [Google Scholar] [CrossRef]
- Neves, C.V.; Gaylarde, C.C.; Baptista Neto, J.A.; Vieira, K.S.; Pierri, B.; Waite, C.C.C.; Scott, D.C.; da Fonseca, E.M. The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biol. Secur. 2022, 1, 100080. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Pires, A.; Cuccaro, A.; Sole, M.; Freitas, R. Micro(nano)plastics and plastic additives effects in marine annelids: A literature review. Environ. Res. 2022, 214, 113642. [Google Scholar] [CrossRef]
- Pisani, X.G.; Lompré, J.S.; Pires, A.; Greco, L.L. Plastics in scene: A review of the effect of plastics in aquatic crustaceans. Environ. Res. 2022, 212, 113484. [Google Scholar] [CrossRef]
- Benson, N.U.; Agboola, O.D.; Fred-Ahmadu, O.H.; De-la-Torre, G.E.; Oluwalana, A.; Williams, A.B. Micro(nano)plastics Prevalence, Food Web Interactions, and Toxicity Assessment in Aquatic Organisms: A Review. Front. Mar. Sci. 2022, 9, 851281. [Google Scholar] [CrossRef]
- Ferreira, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Nanoplastics and marine organisms: What has been studied? Environ. Toxicol. Pharmacol. 2019, 67, 1–7. [Google Scholar] [CrossRef]
- Riveiro, A.; Chantada, A.; Soto, R.; del Val, J.; Arias-González, F.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface texturing of thermoplastics to improve biological performance. In Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 29–56. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Pawar, E. Related papers Strength Appraisal of Fibre Reinforced Concrete with Partial Replacement of OPC with Miner… A Review Article on Acrylic PMMA. IOSR J. Mech. Civ. Eng. IOSR-JMCE 2016, 13, 1–4. [Google Scholar]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Hassanzadeh Tabrizi, S.A.; Ismail, A.F.; Seifalian, A.; RamaKrishna, S.; Berto, F. Poly(methyl methacrylate) bone cement, its rise, growth, downfall and future. Polym. Int. 2021, 70, 1182–1201. [Google Scholar] [CrossRef]
- Avella, M.; Errico, M.E.; Martuscelli, E. Novel PMMA/CaCO3 Nanocomposites Abrasion Resistant Prepared by an in Situ Polymerization Process. Nano Lett. 2001, 1, 213–217. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Cheng, R.; Luo, Q.; Luo, Y.; Chen, Y.; Chen, X.; Sun, Z.; Huang, S. Eu3+-doped NaGdF4 nanocrystal down-converting layer for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 17454–17462. [Google Scholar] [CrossRef] [PubMed]
- Perween, M.; Parmar, D.B.; Bhadu, G.R.; Srivastava, D.N. Polymer–graphite composite: A versatile use and throw plastic chip electrode. Analyst 2014, 139, 5919–5926. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Hermsen, E.; Pompe, R.; Besseling, E.; Koelmans, A.A. Detection of low numbers of microplastics in North Sea fish using strict quality assurance criteria. Mar. Pollut. Bull. 2017, 122, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Barría, C.; Martins, M.A.; Franco-Martínez, L.; Barreto, A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. Hazard. Mater. 2021, 403, 123590. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.S.; Pires, A.; Vethaak, A.D.; Martínez-Gómez, C.; Almeida, M.; Pinto, R.; Figueira, E.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on the polychaete Hediste diversicolor: Behavioural, regenerative, and biochemical responses. Aquat. Toxicol. 2023, 265, 106743. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, P. Biodiversity and functioning of polychaetes in benthic sediments. Biodivers. Conserv. 1998, 7, 1133–1145. [Google Scholar] [CrossRef]
- Dean, H.K. The use of polychaetes (Annelida) as indicator species of marine pollution: A review. Rev. Biol. Trop. 2008, 56, 11–38. [Google Scholar]
- Silva, M.S.S.; Pires, A.; Almeida, M.; Oliveira, M. The use of Hediste diversicolor in the study of contaminants. Mar. Environ. Res. 2020, 159, 105013. [Google Scholar] [CrossRef] [PubMed]
- Scaps, P. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O.F. Müller) (Annelida: Polychaeta). Hydrobiologia 2002, 470, 203–218. [Google Scholar] [CrossRef]
- Christensen, B.; Vedel, A.; Kristensen, E. Carbon and nitrogen fluxes in sediment inhabited by suspension-feeding (Nereis diversicolor) and non-suspension-feeding (N. virens) polychaetes. Mar. Ecol. Prog. Ser. 2000, 192, 203–217. [Google Scholar] [CrossRef]
- Kristensen, E.; Kostka, J.E. Macrofaunal Burrows and Irriation in Marine Sediment: Microbiological and Biogeochemical Interactions. In Interactions Between Macro- and Microorganisms in Marine Sediments; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 125–157. [Google Scholar] [CrossRef]
- Vasquez-Cardenas, D.; Quintana, C.O.; Meysman, F.J.R.; Kristensen, E.; Boschker, H.T.S. Species-specific effects of two bioturbating polychaetes on sediment chemoautotrophic bacteria. Mar. Ecol. Prog. Ser. 2016, 549, 55–68. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Pellini, G.; Salvalaggio, V.; Fabi, G. Comparative effects of ingested PVC micro particles with and without adsorbed benzo(a)pyrene vs. spiked sediments on the cellular and sub cellular processes of the benthic organism Hediste diversicolor. Front. Mar. Sci. 2018, 5, 99. [Google Scholar] [CrossRef]
- Auffret, M.; Cachot, J.; Saint-Louis, R. (Eds.) Multi-stressors in freshwater and transitional environments: From legacy pollutants to emerging ones. In Proceedings of the International Francophone Symposium on Aquatic Ecotoxicology (EcoBIM 2018), Bordeaux, France, 22–25 May 2018. [Google Scholar]
- Silva, M.S.S.; Oliveira, M.; Lopéz, D.; Martins, M.; Figueira, E.; Pires, A. Do nanoplastics impact the ability of the polychaeta Hediste diversicolor to regenerate? Ecol. Indic. 2020, 110, 105921. [Google Scholar] [CrossRef]
- Silva, M.S.S.; Oliveira, M.; Valente, P.; Figueira, E.; Martins, M.; Pires, A. Behavior and biochemical responses of the polychaeta Hediste diversicolor to polystyrene nanoplastics. Sci. Total Environ. 2020, 707, 134434. [Google Scholar] [CrossRef] [PubMed]
- Missawi, O.; Bousserrhine, N.; Zitouni, N.; Maisano, M.; Boughattas, I.; De Marco, G.; Cappello, T.; Belbekhouche, S.; Guerrouache, M.; Alphonse, V.; et al. Uptake, accumulation and associated cellular alterations of environmental samples of microplastics in the seaworm Hediste diversicolor. J. Hazard. Mater. 2021, 406, 124287. [Google Scholar] [CrossRef]
- Muller-karanassos, C.; Arundel, W.; Lindeque, P.K.; Vance, T.; Turner, A.; Cole, M. Environmental concentrations of antifouling paint particles are toxic to sediment-dwelling invertebrates. Environ. Pollut. 2021, 268, 115754. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Vorobyova, L. Influence the North-western Part of the Black Sea Habitat Factors on the Meiobenthic Polychaetes. Turk. J. Fish. Aquat. Sci. 2023, 23, 9. [Google Scholar] [CrossRef]
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Hediste diversicolor as bioindicator of pharmaceutical pollution: Results from single and combined exposure to carbamazepine and caffeine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 188, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pires, A. Studying Annelida Body Regeneration Under Environmental Stress in Diopatra neapolitana. In Whole-Body Regeneration. Methods in Molecular Biology; Blanchoud, S., Galliot, B., Eds.; Humana: New York, NY, USA, 2022; Volume 2450. [Google Scholar] [CrossRef]
- Diepens, N.J.; van den Heuvel-Greve, M.J.; Koelmans, A.A. Modeling of Bioaccumulation in Marine Benthic Invertebrates Using a Multispecies Experimental Approach. Environ. Sci. Technol. 2015, 49, 13575–13585. [Google Scholar] [CrossRef]
- Bartels-Hardege, H.D.; Zeeck, E. Reproductive behaviour of Nereis diversicolor (Annelida: Polychaeta). Mar. Biol. 1990, 106, 409–412. [Google Scholar] [CrossRef]
- Santos, A.; Granada, L.; Baptista, T.; Anjos, C.; Simões, T.; Tecelão, C.; Fidalgo e Costa, P.; Costa, J.L.; Pombo, A. Effect of three diets on the growth and fatty acid profile of the common ragworm Hediste diversicolor (O.F. Müller, 1776). Aquaculture 2016, 465, 37–42. [Google Scholar] [CrossRef]
- Manuel, P.; Almeida, M.; Martins, M.; Oliveira, M. Effects of nanoplastics on zebrafish embryo-larval stages: A case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res. 2022, 213, 113584. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, C.; Melnic, I.; Tamayo-Belda, M.; Oliveira, M.; Martins, M.A.; Lopes, I. Polymethylmethacrylate nanoplastics can cause developmental malformations in early life stages of Xenopus laevis. Sci. Total Environ. 2022, 806, 150491. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.L.; Martins, M.A.; Tort, L.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J.C.; Brandts, I.; Barría, C.; Martins, M.A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Short-term exposure to polymethylmethacrylate nanoplastics alters muscle antioxidant response, development and growth in Sparus aurata. Mar. Pollut. Bull. 2021, 172, 112918. [Google Scholar] [CrossRef]
- Morgado, V.; Gomes, L.; Bettencourt da Silva, R.J.N.; Palma, C. Microplastics contamination in sediments from Portuguese inland waters: Physical-chemical characterisation and distribution. Sci. Total Environ. 2022, 832, 155053. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of nanoplastics in the aquatic environment and possible impacts on aquatic organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Yakovenko, N.; Caley, T.; Guillet, C.; Châtel, A.; Mouneyrac, C. Accumulation and immunotoxicity of microplastics in the estuarine worm Hediste diversicolor in environmentally relevant conditions of exposure. Environ. Sci. Pollut. Res. 2020, 27, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Cardoso, D.N.; Soares, A.M.V.M.; Loureiro, S. Effects of short-term exposure to fluoxetine and carbamazepine to the collembolan Folsomia candida. Chemosphere 2015, 120, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.W.; Hogden, C.G. The biuret reaction in the determination of serum proteins I. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of Acetylcholinesterse activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Acetylcholinesterase Activity in Juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef]
- King, F.D.; Packard, T.T. Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels1. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ahmad, I.; Maria, V.L.; Pacheco, M.; Santos, M.A. Antioxidant responses versus DNA damage and lipid peroxidation in golden grey mullet liver: A field study at ria de aveiro (Portugal). Arch. Environ. Contam. Toxicol. 2010, 59, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Liu, N.; Jing, W.; Wang, L.; Zhou, F. Hydrogen peroxide leads to cell damage and apoptosis in the gill of the freshwater crab Sinopotamon henanense (Crustacea, Decapoda). Hydrobiologia 2014, 741, 13–21. [Google Scholar] [CrossRef]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Huvet, A.; Paul-Pont, I. Surface functionalization determines behavior of nanoplastic solutions in model aquatic environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Almeida, M. The why and how of micro(nano)plastic research. TrAC Trends Anal. Chem. 2019, 114, 196–201. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; el Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, C.; Wu, Y.; Guo, X. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. J. Hazard. Mater. 2020, 392, 122418. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.B.; Fahrenson, C.; Givelet, L.; Herrmann, T.; Loeschner, K.; Böhmert, L.; Thünemann, A.F.; Braeuning, A.; Sieg, H. Beyond microplastics—Investigation on health impacts of submicron and nanoplastic particles after oral uptake in vitro. Microplast. Nanoplast. 2022, 2, 16. [Google Scholar] [CrossRef]
- Yu, Y.; Astner, A.F.; Zahid, T.M.; Chowdhury, I.; Hayes, D.G.; Flury, M. Aggregation kinetics and stability of biodegradable nanoplastics in aquatic environments: Effects of UV-weathering and proteins. Water Res. 2023, 239, 120018. [Google Scholar] [CrossRef]
- Valente, P.; Cardoso, P.; Giménez, V.; Silva, M.S.S.; Sá, C.; Figueira, E.; Pires, A. Biochemical and Behavioural Alterations Induced by Arsenic and Temperature in Hediste diversicolor of Different Growth Stages. Int. J. Environ. Res. Public Health 2022, 19, 15426. [Google Scholar] [CrossRef]
- Pires, A.; Figueira, E.; Silva, M.S.S.; Sá, C.; Marques, P.A.A.P. Effects of graphene oxide nanosheets in the polychaete Hediste diversicolor: Behavioural, physiological and biochemical responses. Environ. Pollut. 2022, 299, 118869. [Google Scholar] [CrossRef]
- Burlinson, F.C.; Lawrence, A.J. Development and validation of a behavioural assay to measure the tolerance of Hediste diversicolor to copper. Environ. Pollut. 2007, 145, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lacerda, M.N.; Nogueira, A.J.A.; Soares, A.M.V.M. In vitro and in vivo inhibition of Daphnia magna acetylcholinesterase by surfactant agents: Possible implications for contamination biomonitoring. Sci. Total Environ. 2000, 247, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, M.; Roméo, M.; Amiard-Triquet, C. Effects of copper on the burrowing behavior of estuarine and coastal invertebrates, the polychaete Nereis diversicolor and the bivalve Scrobicularia plana. Hum. Ecol. Risk Assess. 2009, 15, 11–26. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.Y.; Demirbas, D.; Heinrich, R. Multifaceted promotion of apoptosis by acetylcholinesterase. Front. Cell Death 2023, 2, 1169966. [Google Scholar] [CrossRef]
- Correia, A.D.; Costa, M.H.; Luis, O.J.; Livingstone, D.R. Age-related changes in antioxidant enzyme activities, fatty acid composition and lipid peroxidation in whole body Gammarus locusta (Crustacea: Amphipoda). J. Exp. Mar. Biol. Ecol. 2003, 289, 83–101. [Google Scholar] [CrossRef]
- Sokolova, I.M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Bošnjak, I.; Sepčić, K.; Jaklič, M.; Cvitanić, M.; Lušić, J.; Lajtner, J.; Simčič, T.; Hudina, S. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci. Total Environ. 2016, 543, 449–459. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Jakubowska, M.; Hallmann, A.; Dąbrowska, A. Do the graphene nanoflakes pose a potential threat to the polychaete Hediste diversicolor? Chemosphere 2021, 269, 128685. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Brambilla, L.; Rodriguez-Douton, M.J.; Rossella, F.; Tommasini, M.; Furtado, C.; Soares, A.M.V.M.; et al. Physiological and biochemical impacts of graphene oxide in polychaetes: The case of Diopatra neapolitana. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 193, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner, J.M.; Rowenczyk, L.; Petosa, A.R.; Claveau-Mallet, D.; Hernandez, L.M.; Wilkinson, K.J.; Tufenkji, N. Mechanistic understanding of the aggregation kinetics of nanoplastics in marine environments: Comparing synthetic and natural water matrices. J. Hazard. Mater. Adv. 2022, 7, 100115. [Google Scholar] [CrossRef]
- Lai, Y.; Dong, L.; Sheng, X.; Li, Q.; Li, P.; Hao, Z.; Yu, S.; Liu, J. Swelling-Induced Fragmentation and Polymer Leakage of Nanoplastics in Seawater. Environ. Sci. Technol. 2022, 56, 17694–17701. [Google Scholar] [CrossRef] [PubMed]
- Alice, P.; Maud, G.; Dominique, B.; Julien, G. Micro- And nanoplastic transfer in freezing saltwater: Implications for their fate in polar waters. Environ. Sci. Process. Impacts 2021, 23, 1759–1770. [Google Scholar] [CrossRef]
- Shupe, H.J.; Boenisch, K.M.; Harper, B.J.; Brander, S.M.; Harper, S.L. Effect of Nanoplastic Type and Surface Chemistry on Particle Agglomeration over a Salinity Gradient. Environ. Toxicol. Chem. 2021, 40, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Silva, M.S.S.; Oliveira, M.; Almeida, H.; Vethaak, A.D.; Martínez-Gómez, C.; Figueira, E.; Pires, A. Does parental exposure to nanoplastics modulate the response of Hediste diversicolor to other contaminants: A case study with arsenic. Environ. Res. 2022, 214, 113764. [Google Scholar] [CrossRef]
- De Marchi, L.; Oliva, M.; Freitas, R.; Neto, V.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Pretti, C. Toxicity evaluation of carboxylated carbon nanotubes to the reef-forming tubeworm Ficopomatus enigmaticus (Fauvel, 1923). Mar. Environ. Res. 2019, 143, 1–9. [Google Scholar] [CrossRef]
- Giménez, V.; Cardoso, P.; Carina, S.; Patinha, C.; Ferreira, E.; Figueira, E.; Pires, A. Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana. Biology 2022, 11, 1153. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, B.; Oliveira, M.; Frazão, C.; Almeida, M.; Pinto, R.J.B.; Figueira, E.; Pires, A. The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA). Toxics 2024, 12, 352. https://doi.org/10.3390/toxics12050352
Neves B, Oliveira M, Frazão C, Almeida M, Pinto RJB, Figueira E, Pires A. The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA). Toxics. 2024; 12(5):352. https://doi.org/10.3390/toxics12050352
Chicago/Turabian StyleNeves, Beatriz, Miguel Oliveira, Carolina Frazão, Mónica Almeida, Ricardo J. B. Pinto, Etelvina Figueira, and Adília Pires. 2024. "The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA)" Toxics 12, no. 5: 352. https://doi.org/10.3390/toxics12050352








