Bioreactor Expansion Affects Microbial Succession of Mixotrophic Acidophiles and Bioremediation of Cadmium-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
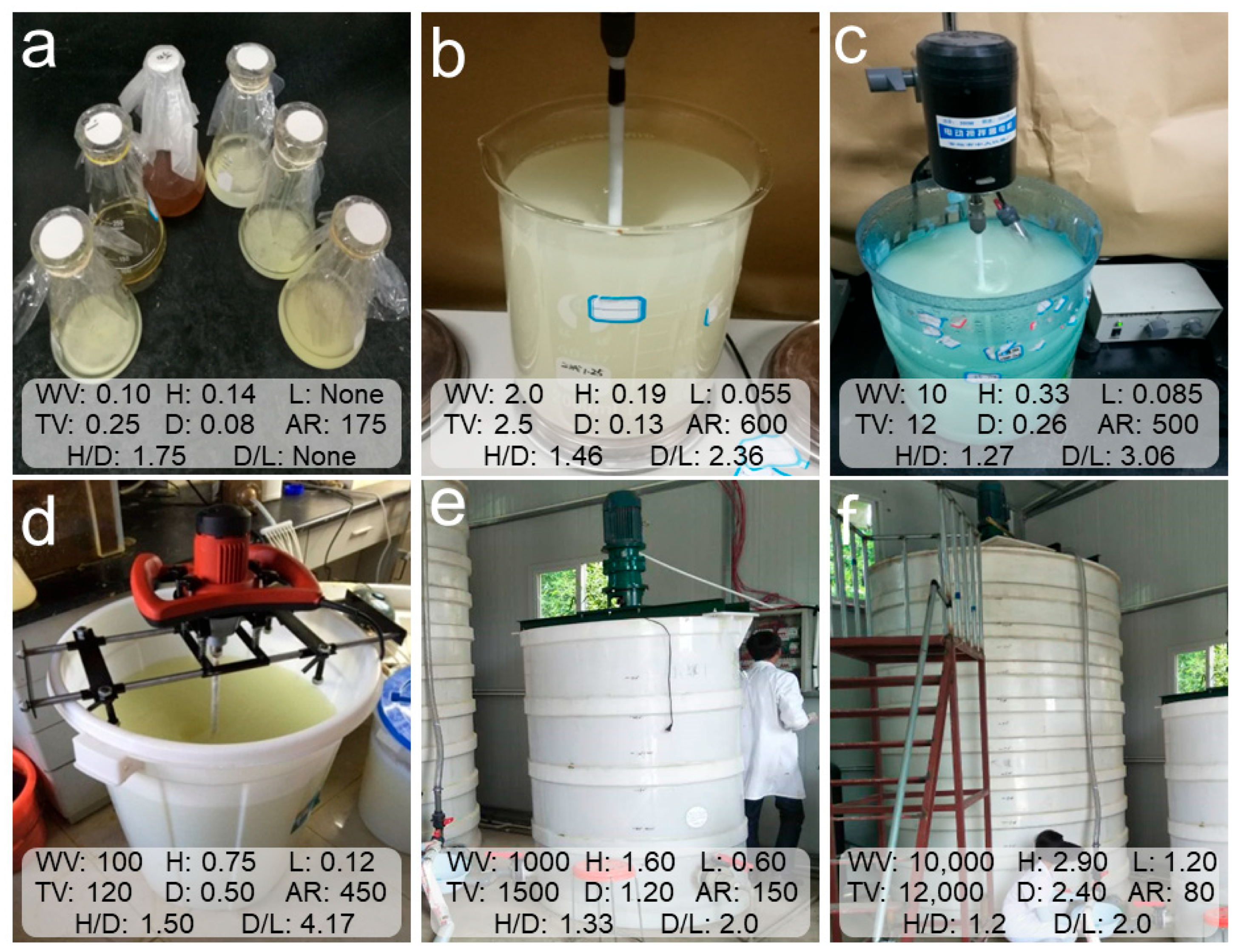
2.1. Initial Inoculum for the Scale-Up Cultivation of Mixotrophic Acidophiles
2.2. Scale-Up Cultivation Process of Mixotrophic Acidophiles
2.3. Bioremediation of Cd-Contaminated Soils
2.4. Chemical Analysis
2.5. Microbial Community Analysis
2.6. Statistical Analysis
3. Results
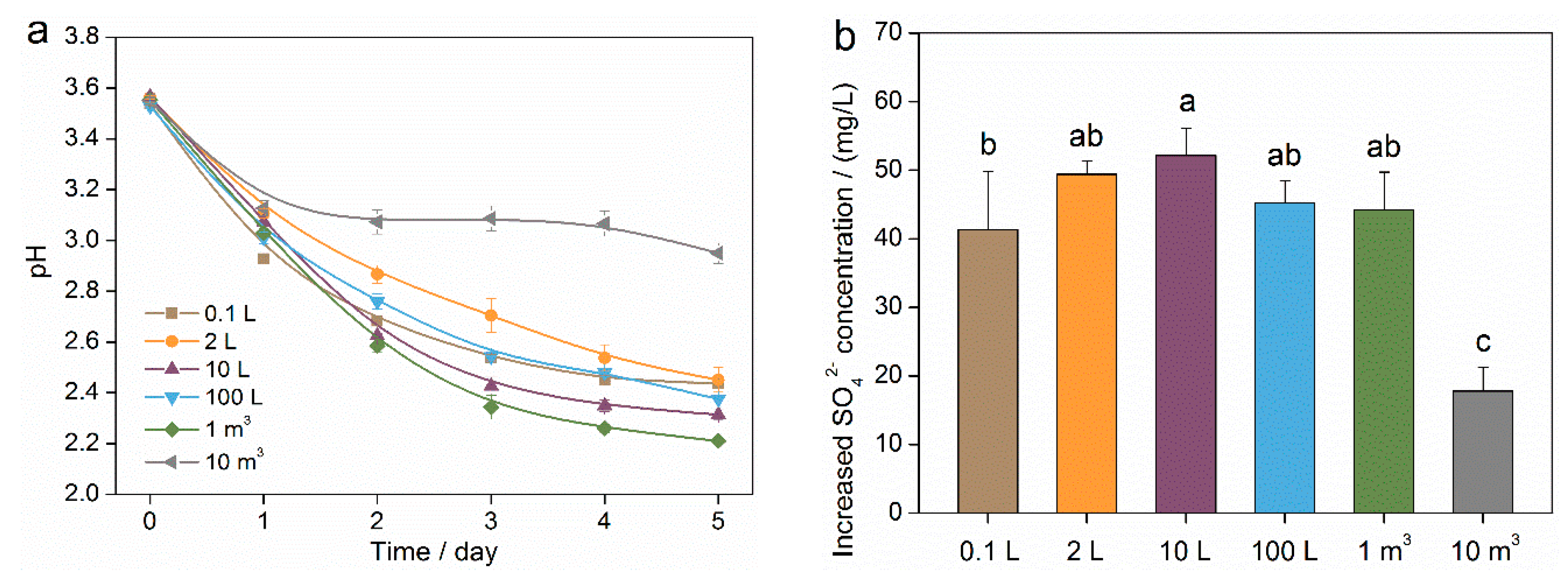
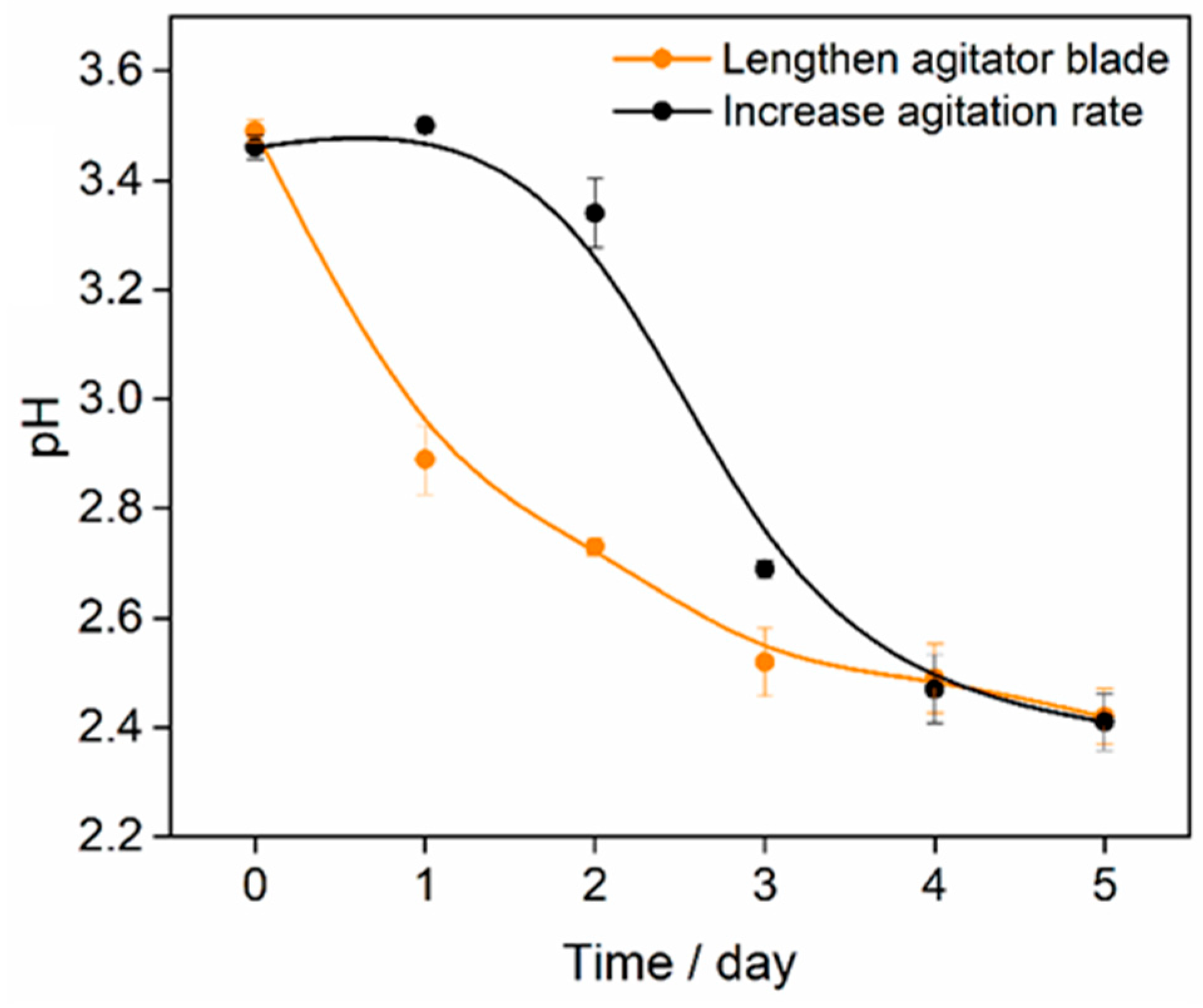
3.1. Properties of Microbial Solution in Scale-Up Cultivation Process of Mixotrophic Acidophiles
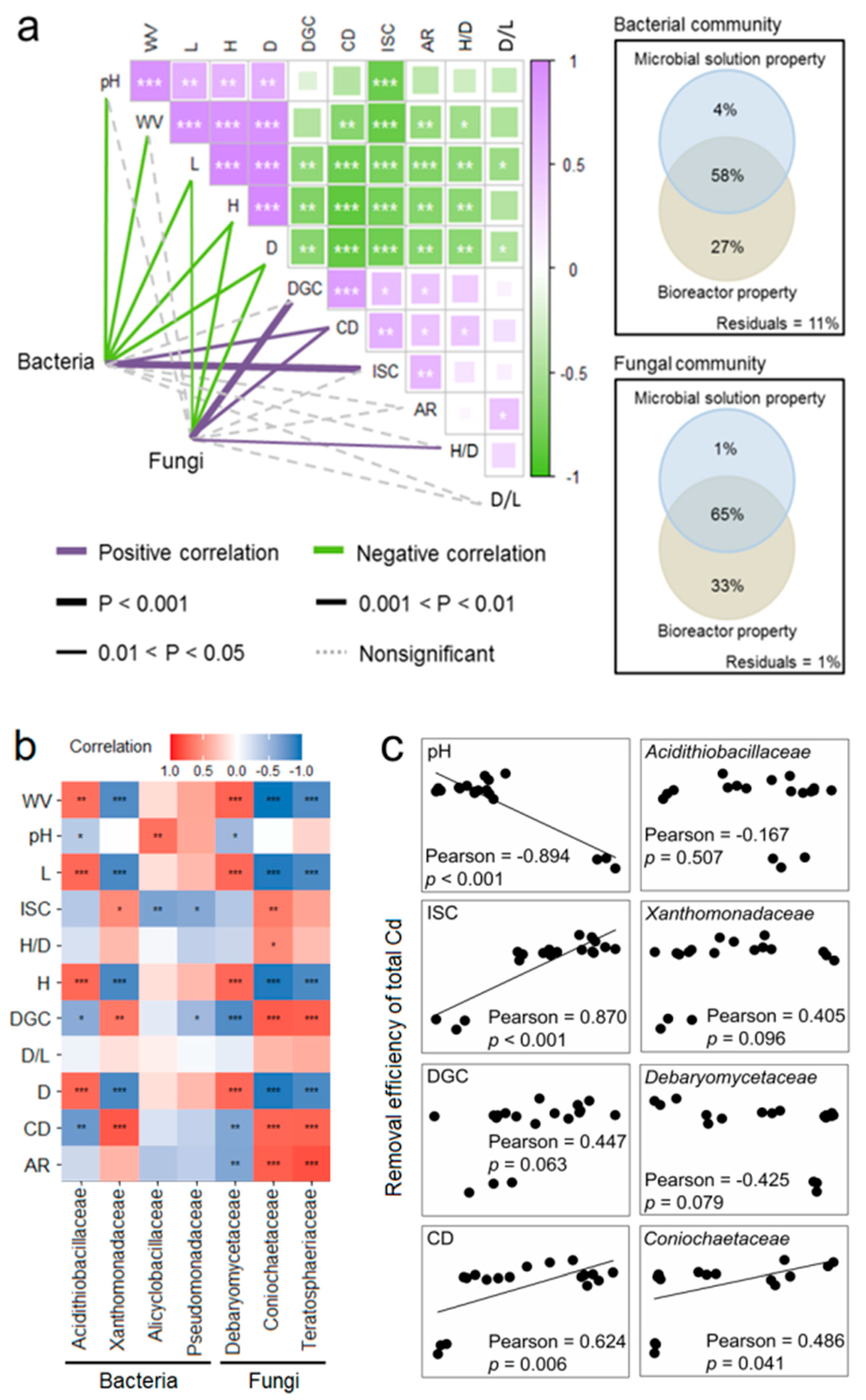
3.2. Microbial Community Diversities and Compositions in Scale-Up Cultivation Process of Mixotrophic Acidophiles
3.3. Soil Removal Efficiencies of Total Cd and DTPA-Cd Using Mixotrophic Acidophiles in Different Scale-Up Cultivation Process
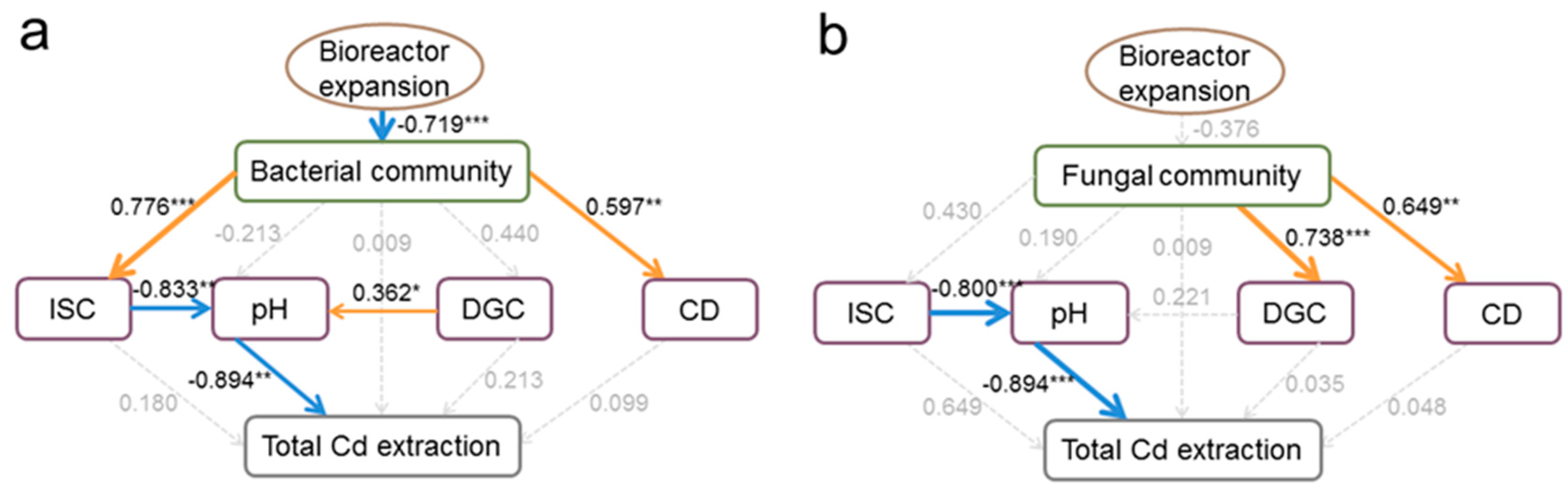
3.4. Links between Microbial Solution Properties, Solution Microbes, and Cd Removal Efficiencies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, S.; Song, C.; Ye, S.; Cheng, C.; Gao, C. The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci. Total Environ. 2022, 806, 150322. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Yan, X.; Liang, T.; Yang, X.; El-Naggar, A.; Liu, J.; Chen, H. Evaluation of potential ecological risks in potential toxic elements contaminated agricultural soils: Correlations between soil contamination and polymetallic mining activity. J. Environ. Manag. 2021, 300, 113679. [Google Scholar] [CrossRef] [PubMed]
- Bind, A.; Kushwaha, A.; Devi, G.; Goswami, S.; Sen, B.; Prakash, V. Biosorption valorization of floating and submerged macrophytes for heavy-metal removal in a multi-component system. Appl. Water Sci. 2019, 9, 95. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.; Antunes, P. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [PubMed]
- Dutta, M.; Kushwaha, A.; Kalita, S.; Devi, G.; Bhuyan, M. Assessment of bioaccumulation and detoxification of cadmium in soil-plant-insect food chain. Bioresour. Technol. Rep. 2019, 7, 100242. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Qian, J.; Huang, T.; Su, F. Study on the remediation of cadmium/mercury contaminated soil by leaching: Effectiveness, conditions, and ecological risks. Water Air Soil Pollut. 2023, 234, 23. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments? Sci. Total Environ. 2016, 563–564, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.Y.; Liao, X.Y.; Li, X.H.; Zheng, S.N.; Zhao, F.H. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Potysz, A.; Van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152. [Google Scholar] [CrossRef]
- Raffa, C.; Chiampo, F.; Shanthakumar, S. Remediation of metal/metalloid-polluted soils: A short review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Brandl, H. Microbial leaching of metals. Biotechnology 2001, 10, 191–224. [Google Scholar]
- Acharya, C.; Kar, R.; Sukla, L. Short Communication: Leaching of chromite overburden with various native bacterial strains. World J. Microbiol. Biotechnol. 1998, 14, 769–771. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Li, Q.; Han, T.; Hu, Y.; Liu, X.; Qin, W.; Chai, L.; Qiu, G. Bioleaching of heavy metals from contaminated alkaline sediment by auto- and heterotrophic bacteria in stirred tank reactor. Trans. Nonferrous Met. Soc. China 2014, 24, 2969–2975. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, P.; Zhang, H.; Liang, Y.; Yin, H.; Liu, X.; Bai, L.; Liu, H.; Jiang, H. Mixotrophic acidophiles increase cadmium soluble fraction and phytoextraction efficiency from cadmium contaminated soils. Sci. Total Environ. 2019, 655, 347–355. [Google Scholar] [CrossRef] [PubMed]
- González-Toledo, S.; Domínguez-Domínguez, J.; García-Almendárez, B.; Prado-Barragán, L.; Regalado-González, C. Optimization of nisin production by Lactococcus lactis UQ2 using supplemented whey as alternative culture medium. J. Food Sci. 2010, 75, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Ren, J.; Lin, W.; Shen, Y.; Wang, J.; Luo, X.; Xie, M. Optimization of fermentation media for nitrite oxidizing bacteria using sequential statistical design. Bioresour. Technol. 2008, 99, 7923–7927. [Google Scholar] [CrossRef]
- Modiri, S.; Kermanshahi, R.; Soudi, M.; Dad, N.; Ebadi, M.; Zahiri, H.; Noghabi, K. Growth optimization of Lactobacillus acidophilus for production of antimicrobial peptide acidocin 4356: Scale up from flask to lab-scale fermenter. Iran. J. Biotechnol. 2021, 19, e2686. [Google Scholar]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of production parameters for probiotic Lactobacillus strains as feed additive. Molecules 2012, 24, 3286. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate radical and its application in decontamination technologies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Yuan, B.; Huang, L.; Liu, X.; Bai, L.; Liu, H.; Jiang, H.; Zhu, P.; Xiao, Y.; Geng, J.; Liu, Q.; et al. Application of mixotrophic acidophiles for the bioremediation of cadmium-contaminated soils elevates cadmium removal, soil nutrient availability, and rice growth. Ecotoxicol. Environ. Saf. 2022, 236, 113499. [Google Scholar] [CrossRef]
- Harris, J.; Kelley, S.; Pace, N. New perspective on uncultured bacterial phylogenetic division OP11. Appl. Environ. Microbiol. 2004, 70, 845–849. [Google Scholar] [CrossRef]
- Cheung, M.; Au, C.; Chu, K.; Kwan, H.; Wong, C. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Bai, L.; Liu, X.; Zhu, P.; Liu, H.; Xiao, Y.; Geng, J.; Liu, Q.; Huang, L.; Jiang, H. Cadmium speciation distribution responses to soil properties and soil microbes of plow layer and plow pan soils in cadmium-contaminated paddy fields. Front. Microbiol. 2021, 12, 774301. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Wang, Q. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Glckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar] [CrossRef]
- Manzoor, A.; Qazi, J.; Haq, I.; Mukhtar, H.; Rasool, A. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Won, S.; Ha, M.; Duc Nguyen, D.; Kang, H.Y. Bioleaching for environmental remediation of toxic metals and metalloids: A review on soils, sediments, and mine tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, Z.; Chen, R. Study of characteristics on metabolism of Penicillium chrysogenum F1 during bioleaching of heavy metals from contaminated soil. Can. J. Microbiol. 2019, 65, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Brejová, B.; Lichancová, H.; Brázdovič, F.; Cillingová, A.; Neboháčová, M.; Tomáška, Ľ.; Vinař, T.; Nosek, J. Draft genome sequence of an obligate psychrophilic yeast, Candida psychrophila NRRL Y-17665T. Genome Announc. 2017, 5, e817–e851. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Park, H.; Kim, K.; Lee, J. The role of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in arsenic bioleaching from soil. Environ. Geochem. Health 2013, 35, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, A.; Joulian, C.; Jacob, J.; Bodenan, F.; D’Hugues, P.; Hedrich, S. Influence of CO2 supplementation on the bioleaching of a copper concentrate from Kupferschiefer Ore. Solid State Phenom. 2017, 262, 242–245. [Google Scholar] [CrossRef]
- Witne, J.Y.; Phillips, C.V. Bioleaching of Ok Tedi copper concentrate in oxygen- and carbon dioxide-enriched air. Miner. Eng. 2001, 14, 25–48. [Google Scholar] [CrossRef]
- Jerez, C. Bioleaching and biomining for the industrial recovery of metals. Compr. Biotechnol. 2017, 3, 758–771. [Google Scholar]
- Peng, T.; Zhou, D.; Liu, Y.; Yu, R.; Qiu, G.; Zeng, W. Effects of pH value on the expression of key iron/sulfur oxidation genes during bioleaching of chalcopyrite on thermophilic condition. Ann. Microbiol. 2019, 69, 627–635. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Si, S.; Liu, G.; Yun, H.; Tu, C.; Li, L.; Luo, Y. Feasibility of a combined solubilization and eluent drainage system to remove Cd and Cu from agricultural soil. Sci. Total Environ. 2022, 807, 150733. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, Y. Reclamation of copper-contaminated soil using EDTA or citric acid coupled with dissolved organic matter solution extracted from distillery sludge. Environ. Pollut. 2013, 178, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, L.; Cheng, Y.; Xiao, X.; Pan, F.; Jiang, K. Effects of pH, pulp density and particle size on solubilization of metals from a Pb/Zn smelting slag using indigenous moderate thermophilic bacteria. Hydrometallurgy 2010, 104, 25–31. [Google Scholar] [CrossRef]



| Item | Mean Value ± SD |
|---|---|
| pH | 6.23 ± 0.06 |
| Organic matter (g/kg) | 17.3 ± 0.5 |
| Total N (g/kg) | 1.74 ± 0.12 |
| Total P (g/kg) | 0.736 ± 0.056 |
| Total K (g/kg) | 12.7 ± 0.3 |
| Cu (mg/kg) | 21.2 ± 0.1 |
| Cd (mg/kg) | 13.2 ± 0.2 |
| DTPA-Cd (mg/kg) | 7.41 ± 0.83 |
| Pb (mg/kg) | 27.8 ± 2.2 |
| Cr (mg/kg) | 125 ± 22 |
| Mn (mg/kg) | 304 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Zhu, P.; Liu, X.; Jiang, L.; Jiang, H.; Liu, H.; Chen, Z. Bioreactor Expansion Affects Microbial Succession of Mixotrophic Acidophiles and Bioremediation of Cadmium-Contaminated Soils. Toxics 2024, 12, 362. https://doi.org/10.3390/toxics12050362
Hao X, Zhu P, Liu X, Jiang L, Jiang H, Liu H, Chen Z. Bioreactor Expansion Affects Microbial Succession of Mixotrophic Acidophiles and Bioremediation of Cadmium-Contaminated Soils. Toxics. 2024; 12(5):362. https://doi.org/10.3390/toxics12050362
Chicago/Turabian StyleHao, Xiaodong, Ping Zhu, Xueduan Liu, Luhua Jiang, Huidan Jiang, Hongwei Liu, and Zhiqun Chen. 2024. "Bioreactor Expansion Affects Microbial Succession of Mixotrophic Acidophiles and Bioremediation of Cadmium-Contaminated Soils" Toxics 12, no. 5: 362. https://doi.org/10.3390/toxics12050362







