Toxic Effect of Cigarette Smoke on Brainstem Nicotinic Receptor Expression: Primary Cause of Sudden Unexplained Perinatal Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
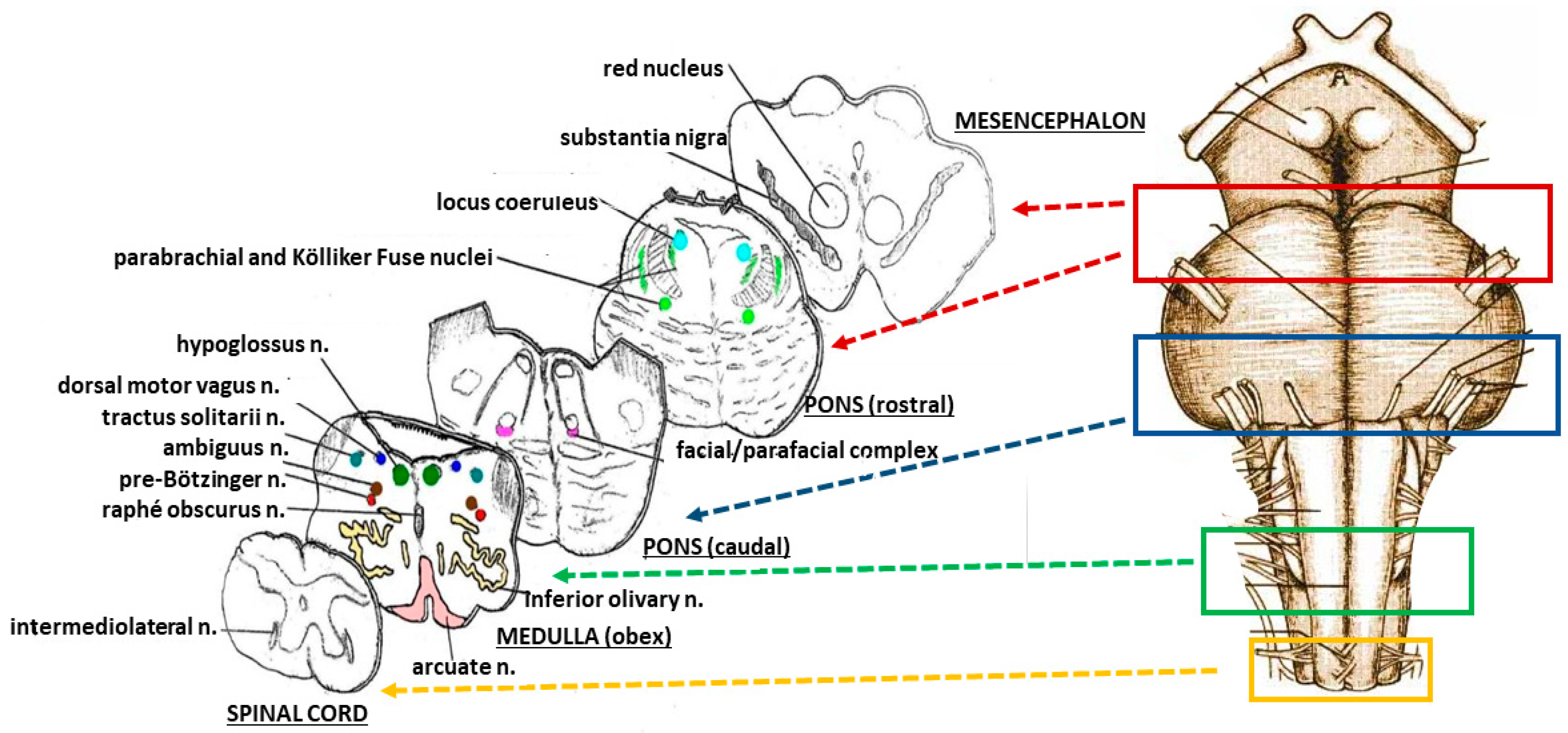
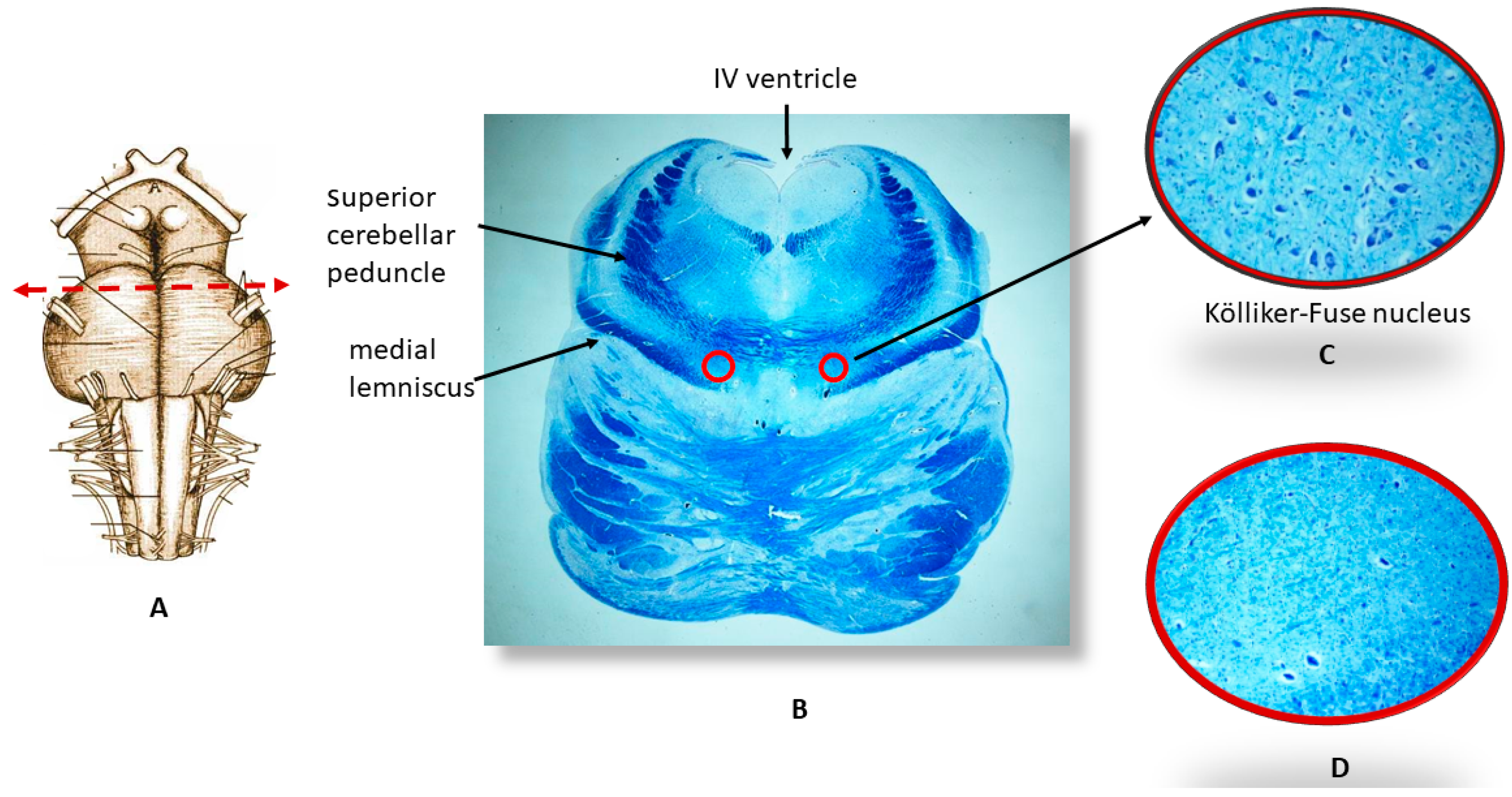
2.2. Neuropathological Protocol
2.3. Immunohistochemical Protocol
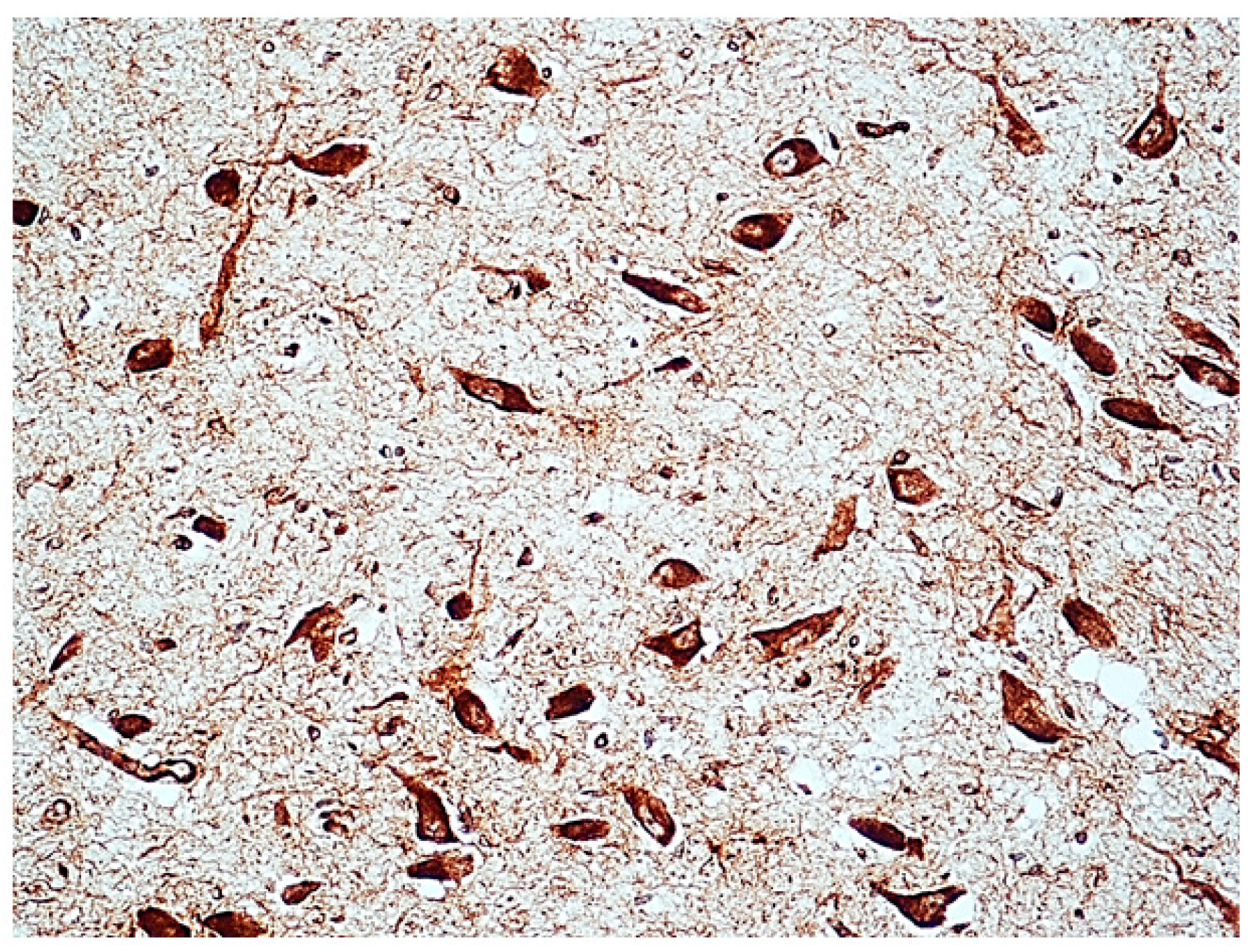
2.3.1. nAChR Immunohistochemistry
2.3.2. nAChR Immunohistochemistry Quantification
2.4. Statistical Analysis
3. Results
3.1. Neuropathological Examination
3.2. Immunohistochemical Expression of α7 Receptors
3.3. Correlations between Immunoexpression of α7-nAChR and Maternal Smoking
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Constitution of the Italian Republic Law n 31. Regulations for Diagnostic Post-Mortem Investigation in Victims of Sudden Infant Death Syndrome (SIDS) and Unexpected Fetal Death. Official Gazette of the Italian Republic, General Series 2006; 34: 4. Available online: http://users.unimi.it/centrolinorossi/files/gazz_ufficiale.pdf (accessed on 16 October 2018).
- Lavezzi, A.M.; Ottaviani, G.; Mauri, M.; Matturri, L. Biopathology of the dentate-olivary complex in sudden unexplained perinatal death and sudden infant death syndrome related to maternal cigarette smoking. Neurol. Res. 2007, 29, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lavezzi, A.M.; Matturri, L.; Del Corno, G.; Johanson, C.E. Vulnerability of fourth ventricle choroid plexus in sudden unexplained fetal and infant death syndromes related to smoking mothers. Int. J. Dev. Neurosci. 2013, 31, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lavezzi, A.M.; Corna, M.F.; Matturri, L. Ependymal alterations in sudden intrauterine unexplained death and sudden infant death syndrome: Possible primary consequence of prenatal exposure to cigarette smoking. Neural. Dev. 2010, 19, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lavezzi, A.M.; Corna, M.F.; Repetti, M.L.; Matturri, L. Cerebellar Purkinje cell vulnerability to prenatal nicotine exposure in sudden unexplained perinatal death. Folia Neuropathol. 2013, 51, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Lavezzi, A.M.; Mecchia, D.; Matturri, L. Neuropathology of the area postrema in sudden intrauterine and infant death syndromes related to tobacco smoke exposure. Auton. Neurosci. 2012, 166, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lavezzi, A.M.; Ottaviani, G.; Matturri, L. Adverse effects of prenatal tobacco smoke exposure on biological parameters of the developing brainstem. Neurobiol. Dis. 2005, 20, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.T. The molecular biology of neuronal nicotinic acetylcholine receptors. Crit. Rev. Toxicol. 1997, 27, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.; Anand, R.; Gerzanich, V.; Peng, X.; Wang, F.; Wells, G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog. Brain Res. 1996, 109, 125–137. [Google Scholar] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Shipton, D.; Tappin, D.M.; Vadiveloo, T.; Crossley, J.A.; Aitken, D.A.; Chalmers, J. Reliability of self-reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. Br. Med. J. 2009, 339, b4347. [Google Scholar] [CrossRef] [PubMed]
- Tzatzarakis, M.N.; Vardavas, C.I.; Terzi, I.; Kavalakis, M.; Kokkinakis, M.; Liesivuori, J.; Tsatsakis, A.M. Hair nicotine/cotinine concentrations as a method of monitoring exposure to tobacco smoke among infants and adults. Hum. Exp. Toxicol. 2012, 31, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Le Novere, N.; Corringer, P.J.; Changeux, J.P. The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 2002, 53, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstrom, J. The structures of neuronal nicotinic receptors. In Neuronal Nicotinic Acetylcholine Receptors; Clementi, F., Fornasari, D., Gotti, C., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000; pp. 101–162. [Google Scholar]
- Broide, R.S.; Lesli, F.M. The alpha7 nicotinic acetylcoline receptor in neuronal plasticity. Mol. Neurobiol. 1999, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Falk, L.; Nordberg, A.; Seiger, Å.; Kjaeldgaard, A.; Hellström-Lindahl, E. The alpha7 nicotinic receptors in human fetal brain and spinal cord. J. Neurochem. 2002, 80, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, M.; Toumbourou, J.W.; Catalano, R.F.; Williams, J.; Patton, G.C. Consequences of youth tobacco use: A review of prospective behavioural studies. Addiction 2006, 101, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.D.; Day, N.L. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009, 22, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.M. Tobacco and pregnancy: Overview of exposures and effects. Birth Defects Res. C 2008, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wisborg, K.; Henriksen, T.B.; Hedegaard, M.; Secher, N.J. Smoking during pregnancy and preterm birth. Br. J. Obstet. Gynaecol. 1996, 103, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.; Day, M. Perinatal complications associated with maternal tobacco use. Semin. Neonatol. 2000, 5, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.R.; Goldstein, H.; Ross, E.M. Cigarette smoking in pregnancy: Its influence on birth weight and perinatal mortality. Br. Med. J. 1972, 2, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Dietz, P.M.; England, L.J.; Shapiro-Mendoza, C.K.; Tong, V.T.; Farr, S.L.; Callaghan, W.M. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am. J. Prev. Med. 2010, 39, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lichtensteiger, W.; Ribary, U.; Schlumpf, M.; Odermatt, B.; Widmer, H.R. Prenatal adverse effects of nicotine on the developing brain. Prog. Brain Res. 1988, 73, 137–157. [Google Scholar] [PubMed]
- Gressens, P.; Laudenbach, V.; Marret, S. Mechanisms of action of tobacco smoke on the developing brain. J. Gynecol. Obstet. Biol. Reprod. (Paris) 2003, 32 (Suppl. 1), 30–32. [Google Scholar]
- Soothill, P.W.; Morafa, W.; Ayida, G.A.; Rodeck, C.H. Maternal smoking and fetal carboxyhaemoglobin and blood gas levels. Br. J. Obstet. Gynaecol. 1996, 103, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Slotkin, T.A. Developmental neurotoxicity of nicotine. In Handbook of Developmental Neurotoxicology; Slikker, W., Chang, W., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 587–615. [Google Scholar]
- Atluri, P.; Fleck, M.W.; Shen, Q.; Mah, S.J.; Stadfelt, D.; Barnes, W.; Goderie, S.K.; Temple, S.; Schneider, A.S. Functional nicotinic acetylcholine receptor expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Dev. Biol. 2001, 240, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Andrews, J.E.; Seidler, F.J.; Slotkin, T.A. Nicotine evokes cell death in embryonic rat brain during neurulation. J. Pharmacol. Exp. Ther. 1998, 287, 1135–1144. [Google Scholar]
- Ezure, K.; Tanaka, I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 2006, 141, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Segers, L.S.; Shannon, R.; Lindsey, B.G. Interactions between rostral pontine and ventral medullary respiratory neurons. J. Neurophysiol. 1985, 54, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Dutschmann, M.; Herbert, H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 2006, 24, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Dutschmann, M.; Dick, T.E. Pontine mechanisms of respiratory control. Compr. Physiol. 2012, 2, 2443–6249. [Google Scholar] [PubMed]
- Mörschel, M.; Dutschmann, M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Victims | Sex | Age Range | Death Diagnosis | Maternal Smoking Habit | ||
|---|---|---|---|---|---|---|
| F | M | Smokers | Nonsmokers | |||
| Fetuses | 20 | 12 | 28–40 gw | SIUD (n = 32) | 23 | 10 |
| 5 | 3 | Control cases (n = 8): | 2 | 6 | ||
| severe chorioamnionitis (n = 5) | ||||||
| congenital heart disease (n = 3) | ||||||
| Newborns | 10 | 12 | 0–2 pm | e-SIDS (n = 22) | 11 | 10 |
| 2 | 4 | Control cases (n = 6): | 2 | 4 | ||
| congenital heart disease (n = 3) | ||||||
| broncopneumonia (n = 1) | ||||||
| pulmonary dysplasia (n = 1) | ||||||
| myocarditis (n = 1) | ||||||
| Brainstem Nuclei with Hypoplasia/Agenesis * | SIUD n = 32[23] | Control Fetal Death n = 8[2] | e-SIDS n = 22[11] | Control Neonatal Death n = 6[2] |
|---|---|---|---|---|
| Kölliker nucleus | 7[7] | 0[0] | 9[9] | 0[0] |
| Raphé nuclei | 5[5] | 0[0] | 4[2] | 0[0] |
| Pre-Bötzinger | 5[2] | 0[0] | 4[4] | 0[0] |
| Facial/parafacial complex | 5[5] | 0[0] | 4[1] | 0[0] |
| Arcuate nucleus | 5[5] | 1[1] | 5[3] | 1[0] |
| α7-nAChR immunopositivity ** | ||||
| “Class 2” Index | 9[9] | 2[2] | 3[2] | 1[1] |
| “Class 3” Index | 14[14] | 0[0] | 8[8] | 0[0] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavezzi, A.M. Toxic Effect of Cigarette Smoke on Brainstem Nicotinic Receptor Expression: Primary Cause of Sudden Unexplained Perinatal Death. Toxics 2018, 6, 63. https://doi.org/10.3390/toxics6040063
Lavezzi AM. Toxic Effect of Cigarette Smoke on Brainstem Nicotinic Receptor Expression: Primary Cause of Sudden Unexplained Perinatal Death. Toxics. 2018; 6(4):63. https://doi.org/10.3390/toxics6040063
Chicago/Turabian StyleLavezzi, Anna Maria. 2018. "Toxic Effect of Cigarette Smoke on Brainstem Nicotinic Receptor Expression: Primary Cause of Sudden Unexplained Perinatal Death" Toxics 6, no. 4: 63. https://doi.org/10.3390/toxics6040063





