Metal and Microelement Biomarkers of Neurodegeneration in Early Life Permethrin-Treated Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Rats
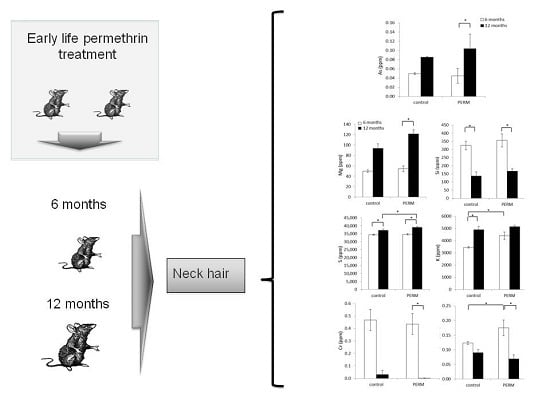
2.3. Treatment and Experimental Design
2.4. Hair Analysis
2.5. Statistical Analysis
3. Results
3.1. General Findings
3.2. PCA Analysis
| Principal Component | Eigenvalue |
|---|---|
| 1 | 6.9296 |
| 2 | 1.5313 |
| 3 | 1.0504 |
| 4 | 0.5982 |
| 5 | 0.2797 |
| 6 | 0.2719 |
| 7 | 0.1736 |
| 8 | 0.1318 |
| 9 | 0.0211 |
| 10 | 0.0124 |
| 11 | 8.56 × 10−8 |

3.3. Metals and Microelements in Hair



| Metals/Microelements | Control (ppm) | PERM (ppm) | Control (ppm) | PERM (ppm) |
|---|---|---|---|---|
| 6 Months | 12 Months | |||
| Na | 658.134 ± 33.84 | 715.742 ± 72.27 | 909.209 ± 96.57 | 785.487 ± 71.05 |
| Al | 117.362 ± 37.24 | 521.173 ± 222.12 | 16.769 ± 5.56 | 29.280 ± 12.16 |
| P | 355.566 ± 5.94 | 393.904 ± 27.39 | 344.279 ± 18.53 | 382.025 ± 8.22 |
| Ca | 262.810 ± 16.53 | 347.534 ± 75.02 | 387.015 ± 169.62 | 1213.493 ± 503.90 |
| Mg | 49.862 ± 2.34 | 54.471 ± 9.00 | 93.547 ± 5.44 | 121.550 * ± 7.79 |
| Cr | 0.469 ± 0.09 | 0.436 ± 0.08 | 0.032 ± 0.03 | 0.000 ± 0.00 |
| Mn | 0.692 ± 0.04 | 0.904 ± 0.08 | 0.529 ± 0.09 | 0.983 ± 0.43 |
| Fe | 19.626 ± 3.01 | 19.494 ± 1.31 | 11.031 ± 0.83 | 19.308 ± 5.06 |
| Ni | 0.331 ± 0.15 | 3.456 ± 2.92 | 0.366 ± 0.11 | 0.544 ± 0.12 |
| Cu | 13.358 ± 0.42 | 18.625 * ± 2.43 | 13.371 ± 0.75 | 16.065 ± 0.58 |
| Zn | 108.667 ± 1.67 | 107.232 ± 30.36 | 162.719 ± 2.13 | 271.370 ° ± 64.67 |
| Se | 0.175 ± 0.01 | 0.158 ± 0.01 | 0.209 ± 0.03 | 0.219 ± 0.03 |
| Rb | 2.631 ± 0.08 | 3.365 ± 0.27 | 4.309 ± 0.15 | 4.766 ± 0.25 |
| Sr | 0.261 ± 0.02 | 0.603 ± 0.08 | 0.249 ± 0.10 | 0.677 ± 0.27 |
| Ag | 0.016 ± 0.01 | 0.032 ± 0.02 | 0.021 ± 0.00 | 0.086 ± 0.05 |
| Cd | 0.001 ± 0.00 | 0.001 ± 0.00 | 0.014 ± 0.01 | 0.030 ± 0.03 |
| Sn | 0.058 ± 0.02 | 0.031 ± 0.01 | 0.031 ± 0.02 | 0.028 ± 0.03 |
| Sb | 0.003 ± 0.00 | 0.006 ± 0.00 | 0.020 ± 0.01 | 0.040 ± 0.03 |
| Pt | 0.003 ± 0.00 | 0.003 ± 0.00 | 0.012 ± 0.01 | 0.025 ± 0.03 |
| Au | 107.047 ± 1.88 | 105.883 ± 0.96 | 0.099 ± 0.02 | 0.128 ± 0.06 |
| Hg | 0.052 ± 0.01 | 0.031 ± 0.00 | 0.057 ± 0.01 | 0.039 ± 0.03 |
| Pb | 0.105 ± 0.02 | 8.744 ± 8.66 | 0.106 ± 0.05 | 0.284 ± 0.08 |
| Na/K | 0.191 ± 0.01 | 0.162 ± 0.01 | 0.186 ± 0.02 | 0.152 ± 0.01 |
| Na/Mg | 13.261 ± 0.69 | 13.168 ± 0.58 | 10.252 ± 1.72 | 6.445 ± 0.29 |
| Ca/K | 0.076 ± 0.00 | 0.077 ± 0.01 | 0.083 ± 0.04 | 0.233 ± 0.10 |
| Ca/Mg | 5.269 ± 0.21 | 6.262 ± 0.81 | 3.895 ± 1.34 | 9.387 ± 3.56 |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ICP | inductively-coupled plasma mass spectrometry |
| PCA | Principal component analysis |
| PERM | Permethrin |
| PC | Principal Component |
| DIM | Dimention |
References
- Martín-Camean, A.; Molina-Villalba, I.; Jos, A.; Iglesias-Linares, A.; Solano, E.; Camean, A.M.; Gil, F. Biomonitorization of chromium, copper, iron, manganese and nickel in scalp hair from orthodontic patients by atomic absorption spectrometry. Environ. Toxicol. Pharmacol. 2014, 37, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Dietz, M.C.; Ihrig, A.; Triebig, G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int. Arch. Occup. Environ. Health 1999, 72, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.H.; Farb, D.H.; Ozer, J.; Feldman, R.G.; Durso, R. Younger age at onset of sporadic Parkinson’s disease among subjects occupationally exposed to metals and pesticides. Interdiscip. Toxicol. 2014, 7, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Costello, S.; Cockburn, M.; Zhang, X.; Bronstein, J.; Ritz, B. Parkinson’s disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 2011, 26, 547–555. [Google Scholar] [CrossRef] [PubMed]
- McMillan, G. Is electric arc welding linked to manganism or Parkinson’s disease? Toxicol. Rev. 2005, 24, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chakraborty, S.; Peres, T.V.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: From C. elegans to humans. Toxicol. Res. 2015, 4, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F. Iron and Parkinson’s disease. Neuro Endocrinol. Lett. 2015, 36, 24–27. [Google Scholar] [PubMed]
- Carloni, M.; Nasuti, C.; Fedeli, D.; Montani, M.; Amici, A.; Vadhana, M.S.; Gabbianelli, R. The impact of early life permethrin exposure on development of neurodegeneration in adulthood. Exp. Gerontol. 2012, 47, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Carloni, M.; Nasuti, C.; Fedeli, D.; Montani, M.; Amici, A.; Vadhana, M.S.; Gabbianelli, R. Early life permethrin exposure induces long-term brain changes in Nurr1, NF-kB and Nrf-2. Brain Res. 2013, 1515, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, G.; Carloni, D.; Nasuti, C.; Gambini, A.; Scocco, V.; Gabbianelli, R. Early life permethrin exposure leads to hypervitaminosis D, nitric oxide and catecholamines impairment. Pestic. Biochem. Physiol. 2013, 107, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, D.; Montani, M.; Carloni, M.; Nasuti, C.; Amici, A.; Gabbianelli, R. Leukocyte Nurr1 as peripheral biomarker of early-life environmental exposure to permethrin insecticide. Biomarkers 2012, 17, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Gabbianelli, R.; Falcioni, M.L.; Di Stefano, A.; Sozio, P.; Cantalamessa, F. Dopaminergic system modulation, behavioural changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 2007, 229, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Carloni, M.; Fedeli, D.; Gabbianelli, R.; di Stefano, A.; Cerasa, L.S.; Isabel, S.; Domingues, V.; Ciccocioppo, R. Effects of early life permethrin exposure on spatial working memory and on monoamine levels in different brain areas of pre-senescent rats. Toxicology 2013, 303, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Ferraro, S.; Giovannetti, R.; Fedeli, D.; Guidi, M.; Ferri, A.; Gabbianelli, R. Metal detection in hair as biomarker to monitor the health status in rats. J. Nutrigenet. Nutrigenom. 2014. [Google Scholar] [CrossRef]
- Barcelos, G.R.; Souza, M.F.; Oliveira, A.Á.; Lengert, A.; Oliveira, M.T.; Camargo, R.B.; Grotto, D.; Valentini, J.; Garcia, S.C.; Braga, G.Ú.; et al. Effects of genetic polymorphisms on antioxidant status and concentrations of the metals in the blood of riverside Amazonian communities co-exposed to Hg and Pb. Environ. Res. 2015, 138, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Dusek, P.; Roos, P.M.; Litwin, T.; Schneider, S.A.; Flaten, T.P.; Aaseth, J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015, 31, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Winters, K.; Tollin, G.; Enemark, J.H. Elucidating the catalytic mechanism of sulfite oxidizing enzymes using structural, spectroscopic, and kinetic analyses. Biochemistry 2010, 49, 7242–5724. [Google Scholar] [CrossRef] [PubMed]
- Goullé, J.P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Lainé, G.; Bouige, D.; Lacroix, C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic. Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Goullé, J.P.; Saussereau, E.; Mahieu, L.; Guerbet, M. Current role of ICP-MS in clinical toxicology and forensic toxicology: A metallic profile. Bioanalysis 2014, 6, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Gellein, K.; Lierhagen, S.; Brevik, P.S.; Teigen, M.; Kaur, P.; Singh, T.; Flaten, T.P.; Syversen, T. Trace Element Profiles in Single Strands of Human Hair Determined by HR-ICP-MS. Biol. Trace Elem. Res. 2008, 123, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Determination of Trace Elements in Hair by ICP-MS. Available online: http://www.youngin.com/application/AN-0812-0133EN.pdf (accessed on 26 January 2016).
- Morgan, M.K. Children’s exposures to pyrethroid insecticides at home: A review of data collected in published exposure measurement studies conducted in the United States. Int. J. Environ. Res. Public Health 2012, 9, 2964–2985. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Nasuti, C.; Mirto, M.; Caradonna, F.; Gabbianelli, R. Intergenerational effect of early life exposure to permethrin: Changes in global DNA methylation and in Nurr1 gene expression. Toxics 2015, 3, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Nasuti, C.; Vincenzetti, S.; Correia-Sá, L.; Domingues, V.; Fedeli, D.; Ricciutelli, M.; Pucciarelli, S.; Gabbianelli, R. Permethrin pesticide residues in food mediate progressive neuronal disorder. J. Nutr. Nutrigenom. 2015, 8, 1–25. [Google Scholar]
- Qi, Y.; Donahoe, R.J. The environmental fate of arsenic in surface soil contaminated by historical herbicide application. Sci. Total Environ. 2008, 405, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ambeskovic, M.; Fuchs, E.; Beaumier, P.; Gerken, M.; Metz, G.A. Hair trace elementary profiles in aging rodents and primates: Links to altered cell homeodynamics and disease. Biogerontology 2013, 14, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, M.L.; Nasuti, C.; Bergamini, C.; Fato, R.; Lenaz, G.; Gabbianelli, R. The primary role of glutathione against nuclear DNA damage of striatum induced by permethrin in rats. Neuroscience 2010, 168, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Vinenzetti, S.; Nasuti, C.; Fedeli, D.; Ricciutelli, M.; Pucciarelli, S.; Gabbianelli, R. Proteomic analysis for early neurodegenerative biomarker detection in an animal model. Biochimie 2015, 121, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Watson, A.I.; Pedro, L.D.; Powell, J.J. The decrease in silicon concentration of the connective tissues with age in rats is a marker of connective tissue turnover. Bone 2015, 75, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Aizenman, E. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl. Stroke Res. 2014, 5, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, Y.L. Chromium, zinc and magnesium status in type 1 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Yoon, K.S.; Clark, J.M.; Park, Y. Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes. J. Biochem. Mol. Toxicol. 2014, 28, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Manto, M. Abnormal Copper Homeostasis: Mechanisms and Roles in Neurodegeneration. Toxics 2014, 2, 327–345. [Google Scholar] [CrossRef]
- Masahiro, K.; Keiko, K.; Hironari, K.; Susumu, O.; Yutaka, S. Zinc and Neurodegenerative Diseases. In Mental and Behavioural Disorders and Diseases of the Nervous System “Neurodegenerative Diseases”; Kishore, U., Ed.; 2013; Available online: http://www.intechopen.com/books/neurodegenerative-diseases/zinc-and-neurodegenerative-diseases (accessed on 28 January 2016).
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasuti, C.; Ferraro, S.; Giovannetti, R.; Piangerelli, M.; Gabbianelli, R. Metal and Microelement Biomarkers of Neurodegeneration in Early Life Permethrin-Treated Rats. Toxics 2016, 4, 3. https://doi.org/10.3390/toxics4010003
Nasuti C, Ferraro S, Giovannetti R, Piangerelli M, Gabbianelli R. Metal and Microelement Biomarkers of Neurodegeneration in Early Life Permethrin-Treated Rats. Toxics. 2016; 4(1):3. https://doi.org/10.3390/toxics4010003
Chicago/Turabian StyleNasuti, Cinzia, Stefano Ferraro, Rita Giovannetti, Marco Piangerelli, and Rosita Gabbianelli. 2016. "Metal and Microelement Biomarkers of Neurodegeneration in Early Life Permethrin-Treated Rats" Toxics 4, no. 1: 3. https://doi.org/10.3390/toxics4010003
APA StyleNasuti, C., Ferraro, S., Giovannetti, R., Piangerelli, M., & Gabbianelli, R. (2016). Metal and Microelement Biomarkers of Neurodegeneration in Early Life Permethrin-Treated Rats. Toxics, 4(1), 3. https://doi.org/10.3390/toxics4010003