Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity?
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Sampling
2.3. Calculation of BaPeq Toxicity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.X.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Yuan, H.S.; Tao, S.; Li, B.G.; Lang, C.; Cao, J.; Coveney, M.R. Emission and outflow of polycyclic aromatic hydrocarbons from wildfires in China. Atmos. Environ. 2008, 42, 6828–6835. [Google Scholar] [CrossRef]
- See, S.W.; Balasubramanian, R.; Rianawati, E.; Karthikeyan, S.; Streets, D.G. Characterization and source apportionment of particulate matter < or = 2.5 micrometer in Sumatra, Indonesia, during a recent peat fire episode. Environ. Sci. Technol. 2007, 41, 3488–3494. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, K.; Masclet, P.; Mouvier, G. Sources and chemical reactivity of polynuclear aromatic hydrocarbons in the atmosphere—A critical review. Sci. Total Environ. 1984, 32, 103–132. [Google Scholar] [CrossRef]
- Raga, G.B.; Baumgardner, D.; Ulke, A.G.; Brizuela, M.T.; Kucienska, B. The environmental impact of the Puyehue-Cordon Caulle 2011 volcanic eruption on Buenos Aires. Nat. Hazards Earth Syst. Sci. 2013, 13, 2319–2330. [Google Scholar] [CrossRef] [Green Version]
- Stogiannidis, E.; Laane, R. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: An overview of possibilities. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2015; pp. 49–133. [Google Scholar]
- Venkatesan, M.I. Occurrence and possible sources of perylene in marine sediments—A review. Mar. Chem. 1988, 25, 1–27. [Google Scholar] [CrossRef]
- Suess, M.J. The environmental load and cycle of polycyclic aromatic hydrocarbons. Sci. Total Environ. 1976, 6, 239–250. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Biomarkers and Isotopes in the Environment and Human History; Cambridge University Press: New York, NY, USA, 2005; Volume 1. [Google Scholar]
- Bakhtiari, A.R.; Zakaria, M.P.; Yaziz, M.I.; Lajis, M.N.H.; Bi, X.; Rahim, M.C.A. Vertical distribution and source identification of polycyclic aromatic hydrocarbons in anoxic sediment cores of Chini Lake, Malaysia: Perylene as indicator of land plant-derived hydrocarbons. Appl. Geochem. 2009, 24, 1777–1787. [Google Scholar] [CrossRef]
- Zielinska, B.; Sagebiel, J.; Arnott, W.P.; Rogers, C.F.; Kelly, K.E.; Wagner, D.A.; Lighty, J.S.; Sarofim, A.F.; Palmer, G. Phase and size distribution of polycyclic aromatic hydrocarbons in diesel and gasoline vehicle emissions. Environ. Sci. Technol. 2004, 38, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, B.; Sagebiel, J.; McDonald, J.D.; Whitney, K.; Lawson, D.R. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. J. Air Waste Manag. Assoc. 2004, 54, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.M.; Zielinska, B.; Campbell, D.E.; Arnott, W.P.; Sagebiel, J.C.; Mazzoleni, L.; Chow, J.C.; Gabele, P.A.; Crews, W.; Snow, R. Variations in speciated emissions from spark-ignition and compression-ignition motor vehicles in California’s south coast air basin. J. Air Waste Manag. Assoc. 2007, 57, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Dou, H.; Wei, Z.C.; Chang, B.; Qiu, W.X.; Liu, Y.; Tao, S. Emission characteristics of polycyclic aromatic hydrocarbons from combustion of different residential coals in North China. Sci. Total Environ. 2009, 407, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.D.B.; Zielinska, E.; Fujita, M.; Sagebiel, J.C.; Chow, J.C.; Watson, J.G. Fine particle and gaseous emission rates from residential wood combustion. Environ. Sci. Technol. 2000, 34, 2080–2091. [Google Scholar] [CrossRef]
- Johansson, L.S.; Leckner, B.; Gustavsson, L.; Cooper, D.; Tullin, C.; Potter, A. Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 2004, 38, 4183–4195. [Google Scholar] [CrossRef]
- Oanh, N.T.K.; Reutergardh, L.B.; Dung, N.T. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environ. Sci. Technol. 1999, 33, 2703–2709. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Simoneit, B.R.T. Source Profiles of Organic Compounds Emitted upon Combustion of Green Vegetation from Temperate Climate Forests. Environ. Sci. Technol. 2008, 42, 8310–8316. [Google Scholar] [CrossRef] [PubMed]
- Kakareka, S.V.; Kukharchyk, T.I.; Khomich, V.S. Study of PAH emission from the solid fuels combustion in residential furnaces. Environ. Pollut. 2005, 133, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hays, M.D.; Fine, P.M.; Geron, C.D.; Kleeman, M.J.; Gullett, B.K. Open burning of agricultural biomass: Physical and chemical properties of particle-phase emissions. Atmos. Environ. 2005, 39, 6747–6764. [Google Scholar] [CrossRef]
- Tian, J.; Chow, J.C.; Cao, J.; Han, Y.; Ni, H.; Chen, L.W.A.; Wang, X.; Huang, R.J.; Moosmueller, H.; Watson, J.G. A biomass combustion chamber: Design, evaluation, and a case study of wheat straw combustion emission tests. Aerosol Air Qual. Res. 2015, 15, 2104–2114. [Google Scholar] [CrossRef]
- Yang, H.H.; Tsai, C.H.; Chao, M.R.; Su, Y.L.; Chien, S.M. Source identification and size distribution of atmospheric polycyclic aromatic hydrocarbons during rice straw burning period. Atmos. Environ. 2006, 40, 1266–1274. [Google Scholar] [CrossRef]
- Godoi, A.F.L.; Ravindra, K.; Godoi, R.H.M.; Andrade, S.J.; Santiago, -S.M.; van Vaeck, L.; van Grieken, R. Fast chromatographic determination of polycyclic aromatic hydrocarbons in aerosol samples from sugar cane burning. J. Chromatogr. A 2004, 1027, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sahu, S.K.; Bhangare, R.C.; Ajmal, P.Y.; Pandit, G.G. Estimation of polycyclic aromatic hydrocarbons associated with size segregated combustion aerosols generated from household fuels. Microchem. J. 2013, 106, 79–86. [Google Scholar] [CrossRef]
- Singh, D.P.; Gadi, R.; Mandal, T.K.; Saud, T.; Saxena, M.; Sharma, S.K. Emissions estimates of PAH from biomass fuels used in rural sector of Indo-Gangetic Plains of India. Atmos. Environ. 2013, 68, 120–126. [Google Scholar] [CrossRef]
- Venkataraman, C.; Negi, G.; Sardar, S.B.; Rastogi, R. Size distributions of polycyclic aromatic hydrocarbons in aerosol emissions from biofuel combustion. J. Aerosol Sci. 2002, 33, 503–518. [Google Scholar] [CrossRef]
- Masclet, P.; Mouvier, G.; Nikolaou, K. Relative decay index and sources of polycyclic aromatic hydrocarbons. Atmos. Environ. 1986, 20, 439–446. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Shen, H.Z.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.F.; Wang, B.; Zhang, Y.Y.; Chen, Y.C.; Lu, Y.; et al. Global Atmospheric Emissions of Polycyclic Aromatic Hydrocarbons from 1960 to 2008 and Future Predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.M.; Breivik, K.; Munch, J.; Fudala, J. European atmospheric emissions of selected persistent organic pollutants, 1970–1995. Atmos. Environ. 2003, 37, S119–S131. [Google Scholar] [CrossRef]
- Breivik, K.; Vestreng, V.; Rozovskaya, O.; Pacyna, J.M. Atmospheric emissions of some POPs in Europe: A discussion of existing inventories and data needs. Environ. Sci. Policy 2006, 9, 663–674. [Google Scholar] [CrossRef]
- Lammel, G.; Heil, A.; Stemmler, I.; Dvorska, A.; Klanova, J. On the Contribution of Biomass Burning to POPs (PAHs and PCDDs) in Air in Africa. Environ. Sci. Technol. 2013, 47, 11616–11624. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Petry, T.; Schmid, P.; Schlatter, C. The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of polycyclic aromatic hydrocarbons (PAHs). Chemosphere 1996, 32, 639–648. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Working Group on the Evaluation of Carcinogenic Risks to Humans, IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organisation: Geneva, Switzerland, 2010; Volume 94. [Google Scholar]
- Thamaraiselvan, R.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.; Garçon, G.; Saint, -G.F.; Andre, V.; Gosset, P.; Billet, S.; Goff, J.L.; Verdin, A.; Mulliez, P.; Sichel, F. Polycyclic aromatic hydrocarbons within airborne particulate matter (PM2.5) produced DNA bulky stable adducts in a human lung cell coculture model. J. Appl. Toxicol. 2013, 33, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.C. Linking DNA adduct formation and human cancer risk in chemical carcinogenesis. Environ. Mol. Mutagen. 2016, 57, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.B. Molecular epidemiology studies of carcinogenic environmental pollutants—Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage. Mutat. Res. Rev. Mutat. Res. 2003, 544, 397–402. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Chen, S.-C.; Liao, C.-M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Boström, C.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110 (Suppl. S3), 451–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Tao, S.; Dou, H.; Zhang, T.W.; Zhang, X.L.; Dawson, R. Exposure of traffic police to polycyclic aromatic hydrocarbons in Beijing, China. Chemosphere 2007, 66, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Mandalakis, M.; Tsapakis, M.; Tsoga, A.; Stephanou, E.G. Gas-particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos. Environ. 2002, 36, 4023–4035. [Google Scholar] [CrossRef]
- Gregoris, E.; Argiriadis, E.; Vecchiato, M.; Zambon, S.; de Pieri, S.; Donateo, A.; Contini, D.; Piazza, R.; Barbante, C.; Gambaro, A. Gas-particle distributions, sources and health effects of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs) in Venice aerosols. Sci. Total Environ. 2014, 476, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Li, X.D.; Qi, S.H.; Liu, G.Q.; Peng, Z.X. Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou, South China. Sci. Total Environ. 2006, 355, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-K. Sources, distribution and toxicity of polyaromatic hydrocarbons (PAHs) in particulate matter. In Air Pollution; Sciyo: Brussels, Belgium, 2010. [Google Scholar]
- OFR. Office of the Federal Registration (OFR) Appendix A: Priority Pollutants; Federal Register: Washington, DC, USA, 1982.
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharm. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Tiwari, M.; Sahu, S.K.; Pandit, G.G. Inhalation Risk Assessment of PAH Exposure Due to Combustion Aerosols Generated from Household Fuels. Aerosol Air Qual. Res. 2015, 15, 582–590. [Google Scholar] [CrossRef]
- Lakhani, A. Source Apportionment of Particle Bound Polycyclic Aromatic Hydrocarbons at an Industrial Location in Agra, India. Sci. World J. 2012, 781291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.Y.; Kim, Y.P.; Lee, S.B.; Jin, H.C.; Bae, G.N. Seasonal characteristics of the gaseous and particulate PAHs at a roadside station in Seoul, Korea. Atmos. Res. 2012, 116, 142–150. [Google Scholar] [CrossRef]
- US Agency for Toxic Substances and Disease Registry. Toxicological Profile for Naphthalene, 1-Methylnaphthalene, and 2-Methylnaphthalene; US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- Vainio, H.; Heseltine, E.; Wilbourn, J. Priorities for Future IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Environ. Health Perspect. 1994, 102, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.; Grumbein, S.; Chou, B.J.; Herbert, R.K. Toxicity and carcinogenicity study in F344 rats following 2 years of whole-body exposure to naphthalene vapors. Inhal. Toxicol. 2001, 13, 931–950. [Google Scholar]
- Jia, C.; Batterman, S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef]
- Possanzini, M.; di Palo, V.; Gigliucci, P.; Scianò, M.C.T.; Cecinato, A. Determination of phase-distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos. Environ. 2004, 38, 1727–1734. [Google Scholar] [CrossRef]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Marcé, R.M.; Borrull, F. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ. Health Perspect. 2011, 119, 1110–1116. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; Office of Research and Development: Washington, DC, USA, 1993.
- Collins, J.F.; Brown, J.P.; Dawson, S.V.; Marty, M.A. Risk assessment for benzo [a] pyrene. Regul. Toxicol. Pharm. 1991, 13, 170–184. [Google Scholar] [CrossRef]
- McDonald, J.D.; Eide, I.; Seagrave, J.C.; Zielinska, B.; Whitney, K.; Lawson, D.R.; Mauderly, J.L. Relationship between composition and toxicity of motor vehicle emission samples. Environ. Health Perspect. 2004, 112, 1527–1538. [Google Scholar] [CrossRef]
- Samy, S.; Zielinska, B. Secondary organic aerosol production from modern diesel engine emissions. Atmos. Chem. Phys. 2010, 10, 609–625. [Google Scholar] [CrossRef]
- He, J.; Zielinska, B.; Balasubramanian, R. Composition of semi-volatile organic compounds in the urban atmosphere of Singapore: Influence of biomass burning. Atmos. Chem. Phys. 2010, 10, 11401–11413. [Google Scholar] [CrossRef] [Green Version]
- Rinehart, L.R.; Fujita, E.M.; Chow, J.C.; Magliano, K.; Zielinska, B. Spatial distribution of PM 2.5 associated organic compounds in central California. Atmos. Environ. 2006, 40, 290–303. [Google Scholar] [CrossRef]
- McDonald, J.D.; Zielinska, B.; Fujita, E.M.; Sagebiel, J.C.; Chow, J.C.; Watson, J.G. Emissions from charbroiling and grilling of chicken and beef. J. Air Waste Manag. Assoc. 2003, 53, 185–194. [Google Scholar] [CrossRef]
- Kameda, Y.; Shirai, J.; Komai, T.; Nakanishi, J.; Masunaga, S. Atmospheric polycyclic aromatic hydrocarbons: Size distribution, estimation of their risk and their depositions to the human respiratory tract. Sci. Total Environ. 2005, 340, 71–80. [Google Scholar] [CrossRef] [PubMed]
- International Programme on Chemical Safety. OMS Environmental Health Criteria 202: Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbons World Health Organisation; World Health Organisation: Geneva, Switzerland, 1998. [Google Scholar]
- Black, R.R.; Aurell, J.; Holder, A.; George, I.J.; Gullett, B.K.; Hays, M.D.; Geron, C.D.; Tabor, D. Characterization of gas and particle emissions from laboratory burns of peat. Atmos. Environ. 2016, 132, 49–57. [Google Scholar] [CrossRef]
- Cereceda, -B.F.; Fadic, X.; Llanos, A.L.; Dominguez, A.M.; Guevara, J.L.; Vidal, V.; Diaz-Robles, L.A.; Schiappacasse, L.N.; Etcharren, P. Obtaining polycyclic aromatic hydrocarbon concentration ratios and molecular markers for residential wood combustion: Temuco, a case study. J. Air Waste Manag. Assoc. 2012, 62, 44–51. [Google Scholar] [CrossRef]
- Vasilakos, C.; Levi, N.; Maggos, T.; Hatzianestis, J.; Michopoulos, J.; Helmis, C. Gas-particle concentration and characterization of sources of PAHs in the atmosphere of a suburban area in Athens, Greece. J. Hazard. Mater. 2007, 140, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Muendo, M.; Hanai, Y.; Kameda, Y.; Masunaga, S. Polycyclic aromatic hydrocarbons in urban air: Concentration levels, patterns, and source analysis in Nairobi, Kenya. Environ. Forensics 2006, 7, 147–157. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int. 2011, 37, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Meusel, H.; Huang, R.J.; Ho, K.F.; Cao, J.J.; Hoffmann, T.; Wilcke, W. PM25-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: Seasonal variation, sources and cancer risk assessment. Sci. Total Environ. 2014, 473, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.O.; Dookeran, K.M.; Smith, K.A.; Sarofim, A.F.; Taghizadeh, K.; Lafleur, A.L. Measurement of polycyclic aromatic hydrocarbons associated with size-segregated atmospheric aerosols in Massachusetts. Environ. Sci. Technol. 1996, 30, 1023–1031. [Google Scholar] [CrossRef]
- LAWA. Los Angeles World Airports Environmental Services Division, Phase III of the LAX Air Quality and Source Apportionment Study. 2013. Available online: https://www.lawa.org/uploadedFiles/OurLAX/pdf/Vol 2 - Phase III LAX AQSAS 2013 06 18s_v2.pdf (accessed on 7 August 2017).
- Samburova, V.; Connolly, J.; Gyawali, M.; Yatavelli, R.L.N.; Watts, A.C.; Chakrabarty, R.K.; Zielinska, B.; Moosmüller, H.; Khlystov, A. Polycyclic aromatic hydrocarbons in biomass-burning emissions and their contribution to light absorption and aerosol toxicity. Sci. Total Environ. 2016, 568, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, B.; Campbell, D.; Lawson, D.R.; Ireson, R.G.; Weaver, C.S.; Hesterberg, T.W.; Larson, T.; Davey, M.; Liu, L.J.S. Detailed characterization and profiles of crankcase and diesel particulate matter exhaust emissions using speciated organics. Environ. Sci. Technol. 2008, 42, 5661–5666. [Google Scholar] [CrossRef] [PubMed]
- OEHHA. Benzo[a]pyrene as a Toxic Air Contaminant. Part B. Health Effects of Benzo[a]pyrene; Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Air Toxicology and Epidemiology Section: Berkeley, CA, USA, 1993.
- Peters, C.A.; Knightes, C.D.; Brown, D.G. Long-term composition dynamics of PAH-containing NAPLs and implications for risk assessment. Environ. Sci. Technol. 1999, 33, 4499–4507. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, P.; Zhang, Q.; Li, D.; Zhao, L.; Mu, D. Seasonal variation, sources and gas/particle partitioning of polycyclic aromatic hydrocarbons in Guangzhou, China. Sci. Total Environ. 2010, 408, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.F. An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol. Atmos. Environ. 1994, 28, 189–193. [Google Scholar] [CrossRef]
- Lemieux, C.L.; Lambert, I.B.; Lundstedt, S.; Tysklind, M.; White, P.A. Mutagenic hazards of complex polycyclic aromatic hydrocarbon mixtures in contaminated soil. Environ. Toxicol. Chem. 2008, 27, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.C.; Lingenfelter, R.; Cizmas, L.; Falahatpisheh, M.H.; Qian, Y.; Tang, Y.; Garcia, S.; Ramos, K.; Tiffany-Castiglioni, E.; Mumtaz, M.M. Toxicity assessment of complex mixtures remains a goal. Environ. Toxicol. Pharmacol. 2004, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
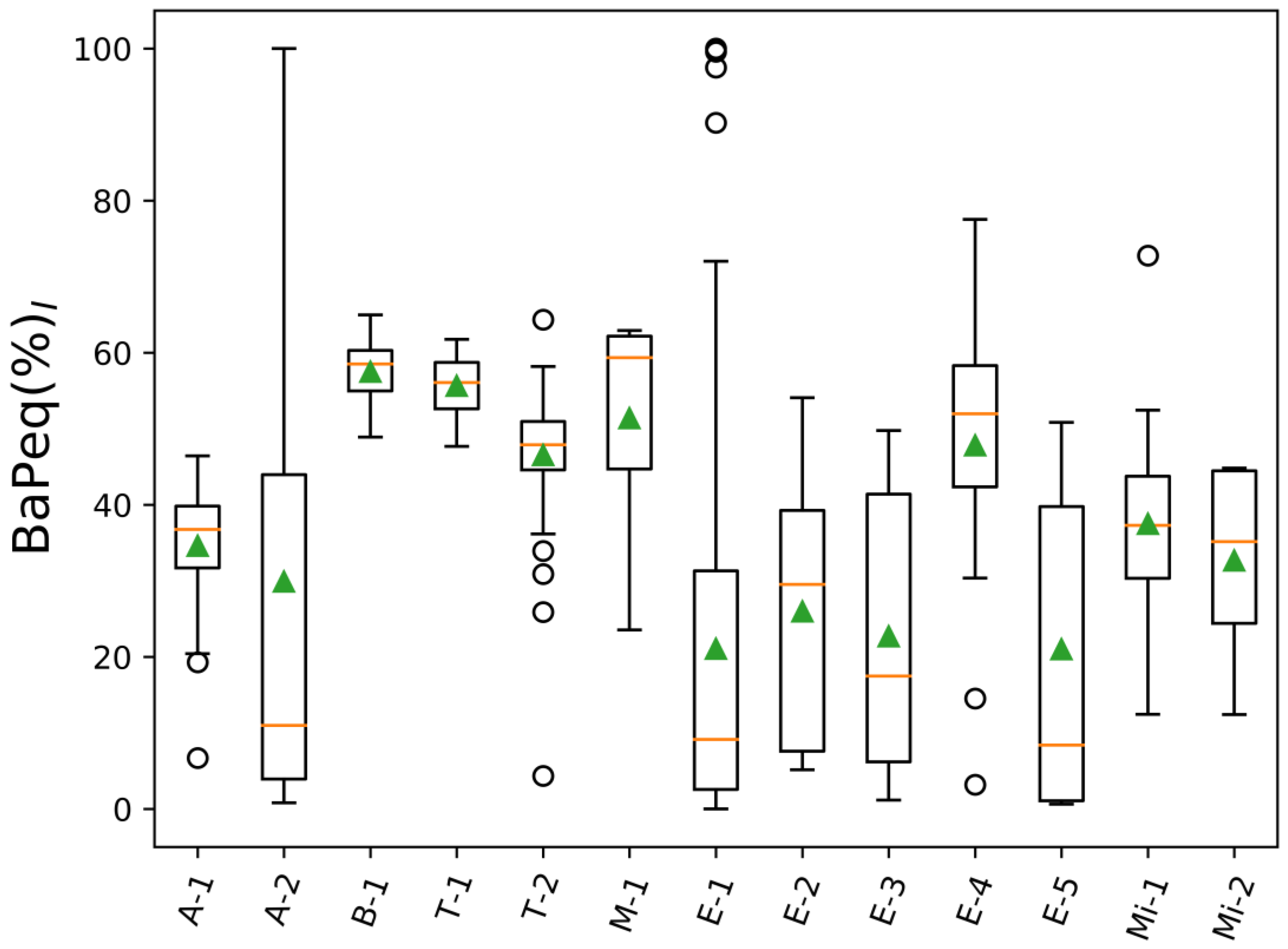
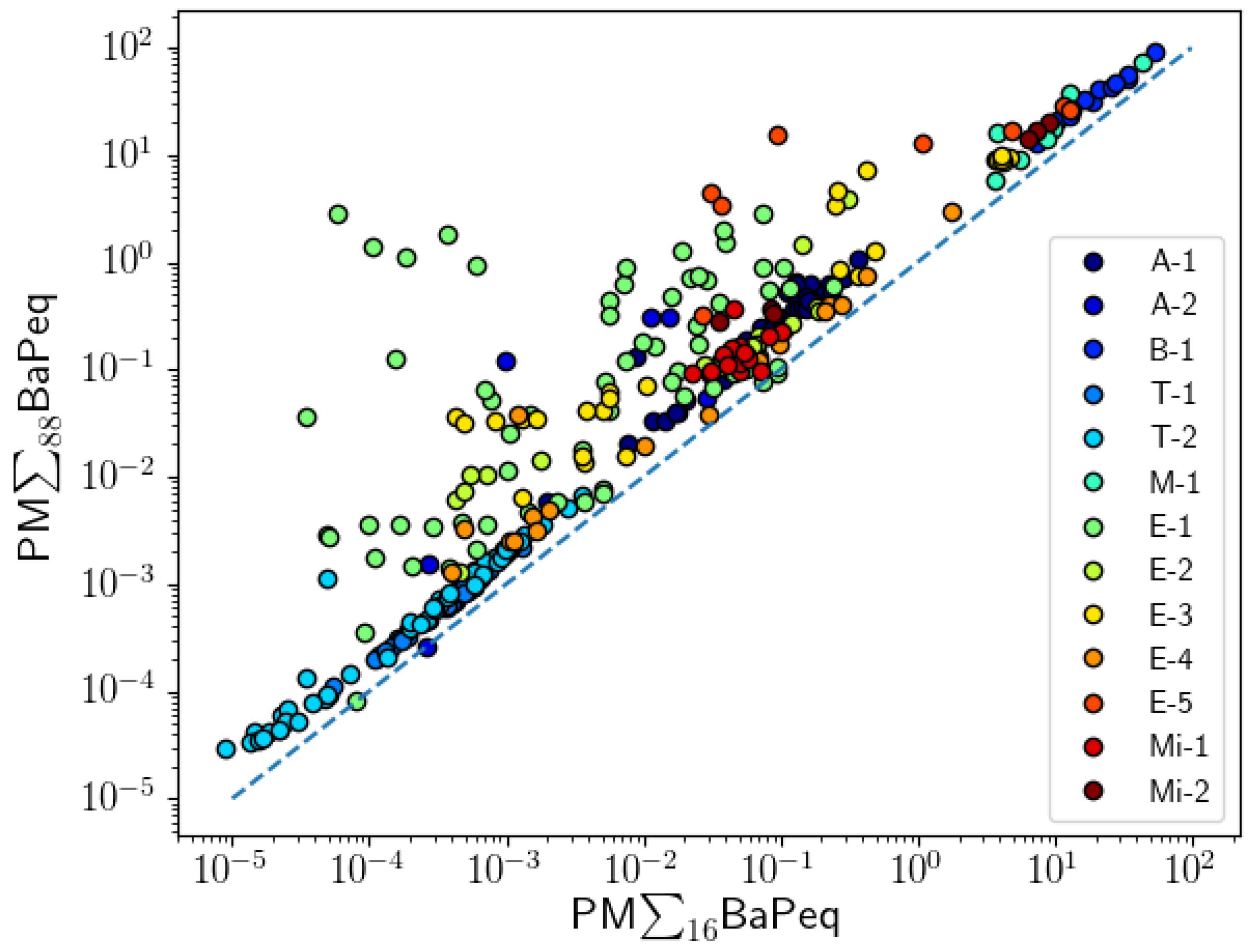

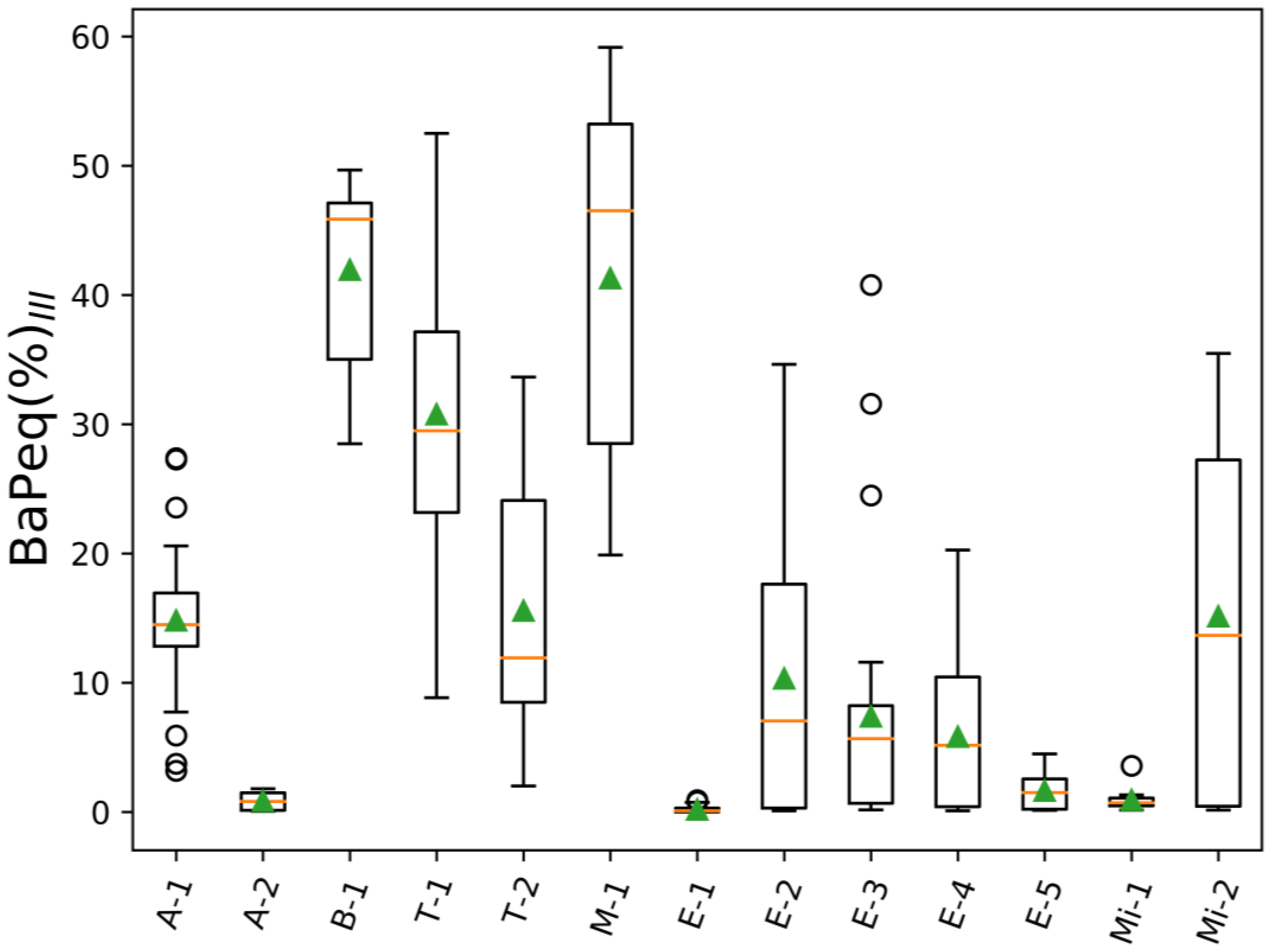
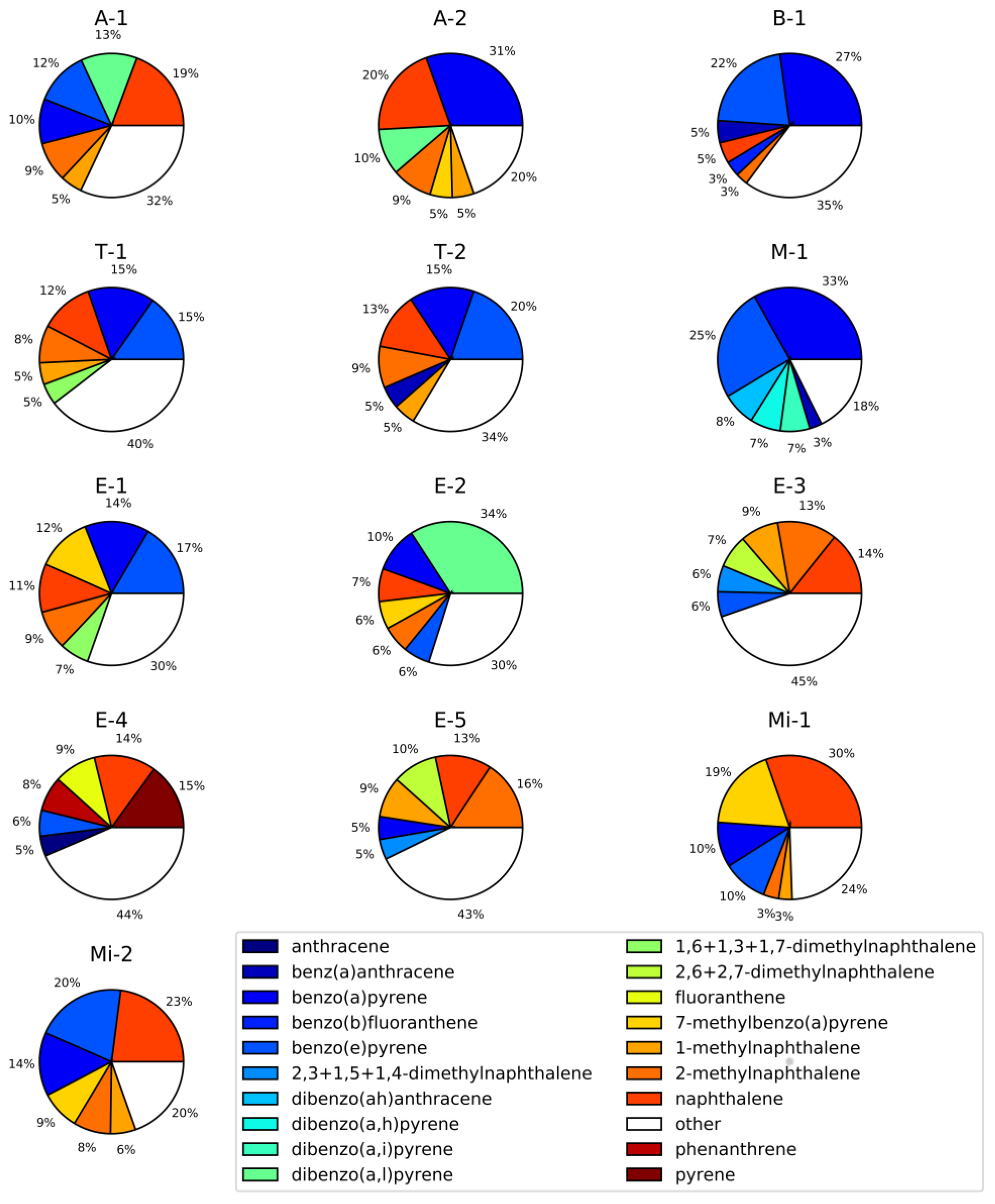

| Project Abbreviation and Reference | Sample Source | Description | Number of Samples | |
|---|---|---|---|---|
| Filters | XAD | |||
| A-1 [77] | Ambient urban air | LAX airport (CA) | 44 | 44 |
| A-2 | Ambient urban air | Reno city (NV) | 6 | 6 |
| B-1 [78] | Controlled biomass burning | DRI combustion chamber (NV) | 11 | 11 |
| T-1 | Tunnel/car emissions | Fort McHenry tunnel (MD), winter 2015 | 46 | 46 |
| T-2 | Tunnel/car emissions | Fort McHenry tunnel (MD), summer 2015 | 50 | 50 |
| M-1 | Meat cooking | - | 7 | 7 |
| E-1 | Engine emissions | Heavy and light duty engines | 71 | 71 |
| E-2 | Engine emissions | Engine emissions (Cummins) | 16 | 16 |
| E-3 | Engine emissions | Engine emissions (Cummins) | 26 | 26 |
| E-4 | Engine emissions | Engine emissions (Cummins) | 17 | 17 |
| E-5 | Engine emissions | Emissions from heavy heavy-duty diesel engines (model year 2007) | 9 | 9 |
| Mi-1 | Mining | Mining operation facility | 16 | 16 |
| Mi-2 | Mining | Mining operation facility | 6 | 6 |
| Project | PM∑16BaPeq/PM∑88BaPeq | PM∑16BaPeq/(PM + GAS)∑88BaPeq | ||
|---|---|---|---|---|
| r_Value | p_Value | r_Value | p_Value | |
| A-1 | 0.917 | 0.0000 | 0.841 | 0.0000 |
| A-2 | 0.657 | 0.1562 | 0.829 | 0.0416 |
| B-1 | 0.991 | 0.0000 | 0.936 | 0.0000 |
| T-1 | 0.981 | 0.0000 | 0.848 | 0.0000 |
| T-2 | 0.987 | 0.0000 | 0.919 | 0.0000 |
| M-1 | 0.893 | 0.0068 | 0.857 | 0.0137 |
| E-1 | 0.507 | 0.0000 | 0.054 | 0.6580 |
| E-2 | 0.982 | 0.0000 | 0.688 | 0.0032 |
| E-3 | 0.888 | 0.0000 | 0.857 | 0.0000 |
| E-4 | 0.941 | 0.0000 | 0.838 | 0.0000 |
| E-5 | 0.917 | 0.0005 | 0.917 | 0.0005 |
| Mi-1 | 0.450 | 0.0803 | −0.297 | 0.2639 |
| Mi-2 | 0.943 | 0.0048 | 0.543 | 0.2657 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. https://doi.org/10.3390/toxics5030017
Samburova V, Zielinska B, Khlystov A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics. 2017; 5(3):17. https://doi.org/10.3390/toxics5030017
Chicago/Turabian StyleSamburova, Vera, Barbara Zielinska, and Andrey Khlystov. 2017. "Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity?" Toxics 5, no. 3: 17. https://doi.org/10.3390/toxics5030017