Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster
Abstract
:1. Introduction
Specific Hypotheses
- H1-A: Genetically modified corn increases mortality.
- H1-B: Genetically modified corn decreases male courtship behavior.
- H1-C: Genetically modified corn decreases mating behavior.
- H1-D: The genetically modified corn used contains glyphosate residue.
- H2-A: Exposure to glyphosate-based herbicide increases mortality.
- H2-Ai: Mortality depends on herbicide formulation.
- H2-B: Mortality depends on D. melanogaster genetic background.
2. Materials and Methods
2.1. Stock Maintenance
2.2. Experimental Set-up to Assess Genetically Modified Corn Toxicity
2.2.1. Mortality
2.2.2. Female Reproductive Behavior
2.2.3. Male Reproductive Behavior
2.3. Analysis of Glyphosate Residue on Corn
2.4. Experimental Set-Up for Herbicide Toxicity
2.4.1. Mortality
2.4.2. Genetic Background
2.4.3. Herbicide Formulation
3. Results
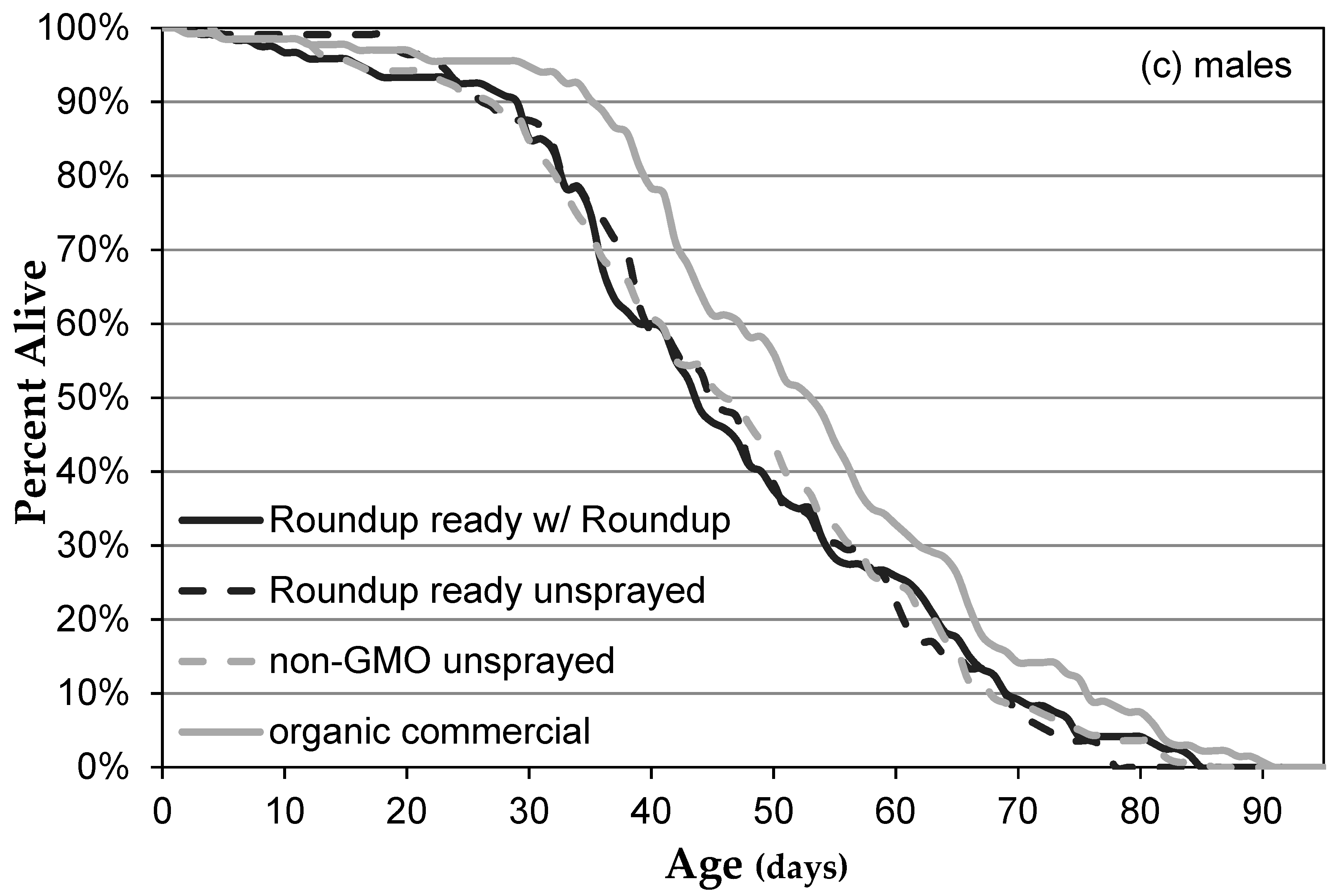
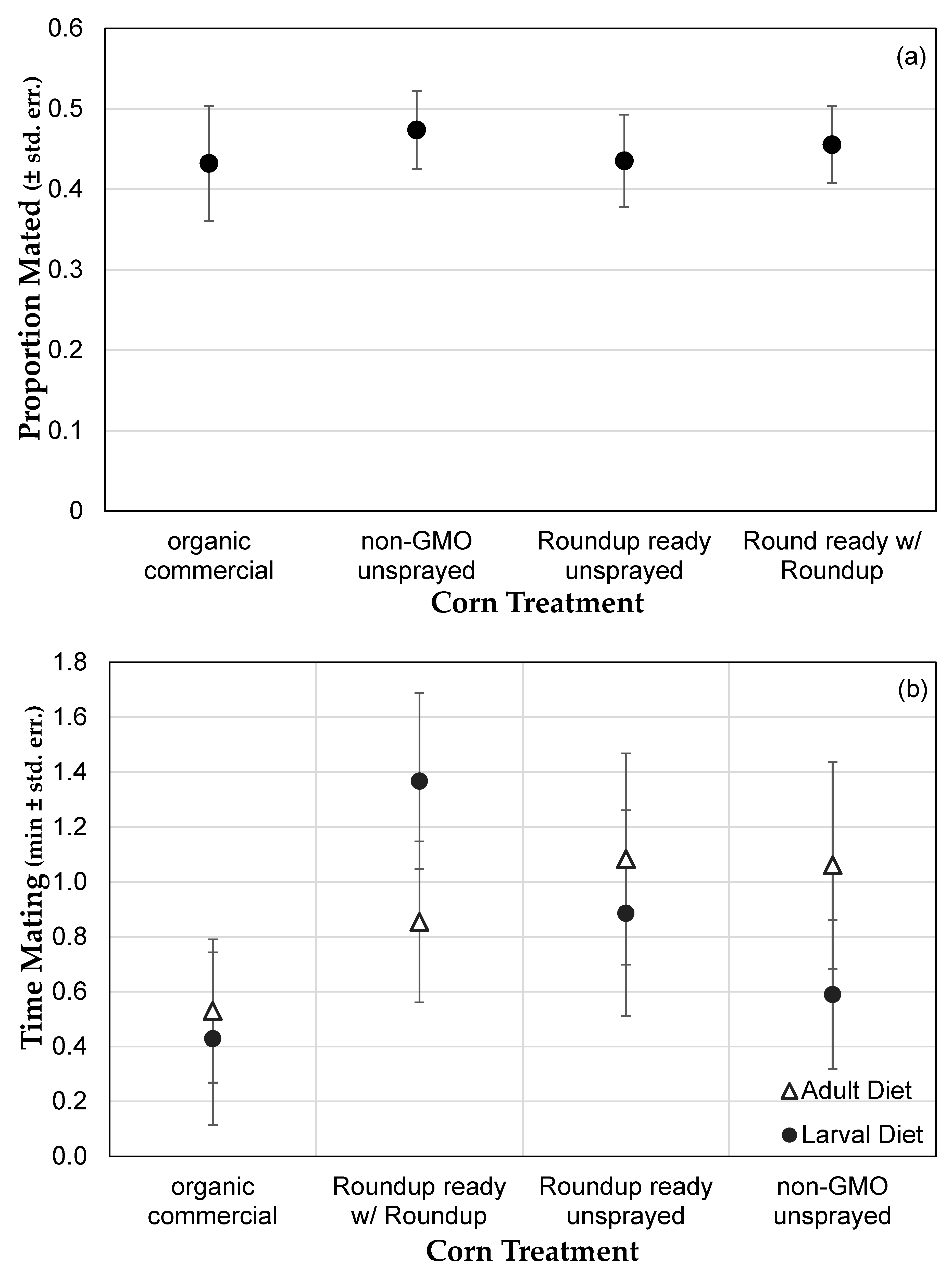
3.1. Genetically Modified NK603 Corn Does Not Harm D. melanogaster’s Lifespan or Reproductive Behavior
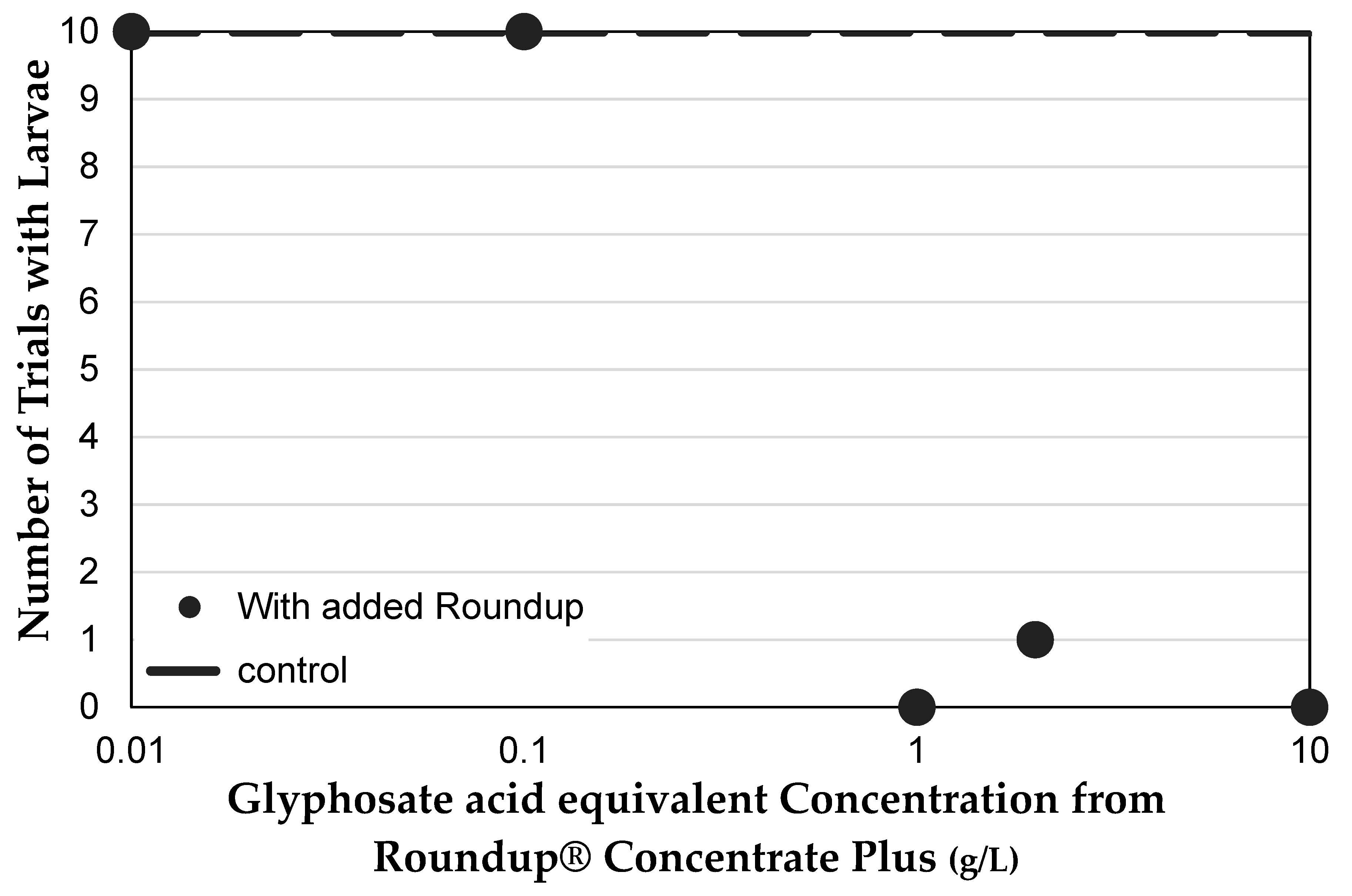
3.2. The NK603 Genetically Modified Corn Used Contains Low Concentration Glyphosate Residue
3.3. Herbicide Exposure Increases Drosophila melanogaster Mortality
3.3.1. Roundup® Exposure Causes a Dose-Dependent Mortality Increase
3.3.2. Genetic Background Does Not Influence Sensitivity to Herbicide
3.3.3. Herbicide Formulations Differ in Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA ERS, United States Department of Agriculture, Economic Research Service. Adoption of Genetically Modified Crops in the US. 2017. Available online: https://www ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us.aspx. (accessed on 20 April 2018).
- Fernandez-Cornejo, J.; Wechsler, S.J.; Milkove, D.L. The Adoption of Genetically Engineered Alfalfa, Canola and Sugarbeets in the United States; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2016.
- ISAAA. ISAAA Brief No. 52: Global Status of Commercialized Biotech/GM Crops: 2016; ISAAA: Ithaca, NY, USA, 2017. [Google Scholar]
- Domingo, H.L. Safety assessment of GM plants: An updated review of the Scientific literature. Food Chem. Toxicol. 2016, 95, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Snell, C.; Bernheim, A.; Bergé, J.B.; Kuntz, M.; Pascal, G.; Paris, A.; Ricroch, A.E. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: A literature review. Food Chem. Toxicol. 2012, 50, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, S.; Swiatkiewicz, M.; Arczewska-Wlosek, A.; Jozefiak, D. Genetically modified feeds and their effect on the metabolic parameters of food-producing animals: A review of recent studies. Anim. Feed Sci. Technol. 2014, 198, 1–19. [Google Scholar] [CrossRef]
- De Maria, N.; Becerril, J.M.; Garca-Plazaola, J.I.; Hernandez, A.H.; de Felipe, M.R.; Fernandez-Pascual, M. New insights on glyphosate mode of action in nodular metabolism: Role of shikimate accumulation. J. Agric. Food Chem. 2006, 54, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- De Vos, C.J.; Swanenburg, M. Health effects of feeding genetically modified (GM) crops to livestock animals: A review. Food Chem. Toxicol. 2018, 117, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Séralini, G.E.; Clair, E.; Mesnage, R.; Gress, S.; Defarge, N.; de Vendomois, J.S. Republished study: Long-term toxicity of a Roundup® herbicide and a Roundup®-tolerant genetically modified maize. Environ. Sci. Eur. 2014, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.M.; Nawaz, M.A.; Tutelyan, V.A.; Golokhvast, K.S.; Kalantzi, O.I.; Chung, D.H.; Chung, G. Impact on environment, ecosystem, diversity and health from culturing and using GMOs as feed and food. Food Chem. Toxicol. 2017, 107, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdziarski, I.M.; Edwards, J.W.; Carman, J.A.; Haynes, J.I. GM crops and the rat digestive tract: A critical review. Environ. Int. 2014, 73, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shi, F. Do genetically modified crops affect animal reproduction? A review of the ongoing debate. Animal 2011, 5, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Tudisco, R.; Lombardi, P.; Bovera, F.; Cutrignelli, M.I.; Mastellone, V.; Terzi, V.; Infascelli, F. Genetically modified soya bean in rabbit feeding: Detection of DNA fragments and evaluation of metabolic effects by enzymatic analysis. Anim. Sci. 2006, 82, 193–199. [Google Scholar] [CrossRef]
- Wang, E.H.; Yu, Z.; Hu, J.; Jia, X.D.; Xu, H.B. A two-generation reproduction study with transgenic Bt rice TT51 in Wistar rats. Food Chem. Toxicol. 2014, 65, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Carman, J.A.; Vlieger, H.R.; Ver Steeg, L.J.; Sneller, V.E.; Robinson, G.W.; Clinch-Jones, C.A.; Edwards, J.W. A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J. Org. Syst. 2013, 8, 38–54. [Google Scholar]
- Ibrahim, M.A.; Okasha, E.F. Effect of genetically modified corn on the jejunal mucosa of adult male albino rat. Exp. Toxicol. Pathol. 2016, 68, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Zdziarski, I.M.; Carman, J.A.; Edwards, J.W. Histopathological Investigation of the Stomach of Rats Fed a 60% Genetically Modified Corn Diet. Food Nutr. Sci. 2018, 9, 763. [Google Scholar] [CrossRef]
- Malatesta, M.; Caporaloni, C.; Rossi, L.; Battistelli, S.; Rocchi, M.B.; Tonucci, F.; Gazzanelli, G. Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. J. Anat. 2002, 201, 409–415. [Google Scholar] [CrossRef]
- Malatesta, M.; Biggiogera, M.; Manuali, E.; Rocchi, M.B.L. Fine structural analyses of pancreatic acinar cell nuclei from mice fed on genetically modified soybean. Eur. J. Histochem. 2003, 47, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Abdo, E.M.; Barbary, O.M.; Shaltout, O.E.S. Feeding study with Bt corn (MON810: Ajeeb YG) on rats: Biochemical analysis and liver histopathology. Food Nutr. Sci. 2014, 5, 185–195. [Google Scholar] [CrossRef]
- Malatesta, M.; Caporaloni, C.; Gavaudan, S.; Rocchi, M.B.; Serafini, S.; Tiberi, C.; Gazzanelli, G. Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell Struct. Funct. 2002, 27, 173–180. [Google Scholar] [CrossRef]
- Malatesta, M.; Boraldi, F.; Annovi, G.; Baldelli, B.; Battistelli, S.; Biggiogera, M.; Quaglino, D. A long-term study on female mice fed on a genetically modified soybean: Effects on liver ageing. Histochem. Cell Biol. 2008, 130, 967–977. [Google Scholar] [CrossRef]
- Oraby, H.; Kandil, M.; Shaffie, N.; Ghaly, I. Biological impact of feeding rats with a genetically modified-based diet. Turk. J. Biol. 2015, 39, 265–275. [Google Scholar] [CrossRef]
- Séralini, G.E.; Cellier, D.; De Vendômois, J.S. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch. Environ. Contam. Toxicol. 2007, 52, 596–602. [Google Scholar] [CrossRef] [PubMed]
- De Vendômois Joël, S.; François, R.; Dominique, C.; Gilles-Eric, S. A comparison of the effects of three GM corn varieties on mammalian health. Int. J. Biol. Sci. 2009, 5, 706–726. [Google Scholar] [CrossRef] [PubMed]
- Adel-Patient, K.; Guimaraes, V.D.; Paris, A.; Drumare, M.F.; Ah-Leung, S.; Lamourette, P.; Créminon, C. Immunological and metabolomic impacts of administration of Cry1Ab protein and MON 810 maize in mouse. PLoS ONE 2011, 6, e16346. [Google Scholar] [CrossRef] [PubMed]
- Tudisco, R.; Calabrò, S.; Cutrignelli, M.I.; Moniello, G.; Grossi, M.; Mastellone, V.; Infascelli, F. Genetically modified soybean in a goat diet: Influence on kid performance. Small Rumin. Res. 2015, 126, 67–74. [Google Scholar] [CrossRef]
- Tulinská, J.; Adel-Patient, K.; Bernard, H.; Líšková, A.; Kuricová, M.; Ilavská, S.; Aláčová, R. Humoral and cellular immune response in Wistar Han RCC rats fed two genetically modified maize MON810 varieties for 90 days (EU 7th Framework Programme project GRACE). Arch. Toxicol. 2018, 92, 2385–2399. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, T.; Rover, C.M.; Semenchuk, P.R. Daphnia magna negatively affected by chronic exposure to purified Cry-toxins. Food Chem. Toxicol. 2016, 91, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.; Cisterna, B.; Malatesta, M.; Martin, T.E.; Biggiogera, M. Ultrastructural analysis of testes from mice fed on genetically modified soybean. Eur. J. Histochem. 2009, 48, 449–454. [Google Scholar] [CrossRef]
- Cyran, N.; Gülly, C.; Handl, S.; Hofstätter, G.; Meyer, F.; Skalicky, M.; Steinborn, R. 2008. Biological Effects Of Transgenic Maize NK603xMON810 Fed in Long Term Reproduction Studies in Mice. Unpublished report: Institute fur Ernahrung, Austria. Available online: http://biologia.ucr.ac.cr/profesores/Garcia%20Jaime/TRANSGENICOS/SALUD-TOXICOLOGIA_EN_RATAS-2008.pdf (accessed on 23 January 2019).
- Trabalza-Marinucci, M.; Brandi, G.; Rondini, C.; Avellini, L.; Giammarini, C.; Costarelli, S.; Malatesta, M. A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci. 2008, 113, 178–190. [Google Scholar] [CrossRef]
- Mesnage, R.; Agapito-Tenfen, S.Z.; Vilperte, V.; Renney, G.; Ward, M.; Séralini, G.E.; Antoniou, M.N. An integrated multi-omics analysis of the NK603 Roundup®-tolerant GM maize reveals metabolism disturbances caused by the transformation process. Sci. Rep. 2016, 19, 37855. [Google Scholar] [CrossRef]
- Mesnage, R.; Arno, M.; Séralini, G.E.; Antoniou, M.N. Transcriptome and metabolome analysis of liver and kidneys of rats chronically fed NK603 Roundup®-tolerant genetically modified maize. Environ. Sci. Eur. 2017, 29, 6. [Google Scholar] [CrossRef]
- Cuhra, M. Review of GMO safety assessment studies: Glyphosate residues in Roundup® Ready crops is an ignored issue. Environ. Sci. Eur. 2015, 27, 20. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Rock, B.; Suriyan, J.; Vijay, B.; Thalha, N.; Elango, S. Organic Food and Health: A Systematic Review. J. Community Med. Health Educ. 2017, 7, 2161–2711. [Google Scholar] [CrossRef]
- Tiryaki, O. Pesticide Residues and Organic Production. J. Biol. Environ. Sci. 2017, 11, 11–23. [Google Scholar]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage 2008–2012 Market Estimates; US Environmental Protection Agency: Washington, DC, USA, 2017.
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Rowlands, H. Glyphosate: Unsafe on Any Plate. Food Democr. Now 2016, 1–29. [Google Scholar]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup® Ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Für Verbrauch. Und Lebensm. 2015, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults–Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef]
- Mills, P.J.; Kania-Korwel, I.; Fagan, J.; McEvoy, L.K.; Laughlin, G.A.; Barrett-Connor, E. Excretion of the herbicide glyphosate in older adults between 1993 and 2016. JAMA 2017, 318, 1610–1611. [Google Scholar] [CrossRef]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.W.; Lutz, W.; Shehata, A.A. Detection of glyphosate residues in animals and humans. J. Environ. Anal. Toxicol. 2014, 4, 1. [Google Scholar] [CrossRef]
- Chhabra, R.; Kolli, S.; Bauer, J.H. Organically grown food provides health benefits to Drosophila melanogaster. PLoS ONE 2013, 8, e52988. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.D. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicol. Teratol. 2010, 32, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Rogina, B.; Helfand, S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Pro. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.N.S.; Coogan, C.; Chamseddin, K.; Fernandez-Kim, S.O.; Kolli, S.; Keller, J.N.; Bauer, J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta 2012, 1822, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Oldham, S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef]
- Bier, E.; Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dionne, M.S.; Schneider, D.S. Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis. Model. Mech. 2008, 1, 43–49. [Google Scholar] [CrossRef]
- Grice, S.J.; Liu, J.L.; Webber, C. Synergistic interactions between Drosophila orthologues of genes spanned by de novo human CNVs support multiple-hit models of autism. PLoS Genet. 2015, 11, e1004998. [Google Scholar] [CrossRef]
- Bilen, J.; Bonini, N.M. Drosophila as a model for human nEur.odegenerative disease. Annu. Rev. Genet. 2005, 39, 153–171. [Google Scholar] [CrossRef]
- Manning, A. Reproductive and parental behaviour: The control of sexual receptivity in female Drosophila. Anim. Behav. 1962, 10, 382. [Google Scholar] [CrossRef]
- Lee, G.; Villella, A.; Taylor, B.J.; Hall, J.C. New reproductive anomalies in fruitless-mutant Drosophila males: Extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J. Neurobiol. 2001, 47, 121–149. [Google Scholar] [CrossRef]
- Talyn, B.C.; Dowse, H.B. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim. Behav. 2004, 68, 1165–1180. [Google Scholar] [CrossRef]
- Zhao, Y.; Bretz, C.A.; Hawksworth, S.A.; Hirsh, J.; Johnson, E.C. Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS ONE 2010, 5, e9141. [Google Scholar] [CrossRef]
- Elens, A.A.; Wattiaux, J.M. Direct observation of sexual isolation. Drosoph. Inf. 1964, 39, 118–119. [Google Scholar]
- Mesnage, R.; Defarge, N.; De Vendômois, J.S.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Bernay, B.; Séralini, G. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Broughton, S.J.; Piper, M.D.; Ikeya, T.; Bass, T.M.; Jacobson, J.; Driege, Y.; Partridge, L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 3105–3110. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef]
- Defays, R.; Gómez, F.H.; Sambucetti, P.; Scannapieco, A.C.; Loeschcke, V.; Norry, F.M. Quantitative trait loci for longevity in heat-stressed Drosophila melanogaster. Exp. Gerontol. 2011, 46, 819–826. [Google Scholar] [CrossRef]
- Travers, L.M.; Garcia-Gonzalez, F.; Simmons, L.W. Live fast die young life history in females: Evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci. Rep. 2015, 20, 15469. [Google Scholar] [CrossRef]
- Viña, J.; Borrás, C.; Gambini, J.; Sastre, J.; Pallardó, F.V. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005, 579, 2541–2545. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Krogdahl, Å.; Sissener, N.H.; Kortner, T.M.; Gelencser, E.; Hemre, G.I.; Bakke, A.M. Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br. J. Nutr. 2013, 109, 1408–1423. [Google Scholar] [CrossRef]
- Santos-Vigil, K.I.; Ilhuicatzi-Alvarado, D.; García-Hernández, A.L.; Herrera-García, J.S.; Moreno-Fierros, L. Study of the allergenic potential of Bacillus thuringiensis Cry1Ac toxin following intra-gastric administration in a murine model of food-allergy. Int. immunopharmacol. 2018, 61, 185–196. [Google Scholar] [CrossRef]
- Latham, J.R.; Wilson, A.K.; Steinbrecher, R.A. The mutational consequences of plant transformation. J. Biomed. Biotechnol. 2006. [Google Scholar] [CrossRef]
- Wilson, A.K.; Latham, J.R.; Steinbrecher, R.A. Transformation-induced mutations in transgenic plants: Analysis and biosafety implications. Biotechnol. Genet. Eng. Rev. 2006, 23, 209–238. [Google Scholar] [CrossRef]
- Zolla, L.; Rinalducci, S.; Antonioli, P.; Righetti, P.G. Proteomics as a complementary tool for identifying unintended side effects occurring in transgenic maize seeds as a result of genetic modifications. J. Proteome Res. 2008, 7, 1850–1861. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, C.; Zhu, Z.; Wu, Z.; Zhou, J.; Zhao, Y.; Xu, G. Metabolic profiling based on LC/MS to evaluate unintended effects of transgenic rice with cry1Ac and sck genes. Plant Mol. Biol. 2012, 78, 477–487. [Google Scholar] [CrossRef]
- Gong, C.Y.; Wang, T. Proteomic evaluation of genetically modified crops: Current status and challenges. Front. Plant Sci. 2013, 4, 41. [Google Scholar] [CrossRef]
- Mathur, C.; Kathuria, P.C.; Dahiya, P.; Singh, A.B. Lack of detectable allergenicity in genetically modified maize containing “cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS ONE 2015, 10, e0117340. [Google Scholar] [CrossRef]
- Hao, W.; Li, F.; Yan, W.; Li, C.; Hao, D. Comparative metabolic profiling of four transgenic maize lines and two non-transgenic maize lines using high-performance liquid chromatography mass spectrometry. Acta Physiol. Plant. 2017, 39, 167. [Google Scholar] [CrossRef]
- Lee, T.H.; Ho, H.K.; Leung, T.F. Genetically modified foods and allergy. Hong Kong Med. J. 2017, 23, 291–295. [Google Scholar] [CrossRef]
- Mou, H.; Smith, J.L.; Peng, L.; Yin, H.; Moore, J.; Zhang, X.O.; Li, Y. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol. 2017, 18, 108. [Google Scholar] [CrossRef]
- Schaefer, K.A.; Wu, W.H.; Colgan, D.F.; Tsang, S.H.; Bassuk, A.G.; Mahajan, V.B. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat. Methods 2017, 14, 547–548. [Google Scholar] [CrossRef]
- Shin, H.Y.; Wang, C.; Lee, H.K.; Yoo, K.H.; Zeng, X.; Kuhns, T.; Hennighausen, L. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat. Commun. 2017, 8, 15464. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 899. [Google Scholar] [CrossRef]
- De Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.S.; da Rosa, C.E. Glyphosate-based herbicide exposure causes antioxidant defense responses in the fruit fly. Drosoph. Melanogaster Comp. Biochem. Physiol. C Toxicol. Pharm. 2016, 185, 94–101. [Google Scholar] [CrossRef]
- De Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.S.; da Rosa, C.E. Corrigendum to “Glyphosate-based herbicide exposure causes antioxidant defense responses in the fruit fly Drosophila melanogaster previously published at CBPC”[Comp. Biochem Physiol. C 185–186 (2016) 94–101]. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2018, 205, 70–73. [Google Scholar] [CrossRef]
- Casabe, N.; Piola, L.; Fuchs, J.; Oneto, M.L.; Pamparato, L.; Basack, S.; Kesten, E. Ecotoxicological assessment of the effects of glyphosate and chlorpyrifos in an Argentine soya field. J. Soils Sediments 2007, 7, 232–239. [Google Scholar] [CrossRef]
- Hansen, L.R.; Roslev, P. Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat. Toxicol. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Herbert, L.H.; Vázquez, D.E.; Arenas, A.; Farina, W.M. Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J. Exp. Biol. 2014, 217 Pt 19, 3457–3464. [Google Scholar] [CrossRef]
- Michalková, V.; Pekár, S. How glyphosate altered the behaviour of agrobiont spiders (Araneae: Lycosidae) and beetles (Coleoptera: Carabidae). Biol. Control 2009, 51, 444–449. [Google Scholar] [CrossRef]
- Benamú, M.A.; Schneider, M.I.; Sánchez, N.E. Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 2010, 78, 871–876. [Google Scholar] [CrossRef]
- Griesinger, L.M.; Evans, S.C.; Rypstra, A.L. Effects of a glyphosate-based herbicide on mate location in a wolf spider that inhabits agroecosystems. Chemosphere 2011, 84, 1461–1466. [Google Scholar] [CrossRef]
- Mikó, Z.; Ujszegi, J.; Gál, Z.; Hettyey, A. Effects of a glyphosate-based herbicide and predation threat on the behaviour of agile frog tadpoles. Ecotoxicol. Environ. Saf. 2017, 140, 96–102. [Google Scholar] [CrossRef]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; De Oliveira, P.A.; Rieg, C.E.H.; Wilhelm, F.D. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Aitbali, Y.; Ba-M’hamed, S.; Elhidar, N.; Nafis, A.; Soraa, N.; Bennis, M. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018, 67, 44–49. [Google Scholar] [CrossRef]
- Abraham, J.; Benhotons, G.S.; Krampah, I.; Tagba, J.; Amissah, C.; Abraham, J.D. Commercially formulated glyphosate can kill non-target pollinator bees under laboratory conditions. Entomol. Exp. Appl. 2018, 166, 695–702. [Google Scholar] [CrossRef]
- Adam, A.; Marzuki, A.; Abdul, H.R.; Abdul, M.A. The oral and intratracheal toxicities of ROUNDUP® and its components to rats. Vet. Hum. Toxicol. 1997, 39, 147–151. [Google Scholar]
- Dajoraitė, G.; Žaltauskaitė, J.; Zigmontienė, A. Herbicide glyphosate impact to earthworm (E. fetida)/Herbicido glifosato poveikio kompostiniam sliekui (E. fetida L.) tyrimai ir vertinimas. Moksl. Liet. Ateitis Sci. Future Lith. 2016, 8, 370–375. [Google Scholar] [CrossRef]
- García-Torres, T.; Giuffré, L.; Romaniuk, R.; Ríos, R.P.; Pagano, E.A. Exposure assessment to glyphosate of two species of annelids. Bull. Environ. Contam. Toxicol. 2014, 93, 209–214. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Séralini, G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Séralini, G.E. Differential effects of glyphosate and Roundup® on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef]
- Wehtje, G.; Altland, J.E.; Gilliam, C.H. Interaction of glyphosate and pelargonic acid in ready-to-use weed control products. Weed Technol. 2009, 23, 544–549. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Heldreth, A.B.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Hill, R.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Final report of the Cosmetic Ingredient Review Expert Panel on the safety assessment of pelargonic acid (nonanoic acid) and nonanoate esters. Int. J. Toxicol. 2011, 30 (Suppl. 6), 228S–269S. [Google Scholar] [CrossRef]
- Techer, D.; Milla, S.; Fontaine, P.; Viot, S.; Thomas, M. Acute toxicity and sublethal effects of gallic and pelargonic acids on the zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2015, 22, 5020–5029. [Google Scholar] [CrossRef]
- Techer, D.; Milla, S.; Fontaine, P.; Viot, S.; Thomas, M. Influence of waterborne gallic and pelargonic acid exposures on biochemical and reproductive parameters in the zebrafish (Danio rerio). Environ. Toxicol. 2017, 32, 227–240. [Google Scholar] [CrossRef]
- Cai, W.; Ji, Y.; Song, X.; Guo, H.; Han, L.; Zhang, F.; Xu, M. Effects of glyphosate exposure on sperm concentration in rodents: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2017, 55, 148–155. [Google Scholar] [CrossRef]
- Owagboriaye, F.O.; Dedeke, G.A.; Ademolu, K.O.; Olujimi, O.O.; Ashidi, J.S.; Adeyinka, A.A. Reproductive toxicity of Roundup® herbicide exposure in male albino rat. Exptoxicol. Pathol. 2017, 69, 461–468. [Google Scholar] [CrossRef]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2009, 84, 309–317. [Google Scholar] [CrossRef]
- Clair, É.; Mesnage, R.; Travert, C.; Séralini, G. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. In Vitro 2012, 26, 269–279. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Telles, L.F.; Hess, R.A.; Mahecha, G.A.; Oliveira, C.A. Effects of the herbicide Roundup® on the epididymal region of drakes Anas platyrhynchos. Reprod. Toxicol. 2007, 23, 182–191. [Google Scholar] [CrossRef]
- Romano, M.A.; Romano, R.M.; Santos, L.D.; Wisniewski, P.; Campos, D.A.; de Souza, P.B.; de Oliveira, C.A. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 2012, 86, 663–673. [Google Scholar] [CrossRef]
- Dai, P.; Hu, P.; Tang, J.; Li, Y.; Li, C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem. 2016, 118, 519–526. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, N.; Yin, L.; Zhang, W.; Han, F.; Liu, W.; Liu, J. A commercial Roundup® formulation induced male germ cell apoptosis by promoting the expression of XAF1 in adult mice. Toxicol. Lett. 2018, 15, 163–172. [Google Scholar] [CrossRef]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Vassiou, K. The in vitro impact of the herbicide Roundup® on human sperm motility and sperm mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef]
- Druart, C.; Gimbert, F.; Scheifler, R.; De VauflEury, A. A full life-cycle bioassay with Cantareus aspersus shows reproductive effects of a glyphosate-based herbicide suggesting potential endocrine disruption. Environ. Pollut. 2017, 226, 240–249. [Google Scholar] [CrossRef]
- Schimpf, M.G.; Milesi, M.M.; Ingaramo, P.I.; Luque, E.H.; Varayoud, J. Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus. Toxicology 2017, 376, 2–14. [Google Scholar] [CrossRef]
- Milesi, M.M.; Lorenz, V.; Pacini, G.; Repetti, M.R.; Demonte, L.D.; Varayoud, J.; Luque, E.H. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch. Toxicol. 2018, 92, 2629–2643. [Google Scholar] [CrossRef]
- Baier, F.; Jedinger, M.; Gruber, E.; Zaller, J.G. Temperature-Dependence of Glyphosate-Based Herbicides Effects on Egg and Tadpole Growth of Common Toads. Front. Environ. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2017, 401–426. [Google Scholar] [CrossRef]
- Torretta, V.; Katsoyiannis, I.A.; Viotti, P.; Rada, E.C. Critical Review of the Effects of Glyphosate Exposure to the Environment and Humans through the Food Supply Chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sritana, N.; Suriyo, T.; Kanitwithayanun, J.; Somgvasin, B.H.; Thiantanawat, A.; Satayavivad, J. Glyphosate induces growth of estrogen receptor alpha positive cholangiocarcinoma cells via non-genomic estrogen receptor/ERK1/2 signaling pathway. Food Chem. Toxicol. 2018, 118, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. IARC Monographs Volume 112: Evaluation of Five Organophosphate Insecticides and Herbicides. Available online: https://www.iarc.fr/en/media-centre/iarcnews/pdf/MonographVolume112.pdf. (accessed on 23 January 2019).
- De Roos, A.; Zahm, S.H.; Cantor, K.P.; Weisenburger, D.D.; Holmes, F.F.; Burmeister, L.F.; Blair, A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup. Environ. Med. 2003, 60, e11. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Hardell, L.; Carlberg, M.; Åkerman, M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer 2008, 123, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Bukowska, B. The mechanism of DNA damage induced by Roundup® 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells-genotoxic risk assessement. Food Chem. Toxicol. 2018. [Google Scholar] [CrossRef]
- Shaw, W. Elevated urinary glyphosate and clostridia metabolites with altered dopamine metabolism in triplets with autistic spectrum disorder or suspected seizure disorder: A case study. Integr. Med. 2017, 16, 50–57. [Google Scholar]
- Argou-Cardozo, I.; Zeidán-Chuliá, F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med. Sci. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Markel, T.A.; Proctor, C.; Ying, J.; Winchester, P.D. Environmental pesticides increase the risk of developing hypertrophic pyloric stenosis. J. Pediatr. Surg. 2015, 50, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Lozano, V.L.; Defarge, N.; Rocque, L.M.; Mesnage, R.; Hennequin, D.; Cassier, R.; Amiel, C. Sex-dependent impact of Roundup® on the rat gut microbiome. Toxicol. Rep. 2018, 5, 96–107. [Google Scholar] [CrossRef] [PubMed]
- You, M.J.; Shin, G.W.; Lee, C.S. Clostridium tertium bacteremia in a patient with glyphosate ingestion. Am. J. Case Rep. 2015, 16, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Schrödl, W.; Aldin, A.A.; Hafez, H.M.; Krüger, M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr. Microbiol. 2013, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Shehata, A.A.; Schr, W.; Rodloff, A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe 2013, 20, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, W.; Coenen, M.; Schrödl, W.; Shehata, A.A.; Krüger, M. The influence of glyphosate on the microbiota and production of botulinum nEur.otoxin during ruminal fermentation. Curr. Microbiol. 2015, 70, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Manservisi, F.; Panzacchi, S.; Mandrioli, D.; Menghetti, I.; Vornoli, A.; Belpoggi, F. The Ramazzini Institute 13-week pilot study on glyphosate and Roundup® administered at human-equivalent dose to Sprague Dawley rats: Effects on the microbiome. Environ. Health 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol 2015, 16, 103. [Google Scholar] [CrossRef]
- Roberts, D.M.; Buckley, N.A.; Mohamed, F.; Eddleston MGoldstein, D.A.; Mehrsheikh, A.; Dawson, A.H. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin. Toxicol. 2010, 48, 129–136. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.H.; Hong, C.K.; Cho, K.W.; Park, Y.H.; Kim, Y.W.; Hwang, S.Y. Heart rate–corrected QT interval predicts mortality in glyphosate-surfactant herbicide–poisoned patients. Am. J. Emerg. Med. 2014, 32, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Hua, X.; Müller, K.; Wang, H.; Yang, T.; Wang, Q.; Peng, X.; Wang, M.; Pang, Y.; et al. Glyphosate application increased catabolic activity of gram-negative bacteria but impaired soil fungal community. Environ. Sci. Pollut. Res. 2018, 25, 14762–14772. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.J.; Means, N.E.; Kim, S.J. Glyphosate affects soybean root exudation and rhizosphere microorganisms. Int. J. Environ. Anal. Chem. 2005, 85, 1165–1174. [Google Scholar] [CrossRef]
- Kremer, R.J. Soil and Environmental Health after Twenty Years of Intensive Use of Glyphosate. Adv. Plants Agric. Res. 2017, 6. [Google Scholar] [CrossRef]
- Mentler, A.; Paredes, M.; Fuerhacker, M. Adsorption of glyphosate to cambisols, podzols and silica sand. In Proceedings of the ALVA Conference, Vienna, Austria, 14 May 2007; pp. 21–22. [Google Scholar]
- Primost, J.E.; Marino, D.J.; Aparicio, V.C.; Costa, J.L.; Carriquiriborde, P. Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ. Pollut. 2017, 229, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Contardo-Jara, V.; Klingelmann, E.; Wiegand, C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ. Pollut. 2009, 157, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cuhra, M.; Traavik, T.; Bøhn, T. Clone-and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 2013, 22, 251–262. [Google Scholar] [CrossRef]
- Cuhra, M.; Traavik, T.; Bøhn, T. Life cycle fitness differences in Daphnia magna fed Roundup-Ready soybean or conventional soybean or organic soybean. Aquac. Nutr. 2015, 21, 702–713. [Google Scholar] [CrossRef]
- Cuhra, M.; Traavik, T.; Dando, M.; Primicerio, R.; Holderbaum, D.F.; Bøhn, T. Glyphosate-residues in Roundup-Ready soybean impair Daphnia magna life-cycle. J. Agric. Chem. Environ. 2015, 4, 24–36. [Google Scholar] [CrossRef]






| Treatment | Glyphosate (ng/g) | AMPA (ng/g) | Effective Concentration in Corn (ng/g) | Effective Concentration in Medium (μg/L) |
|---|---|---|---|---|
| Organic commercial | Not detected | Not detected | Not detected | Not detected |
| Roundup® Ready w/Roundup® | 5.67 | 1.52 | 8.05 | 0.437 |
| Conventional commercial | 0.42 | 0.21 | 0.74 | Not used in medium |
| Experiment | Formulation (Active Ingredient) (other known ingredients) | Strain | Sex | Exposure | R2 | F Ratio | p-value | LC-50 (g/L) |
|---|---|---|---|---|---|---|---|---|
| Exp. 1 Bonferoni-corrected p = 0.0125 | Roundup Concentrate Plus (glyphosate, diquat) (POEA) | Canton-S | Females | 2 days 7 days | 63.4% 47.6% | 83.3 43.5 | <0.0001 <0.0001 | 11.5 2.67 |
| Males | 2 days 7 days | 86.2% 5.6% | 300 2.84 | <0.0001 0.0985 | 7.12 −5.86a | |||
| Exp. 2 Bonferoni-corrected p = 0.0125 | Roundup Super Concentrate (glyphosate) (POEA) | Canton-S | Both | 2 days 7 days | 0.40% 14.2% | 0.150 6.11t | 0.7007 0.0181 | 199 7.43 |
| Harwick | Both | 2 days 7 days | 2.9% 50.6% | 1.10 37.9 | 0.3008 <0.0001 | 106a 8.97 | ||
| Exp. 3 Bonferoni-corrected p = 0.0042 | Roundup Super Concentrate (glyphosate) (POEA) | Canton-S | Females | 2 days 7 days | 21.6% 92.1% | 9.11 397 | 0.0049 <0.0001 | 23.3 5.43 |
| Males | 2 days 7 days | 8.0% 39.6% | 2.87 22.3 | 0.0999 <0.0001 | 34.9 6.51a | |||
| Roundup Ready to Use (glyphosate, pelargonic acid) | Canton-S | Females | 2 days 7 days | 9.4% 29.7% | 2.19 9.29 | 0.1534 0.0059 | 9.93 3.12 | |
| Males | 2 days 7 days | 29.3% 37.8% | 8.30 13.4 | 0.0092 0.0014 | 6.02 3.41a | |||
| Scythe (pelargonic acid) | Canton-S | Females | 2 days 7 days | 75.1% 53.4% | 99.3 38.9 | <0.0001 <0.0001 | 5.14 3.02 | |
| Males | 2 days 7 days | 75.7% 36.2% | 103 19.3 | <0.0001 <0.0001 | 5.40 7.47a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talyn, B.; Lemon, R.; Badoella, M.; Melchiorre, D.; Villalobos, M.; Elias, R.; Muller, K.; Santos, M.; Melchiorre, E. Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster. Toxics 2019, 7, 38. https://doi.org/10.3390/toxics7030038
Talyn B, Lemon R, Badoella M, Melchiorre D, Villalobos M, Elias R, Muller K, Santos M, Melchiorre E. Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster. Toxics. 2019; 7(3):38. https://doi.org/10.3390/toxics7030038
Chicago/Turabian StyleTalyn, Becky, Rachael Lemon, Maryam Badoella, Darwin Melchiorre, Maryori Villalobos, Raquel Elias, Kelly Muller, Maggie Santos, and Erik Melchiorre. 2019. "Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster" Toxics 7, no. 3: 38. https://doi.org/10.3390/toxics7030038
APA StyleTalyn, B., Lemon, R., Badoella, M., Melchiorre, D., Villalobos, M., Elias, R., Muller, K., Santos, M., & Melchiorre, E. (2019). Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster. Toxics, 7(3), 38. https://doi.org/10.3390/toxics7030038






