Exposure of Larval Zebrafish to the Insecticide Propoxur Induced Developmental Delays that Correlate with Behavioral Abnormalities and Altered Expression of hspb9 and hspb11
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Propoxur Treatment
2.3. Behavior Analysis Procedure
2.4. Acridine Orange (AO) Staining
2.5. Microscopy
2.6. Heat Shock Treatment
2.7. RNA Isolation, Microarray Experiments, and Data Analysis
2.8. Real-Time Reverse Transcription (qRT)-PCR
2.9. Statistical Analysis
3. Results
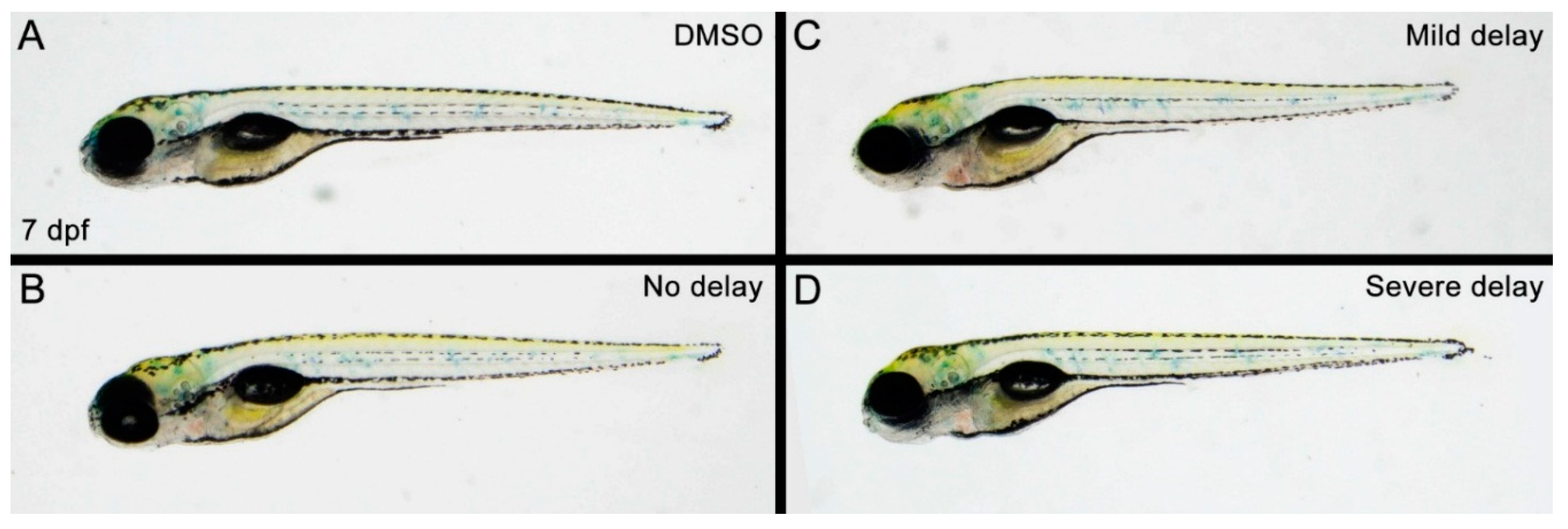
3.1. Propoxur Delays Zebrafish Development in a Dose- and Time-dependent Manner
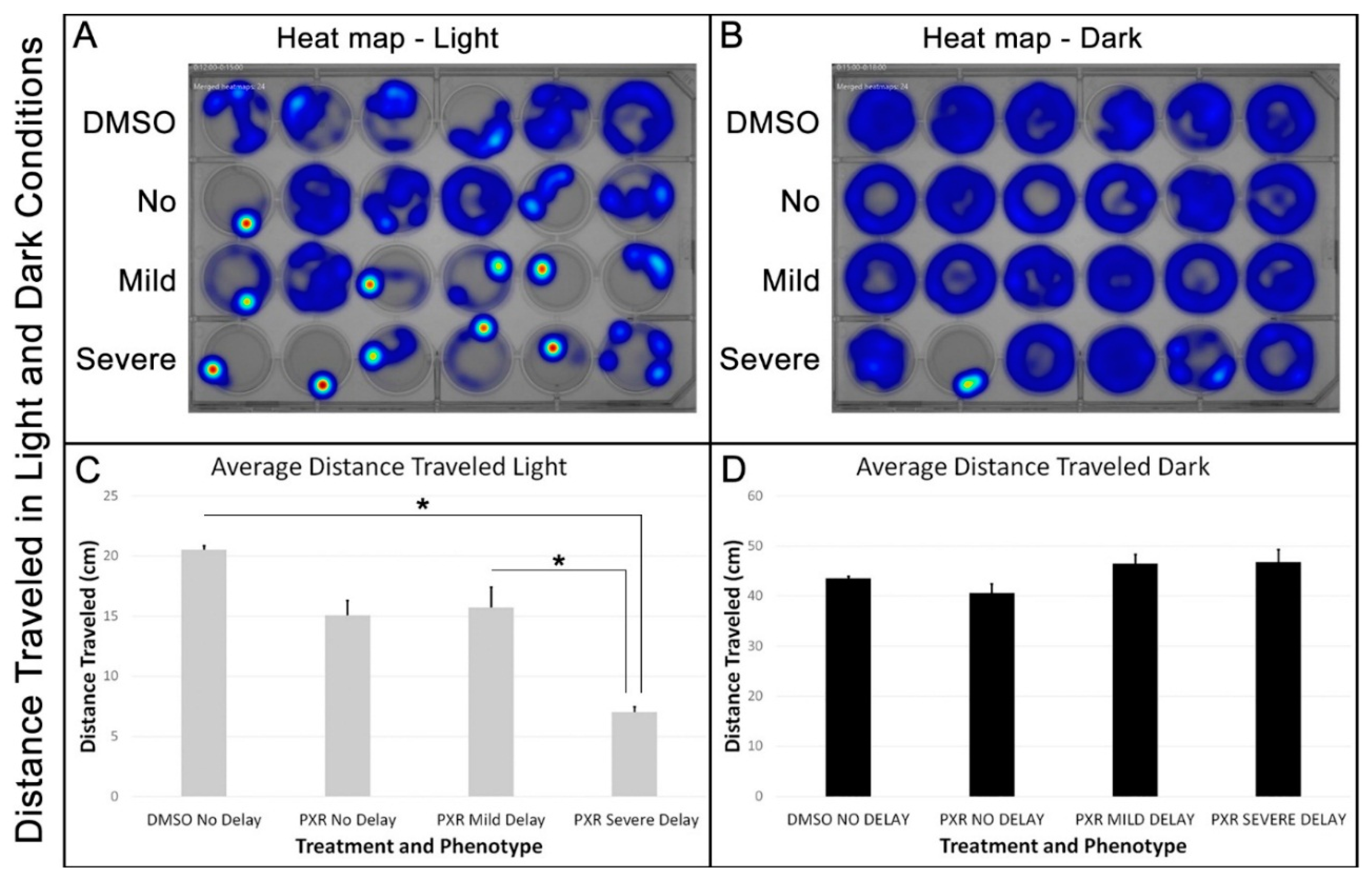
3.2. Zebrafish with the Severely Delayed Phenotypes Develop a Photophobic Response to Light Leading to Reduced Locomotor Activity
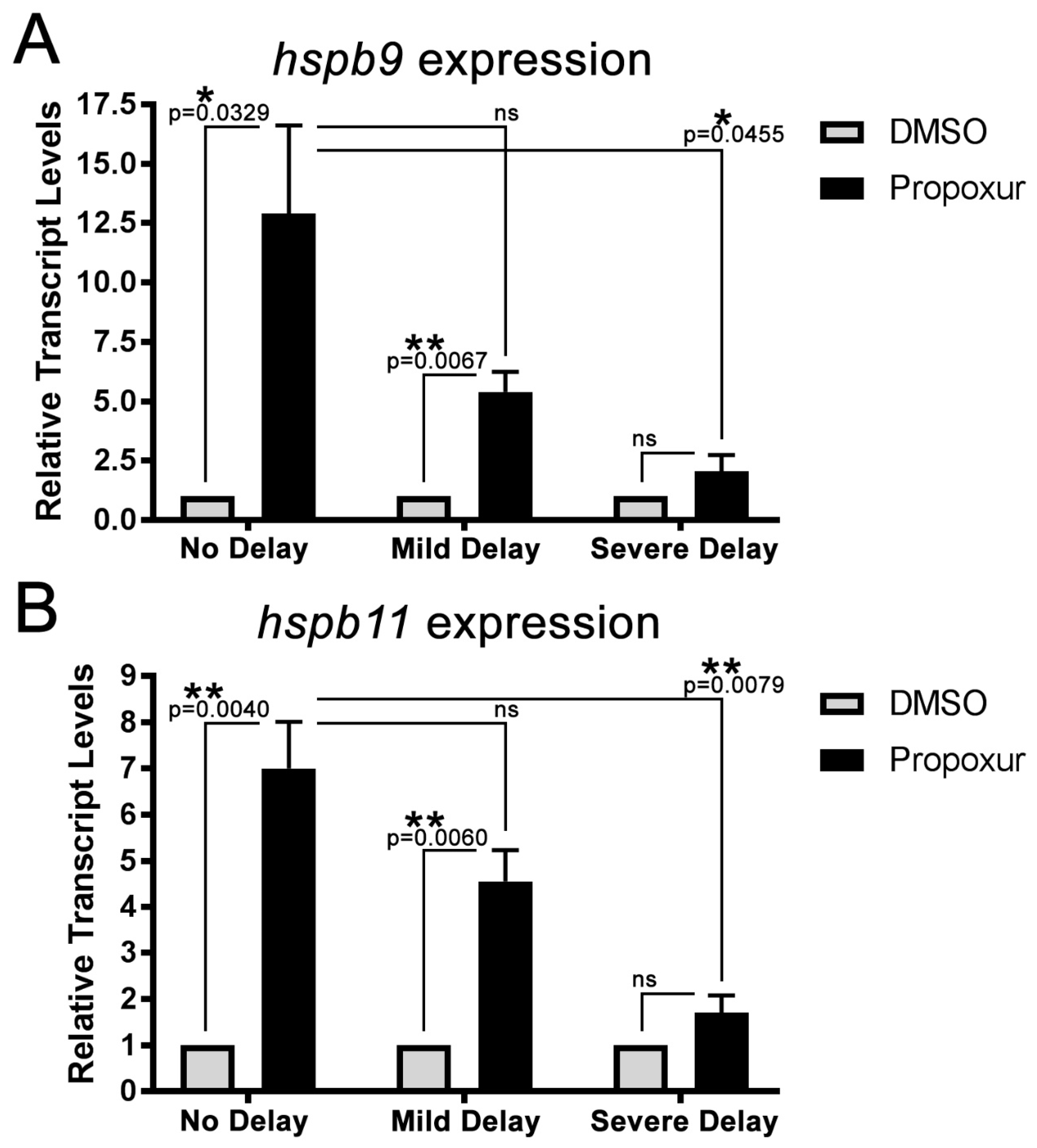
3.3. The Expression of the Small Heat Shock Proteins hspb9 and hspb11 are Increased in Propoxur Treated Embryos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates; United States Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Burns, C.J.; McIntosh, L.J.; Mink, P.J.; Jurek, A.M.; Li, A.A. Pesticide exposure and neurodevelopmental outcomes: Review of the epidemiologic and animal studies. J. Toxicol. Environ. Health Part B 2013, 16, 127–283. [Google Scholar] [CrossRef] [PubMed]
- TOXNET. National Library of Medicine’s Toxicology Data Network; Hazardous Substances Databank; Public Health Service. National Institute of Health. U.S. Department of Health and Human Services. NLM: Bethesda, MD, USA, 1986.
- Hertz-Picciotto, I.; Sass, J.B.; Engel, S.; Bennett, D.H.; Bradman, A.; Eskenazi, B.; Lanphear, B.; Whyatt, R. Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLoS Med. 2018, 15, e1002671. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Harris, M.H.; Gunier, R.B.; Kogut, K.R.; Harley, K.G.; Deardorff, J.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal organophosphate pesticide exposure and traits related to autism spectrum disorders in a population living in proximity to agriculture. Environ. Health Perspect. 2018, 126, 047012. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Rosas, L.G.; Marks, A.R.; Bradman, A.; Harley, K.; Holland, N.; Johnson, C.; Fenster, L.; Barr, D.B. Pesticide toxicity and the developing brain. Basic Clin. Pharm. Toxicol. 2008, 102, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Young, J.G.; Eskenazi, B.; Gladstone, E.A.; Bradman, A.; Pedersen, L.; Johnson, C.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology 2005, 26, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.A.; Garfinkel, R.; Perera, F.P.; Andrews, H.F.; Hoepner, L.; Barr, D.B.; Whitehead, R.; Tang, D.; Whyatt, R.W. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006, 118, e1845–e1859. [Google Scholar] [CrossRef] [PubMed]
- Elmazoudy, R.H.; Attia, A.A.; Abdelgawad, H.S. Evaluation of developmental toxicity induced by anticholinesterase insecticide, diazinon in female rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 534–542. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Rauh, V.; Barr, D.B.; Camann, D.E.; Andrews, H.F.; Garfinkel, R.; Hoepner, L.A.; Diaz, D.; Dietrich, J.; Reyes, A.; et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004, 112, 1125–1132. [Google Scholar] [CrossRef]
- Eskenazi, B.; Harley, K.; Bradman, A.; Weltzien, E.; Jewell, N.P.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004, 112, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Ostrea, E.M., Jr.; Bielawski, D.M.; Posecion, N.C., Jr.; Corrion, M.; Villanueva-Uy, E.; Jin, Y.; Janisse, J.J.; Ager, J.W. A comparison of infant hair, cord blood and meconium analysis to detect fetal exposure to environmental pesticides. Environ. Res. 2008, 106, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrea, E.M., Jr.; Reyes, A.; Villanueva-Uy, E.; Pacifico, R.; Benitez, B.; Ramos, E.; Bernardo, R.C.; Bielawski, D.M.; Delaney-Black, V.; Chiodo, L.; et al. Fetal exposure to propoxur and abnormal child neurodevelopment at 2 years of age. Neurotoxicology 2012, 33, 669–675. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Baygon (Propoxur) Health Advisory; Office of Drinking Water: Washington, DC, USA, 1989. [Google Scholar]
- EPA, U.S. Integrated Risk Information System (IRIS) on Baygon; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999. [Google Scholar]
- Lee, H.Y.; Inselman, A.L.; Kanungo, J.; Hansen, D.K. Alternative models in developmental toxicology. Syst. Biol. Reprod. Med. 2012, 58, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.E.; Van Leeuwen, M. Effects of Sevin (carbaryl insecticide) on early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2002, 53, 267–272. [Google Scholar] [CrossRef]
- Lin, C.C.; Hui, M.N.; Cheng, S.H. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharm. 2007, 222, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Schock, E.N.; Ford, W.C.; Midgley, K.J.; Fader, J.G.; Giavasis, M.N.; McWhorter, M.L. The effects of carbaryl on the development of zebrafish (Danio rerio) embryos. Zebrafish 2012, 9, 169–178. [Google Scholar] [CrossRef]
- Scheil, V.; Zürn, A.; Köhler, H.R.; Triebskorn, R. Embryo development, stress protein (Hsp70) responses, and histopathology in zebrafish (Danio rerio) following exposure to nickel chloride, chlorpyrifos, and binary mixtures of them. Environ. Toxicol. Int. J. 2010, 25, 83–93. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Xu, L.; Wang, J.; Wu, W.; Xu, L.; Yan, Y. Analysis of differentially expressed proteins in zebrafish (Danio rerio) embryos exposed to chlorpyrifos. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 183–189. [Google Scholar] [CrossRef]
- Garcia-Reyero, N.; Escalon, L.; Prats, E.; Faria, M.; Soares, A.M.; Raldúa, D. Targeted gene expression in zebrafish exposed to chlorpyrifos-oxon confirms phenotype-specific mechanisms leading to adverse outcomes. Bull. Environ. Contam. Toxicol. 2016, 96, 707–713. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- MacPhail, R.; Brooks, J.; Hunter, D.; Padnos, B.; Irons, T.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Tucker, B.; Lardelli, M. A rapid apoptosis assay measuring relative acridine orange fluorescence in zebrafish embryos. Zebrafish 2007, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Burket, C.T.; Brewer, J.L.; Sarras, M.P., Jr.; Li, L.; Perry, M.; McDermott, J.P.; Sauer, B.; Hyde, D.R.; Godwin, A.R. Cre-mediated site-specific recombination in zebrafish embryos. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Breitling, R.; Armengaud, P.; Amtmann, A.; Herzyk, P. Rank products: A simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004, 573, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Elicker, K.S.; Hutson, L.D. Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene 2007, 403, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids. Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Haendel, M.A.; Tilton, F.; Bailey, G.S.; Tanguay, R.L. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish. Toxicol. Sci. 2004, 81, 390–400. [Google Scholar] [CrossRef]
- Yee, N.S.; Kazi, A.A.; Yee, R.K. Translating discovery in zebrafish pancreatic development to human pancreatic cancer: Biomarkers, targets, pathogenesis, and therapeutics. Zebrafish 2013, 10, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Altemus, A. The life and work of James F. Didusch. J. Biocommun. 1992, 19, 8–21. [Google Scholar] [PubMed]
- Hill, M.A. Fetal Development. Available online: https://embryology.med.unsw.edu.au/embryology/index.php/Fetal_Development (accessed on 6 June 2019).
- Jomaa, B.; Hermsen, S.A.; Kessels, M.Y.; van den Berg, J.H.; Peijnenburg, A.A.; Aarts, J.M.; Piersma, A.H.; Rietjens, I.M. Developmental toxicity of thyroid-active compounds in a zebrafish embryotoxicity test. Altex Altern. Anim. Exp. 2014, 31, 303–317. [Google Scholar] [CrossRef]
- Cahill, G.M.; Hurd, M.W.; Batchelor, M.M. Circadian rhythmicity in the locomotor activity of larval zebrafish. Neuroreport 1998, 9, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Marvin, M.; O’Rourke, D.; Kurihara, T.; Juliano, C.E.; Harrison, K.L.; Hutson, L.D. Developmental expression patterns of the zebrafish small heat shock proteins. Dev. Dyn. 2008, 237, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Krone, P.H.; Evans, T.G.; Blechinger, S.R. Heat shock gene expression and function during zebrafish embryogenesis. Semin. Cell Dev. Biol. 2003, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of neonicotinoid pesticide exposure on human health: A systematic review. Environ. Health Perspect. 2016, 125, 155–162. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Chang, C.; Lou, J.; Zhao, M.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-cancer and other associated disorders. Environ.Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology 2018, 409, 44–52. [Google Scholar] [CrossRef]
- Fenske, R.A.; Black, K.G.; Elkner, K.P.; Lee, C.-L.; Methner, M.M.; Soto, R. Potential exposure and health risks of infants following indoor residential pesticide applications. Am. J. Public Health 1990, 80, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D.B.; Slotkin, T.A.; Peterson, B.S. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. USA 2012, 109, 7871–7876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garry, V.F. Pesticides and children. Toxicol. Appl. Pharm. 2004, 198, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef]
- Carrillo, G.; Mehta, R.K.; Johnson, N.M. Neurocognitive Effects of Pesticides in Children. In Pediatric Neurotoxicology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–141. [Google Scholar]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Beseler, C.; Bouchard, M.F.; Bellinger, D.C.; Colosio, C.; Grandjean, P.; Harari, R.; Kootbodien, T.; Kromhout, H.; Little, F. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology 2012, 33, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppin, J.A.; LePrevost, C.E. Pesticides and human health. In Environmental Pest Management: Challenges for Agronomists, Ecologists, Economists and Policymakers; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 251. [Google Scholar]
- Bond, G.G.; Dietrich, D.R. Human cost burden of exposure to endocrine disrupting chemicals. A critical review. Arch. Toxicol. 2017, 91, 2745–2762. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Bradman, A.; Salvatore, A.L.; Boeniger, M.; Castorina, R.; Snyder, J.; Barr, D.B.; Jewell, N.P.; Kavanagh-Baird, G.; Striley, C.; Eskenazi, B. Community-based intervention to reduce pesticide exposure to farmworkers and potential take-home exposure to their families. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 79. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental Neurobehavioral Neurotoxicity of Insecticides. In Handbook of Developmental Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 453–466. [Google Scholar]
- Liu, X.; Zhang, Q.; Li, S.; Mi, P.; Chen, D.; Zhao, X.; Feng, X. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 2018, 199, 16–25. [Google Scholar] [CrossRef]
- DeMicco, A.; Cooper, K.R.; Richardson, J.R.; White, L.A. Developmental Neurotoxicity of Pyrethroid Insecticides in Zebrafish Embryos. Toxicol. Sci. 2009, 113, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, W. How stress selects for reversible phenotypic plasticity. J. Evol. Biol. 2005, 18, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, R. Reversible delay of normal development of frog embryos by inhibition of DNA synthesis. J. Exp. Zool. 1966, 161, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ohishi, T.; Akane, H.; Shiraki, A.; Itahashi, M.; Mitsumori, K.; Shibutani, M. Reversible effect of developmental exposure to chlorpyrifos on late-stage neurogenesis in the hippocampal dentate gyrus in mouse offspring. Reprod. Toxicol. 2013, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, E.; Piqué, E.; Gómez-Catalán, J.; Llobet, J.M. Assessment of developmental delay in the zebrafish embryo teratogenicity assay. Toxicol. Vitr. 2013, 27, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.S.; Weis, P. Pollutants as developmental toxicants in aquatic organisms. Environ. Health Perspect. 1987, 71, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Daston, G.P.; Chapin, R.E.; Scialli, A.R.; Piersma, A.H.; Carney, E.W.; Rogers, J.M.; Friedman, J.M. A different approach to validating screening assays for developmental toxicity. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 526–530. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Bellinger, D.C.; Wright, R.O.; Weisskopf, M.G. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 2010, 125, e1270–e1277. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, B.; Marks, A.R.; Bradman, A.; Harley, K.; Barr, D.B.; Johnson, C.; Morga, N.; Jewell, N.P. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environ. Health Perspect. 2007, 115, 792–798. [Google Scholar] [CrossRef]
- Furlong, M.A.; Engel, S.M.; Barr, D.B.; Wolff, M.S. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ. Int. 2014, 70, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middlemore-Risher, M.L.; Buccafusco, J.J.; Terry, A.V. Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol. Teratol. 2010, 32, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Deciphering Developmental Disorders, S.; Fitzgerald, T.W.; Gerety, S.S.; Jones, W.D.; van Kogelenberg, M.; King, D.A.; McRae, J.; Morley, K.I.; Parthiban, V.; Al-Turki, S.; et al. Large-scale discovery of novel genetic causes of developmental disorders. Nature 2014, 519, 223. [Google Scholar] [CrossRef] [PubMed]
- Vester, A.; Caudle, W.M. The Synapse as a Central Target for Neurodevelopmental Susceptibility to Pesticides. Toxics 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Hollins, S.L.; Cairns, M.J. MicroRNA: Small RNA mediators of the brains genomic response to environmental stress. Prog. Neurobiol. 2016, 143, 61–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autier, P. Increasing incidence of cancer in children and competing risks. Lancet Oncol. 2018, 19, 1136–1137. [Google Scholar] [CrossRef] [Green Version]
- Steliarova-Foucher, E.; Fidler, M.M.; Colombet, M.; Lacour, B.; Kaatsch, P.; Piñeros, M.; Soerjomataram, I.; Bray, F.; Coebergh, J.W.; Peris-Bonet, R. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 2018, 19, 1159–1169. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Metayer, C.; Dahl, G.; Wiemels, J.; Miller, M. Childhood leukemia: A preventable disease. Pediatrics 2016, 138, S45. [Google Scholar] [CrossRef]
- Ross, J.A.; Johnson, K.J.; Spector, L.G.; Kersey, J.H. Epidemiology of acute childhood leukemia. In Childhood Leukemia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–26. [Google Scholar]
- Rull, R.P.; Gunier, R.; Von Behren, J.; Hertz, A.; Crouse, V.; Buffler, P.A.; Reynolds, P. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ. Res. 2009, 109, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chang, C.-H.; Tao, L.; Lu, C. Residential exposure to pesticide during childhood and childhood cancers: A meta-analysis. Pediatrics 2015, 136, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Shi, R.; Gao, Y.; Zhang, Y.; Kamijima, M.; Sakai, K.; Wang, G.; Feng, C.; Tian, Y. Pyrethroid Pesticide Exposure and Risk of Childhood Acute Lymphocytic Leukemia in Shanghai. Environ. Sci. Technol. 2012, 46, 13480–13487. [Google Scholar] [CrossRef] [PubMed]
- Wigle Donald, T.; Turner Michelle, C.; Krewski, D. A Systematic Review and Meta-analysis of Childhood Leukemia and Parental Occupational Pesticide Exposure. Environ. Health Perspect. 2009, 117, 1505–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.H.; Colt, J.S.; Metayer, C.; Gunier, R.B.; Lubin, J.; Crouse, V.; Nishioka, M.G.; Reynolds, P.; Buffler, P.A. Residential Exposure to Polychlorinated Biphenyls and Organochlorine Pesticides and Risk of Childhood Leukemia. Environ. Health Perspect. 2009, 117, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldin, O.P.; Nsouli-Maktabi, H.; Genkinger, J.M.; Loffredo, C.A.; Ortega-Garcia, J.A.; Colantino, D.; Barr, D.B.; Luban, N.L.; Shad, A.T.; Nelson, D. Pediatric acute lymphoblastic leukemia and exposure to pesticides. Drug Monit. 2009, 31, 495–501. [Google Scholar] [CrossRef]
- Kumar, A.; Vashist, M.; Rathee, R. Maternal factors and risk of childhood leukemia. Asian Pac. J. Cancer Prev. 2014, 15, 781–784. [Google Scholar] [CrossRef] [PubMed]
- LaFiura, K.M.; Bielawski, D.M.; Posecion, N.C., Jr.; Ostrea, E.M., Jr.; Matherly, L.H.; Taub, J.W.; Ge, Y. Association between prenatal pesticide exposures and the generation of leukemia-associated T (8; 21). Pediatric Blood Cancer 2007, 49, 624–628. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mahanty, A.; Mitra, T.; Parija, S.C.; Mohanty, S. Heat Shock Proteins in Stress in Teleosts. In Regulation of Heat Shock Protein Responses; Asea, A.A.A., Kaur, P., Eds.; Springer: Cham, Germany, 2018; pp. 71–94. [Google Scholar]
- Kluver, N.; Yang, L.; Busch, W.; Scheffler, K.; Renner, P.; Strahle, U.; Scholz, S. Transcriptional response of zebrafish embryos exposed to neurotoxic compounds reveals a muscle activity dependent hspb11 expression. PLoS ONE 2011, 6, e29063. [Google Scholar] [CrossRef]
- Shahid, M.; Takamiya, M.; Stegmaier, J.; Middel, V.; Gradl, M.; Klüver, N.; Mikut, R.; Dickmeis, T.; Scholz, S.; Rastegar, S.; et al. Zebrafish biosensor for toxicant induced muscle hyperactivity. Sci. Rep. 2016, 6, 23768. [Google Scholar] [CrossRef] [Green Version]
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef]
- Di Paolo, C.; Groh, K.J.; Zennegg, M.; Vermeirssen, E.L.M.; Murk, A.J.; Eggen, R.I.L.; Hollert, H.; Werner, I.; Schirmer, K. Early life exposure to PCB126 results in delayed mortality and growth impairment in the zebrafish larvae. Aquat. Toxicol. 2015, 169, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Zhang, Y.; Ye, J.; Huang, C.; Zhao, M.; Liu, W. Dual enantioselective effect of the insecticide bifenthrin on locomotor behavior and development in embryonic–larval zebrafish. Environ. Toxicol. Chem. 2010, 29, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.B.; Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 2015, 49, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, J.; Donerly, S.; Levin, E.D.; Linney, E.A. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol. Teratol. 2011, 33, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Velki, M.; Di Paolo, C.; Nelles, J.; Seiler, T.-B.; Hollert, H. Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 2017, 180, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Eddins, D.; Cerutti, D.; Williams, P.; Linney, E.; Levin, E.D. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010, 32, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horzmann, K.A.; Freeman, J.L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Babin, P.J.; Goizet, C.; Raldua, D. Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog. Neurobiol. 2014, 118, 36–58. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, V.; Inati, S.; Ksendzovsky, A.; Zaghloul, K. Clinical advances in photosensitive epilepsy. Brain Res. 2019, 1703, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Panayiotopoulos, C. Neonatal seizures and neonatal syndromes. In The Epilepsies: Seizures, Syndromes and Management; Bladon Medical Publishing: Oxford, UK, 2005. [Google Scholar]
- Wang, K.; Chen, X.; Liu, J.; Zou, L.-P.; Feng, W.; Cai, L.; Wu, X.; Chen, S.-y. Embryonic exposure to ethanol increases the susceptibility of larval zebrafish to chemically induced seizures. Sci. Rep. 2018, 8, 1845. [Google Scholar] [CrossRef]
- Vijverberg, H.P.; vanden Bercken, J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol. 1990, 21, 105–126. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef]
- Richendrfer, H.; Creton, R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology 2015, 49, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Stewart, A.; Kadri, F.; DiLeo, J.; Min Chung, K.; Cachat, J.; Goodspeed, J.; Suciu, C.; Roy, S.; Gaikwad, S.; Wong, K. The developing utility of zebrafish in modeling neurobehavioral disorders. Int. J. Comp. Psychol. 2010, 23, 104–120. [Google Scholar]
- Fero, K.; Yokogawa, T.; Burgess, H.A. The behavioral repertoire of larval zebrafish. In Zebrafish Models in Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 249–291. [Google Scholar]



| IPA Top Diseases and Biological Functions | |
|---|---|
| Diseases and Disorders | Number of Molecules/% of Genes in the Dataset |
| Organismal Injury and Abnormalities | 14/70% |
| Cancer | 10/50% |
| Neurological Disease | 7/35% |
| Cardiovascular Disease | 6/30% |
| Inflammatory Response | 5/25% |
| Physiological System Development and Function | |
| Hematological System Development and Function | 7/35% |
| Cardiovascular System Development and Function | 4/20% |
| Organismal Development | 10/50% |
| Visual System Development and Function | 3/15% |
| Skeletal and Muscular System Development and Function | 5/25% |
| Diseases and Functions Annotation | p Value | Genes |
|---|---|---|
| Cancer and Organismal Injury and Abnormalities | ||
| Cancer of secretory structure | 3.41 × 10−2 | C6, CSRP3, DARS, FBP2, HSD3B7, IQCH, LGALS1, SOCS3, TSPO, UBE3A |
| Advanced malignant tumor | 4.68 × 10−3 | CSRP3, LGALS1, SOCS3, TSPO, UBE3A |
| Metastasis | 1.55 ×10−2 | CSRP3, LGALS1, SOCS3, UBE3A |
| Neurological Disease | ||
| Seizures | 8.55 × 10−3 | SOCS3, TSPO, UBE3A |
| Amyotrophic lateral sclerosis | 2.05 × 10−2 | FBP2, TSPO |
| Damage of nervous system | 2.45 × 10−2 | SOCS3, TSPO |
| Neurodegeneration | 4.10 × 10−2 | C6, UBE3A |
| Hematological System Development and Function | ||
| Quantity of blood cells | 6.75 × 10−4 | C6, LGALS1, NPR3, SNAI3, SOCS3, TSPO |
| Quantity of leukocytes | 2.77 × 10−3 | C6, LGALS1, SNAI3, SOCS3, TSPO |
| Quantity of phagocytes | 8.89 × 10−4 | C6, LGALS1, SNAI3, SOCS3 |
| Quantity of lymphocytes | 6.93 × 10−3 | LGALS1, SNAI3, SOCS3, TSPO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shields, J.N.; Hales, E.C.; Ranspach, L.E.; Luo, X.; Orr, S.; Runft, D.; Dombkowski, A.; Neely, M.N.; Matherly, L.H.; Taub, J.W.; et al. Exposure of Larval Zebrafish to the Insecticide Propoxur Induced Developmental Delays that Correlate with Behavioral Abnormalities and Altered Expression of hspb9 and hspb11. Toxics 2019, 7, 50. https://doi.org/10.3390/toxics7040050
Shields JN, Hales EC, Ranspach LE, Luo X, Orr S, Runft D, Dombkowski A, Neely MN, Matherly LH, Taub JW, et al. Exposure of Larval Zebrafish to the Insecticide Propoxur Induced Developmental Delays that Correlate with Behavioral Abnormalities and Altered Expression of hspb9 and hspb11. Toxics. 2019; 7(4):50. https://doi.org/10.3390/toxics7040050
Chicago/Turabian StyleShields, Jeremiah N., Eric C. Hales, Lillian E. Ranspach, Xixia Luo, Steven Orr, Donna Runft, Alan Dombkowski, Melody N. Neely, Larry H. Matherly, Jeffrey W. Taub, and et al. 2019. "Exposure of Larval Zebrafish to the Insecticide Propoxur Induced Developmental Delays that Correlate with Behavioral Abnormalities and Altered Expression of hspb9 and hspb11" Toxics 7, no. 4: 50. https://doi.org/10.3390/toxics7040050





