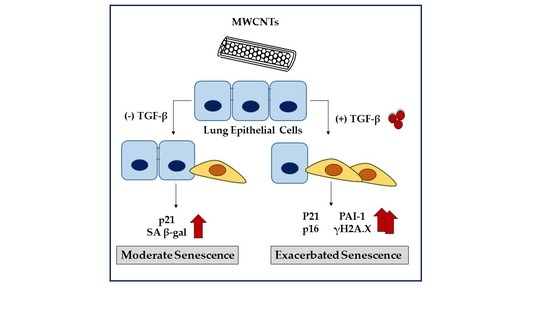
Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. MWCNT Preparation and Characterization
2.2. Cell Viability and ELISA
2.3. Cellular Reactive Oxygen Species (ROS) Production
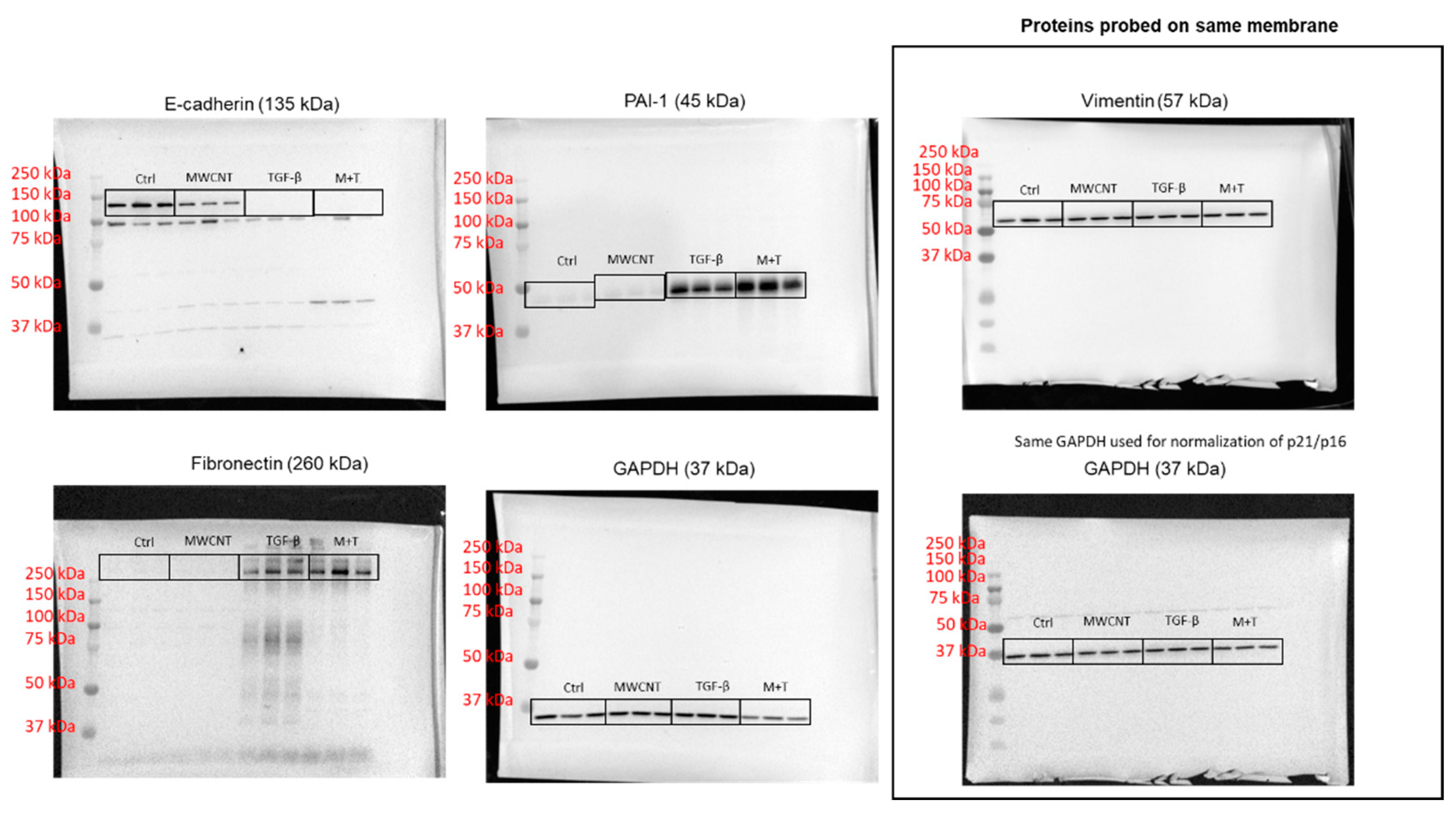
2.4. Western Blotting
2.5. Cellular Senescence Activity Assay
2.6. Statistical Analysis
3. Results
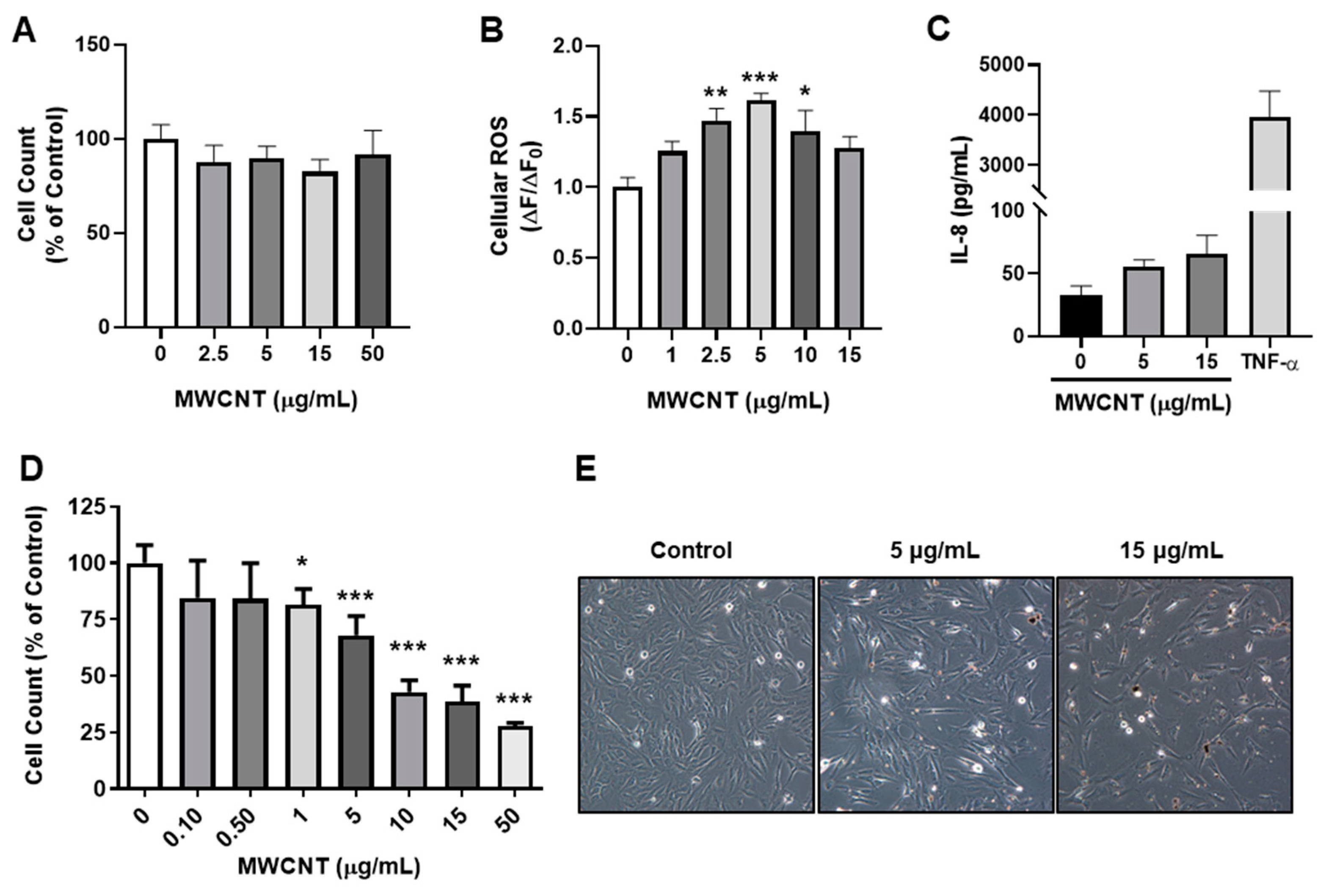
3.1. Cellular Responses to MWCNTs in Human Bronchial Epithelial Cells
3.2. MWCNT and TGF- β Exposure Propagate Cellular Senescence
3.3. MWCNT and TGF- β Induce EMT and Increase ECM Production
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar]
- Kuijpers, E.; Bekker, C.; Fransman, W.; Brouwer, D.; Tromp, P.; Vlaanderen, J.; Godderis, L.; Hoet, P.; Lan, Q.; Silverman, D.; et al. Occupational Exposure to Multi-Walled Carbon Nanotubes During Commercial Production Synthesis and Handling. Ann. Occup. Hyg. 2016, 60, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Wang, L.; Battelli, L.A.; McKinney, W.; Castranova, V.; Porter, D.W. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Porter, D.W.; Batteli, L.A.; Wolfarth, M.G.; Richardson, D.L.; Ma, Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch. Toxicol. 2015, 89, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, K.; Kunitake, R.; Kawasaki, M.; Nomoto, Y.; Hagimoto, N.; Nakanishi, Y.; Hara, N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Cruz, T.; Jia, M.; Tabib, T.; Sembrat, J.; Liu, J.; Bondonese, A.; Kavanagh, J.; Nayra, C.; Bruno, T.; Mora, A.; et al. SASP from lung senescent fibroblasts induces immunesenescence and fibrosis. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- He, Y.; Thummuri, D.; Zheng, G.; Okunieff, P.; Citrin, D.E.; Vujaskovic, Z.; Zhou, D. Cellular senescence and radiation-induced pulmonary fibrosis. Transl. Res. J. Lab. Clin. Med. 2019, 209, 14–21. [Google Scholar] [CrossRef]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef] [Green Version]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Just, A.J.; Ihrie, M.D.; Duke, K.S.; Lee, H.Y.; You, D.J.; Hussain, S.; Kodali, V.K.; Ziemann, C.; Creutzenberg, O.; Vulpoi, A.; et al. The pulmonary toxicity of carboxylated or aminated multi-walled carbon nanotubes in mice is determined by the prior purification method. Part. Fibre Toxicol. 2020, 17, 60. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. TIMP1 promotes multi-walled carbon nanotube-induced lung fibrosis by stimulating fibroblast activation and proliferation. Nanotoxicology 2017, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, Y.; Nie, X.; Braïni, C.; Bai, R.; Chen, C. Multiwall Carbon Nanotubes Directly Promote Fibroblast–Myofibroblast and Epithelial–Mesenchymal Transitions through the Activation of the TGF-β/Smad Signaling Pathway. Small 2015, 11, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.F.; Wu, Y.H.; Hou, Z.Q.; Zhang, Q.Q. ROS and NF-kappaB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem. Biophys. Res. Commun. 2009, 379, 643–648. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y.; Sugino, S.; Fukui, H.; Nishioka, A.; Hagiwara, Y.; Sato, K.; Yoneda, T.; Tada, A.; Koyama, T. Comparison of the effects of multiwall carbon nanotubes on the epithelial cells and macrophages. Nanotoxicology 2019, 13, 861–878. [Google Scholar] [CrossRef]
- Yang, L.; Herrera, J.; Gilbertsen, A.; Xia, H.; Smith, K.; Benyumov, A.; Bitterman, P.B.; Henke, C.A. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L127–L136. [Google Scholar] [CrossRef] [Green Version]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.-P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Lagnado, A.; Leslie, J.; Ruchaud-Sparagano, M.-H.; Victorelli, S.; Hirsova, P.; Ogrodnik, M.; Collins, A.L.; Vizioli, M.G.; Habiballa, L.; Saretzki, G.; et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021, 40, e106048. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Sriram, K.; Wolfarth, M.; Jefferson, A.; Schwegler-Berry, D.; Andrew, M.E.; Castranova, V. A biocompatible medium for nanoparticle dispersion. Nanotoxicology 2008, 2, 144–154. [Google Scholar] [CrossRef]
- Lippmann, M. Effects of fiber characteristics on lung deposition, retention, and disease. Environ. Health Perspect. 1990, 88, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Roursgaard, M.; Andreassi, M.G.; Kermanizadeh, A.; Møller, P. Repair activity of oxidatively damaged DNA and telomere length in human lung epithelial cells after exposure to multi-walled carbon nanotubes. Mutagenesis 2017, 32, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reamon-Buettner, S.M.; Hackbarth, A.; Leonhardt, A.; Braun, A.; Ziemann, C. Cellular senescence as a response to multiwalled carbon nanotube (MWCNT) exposure in human mesothelial cells. Mech. Ageing Dev. 2021, 193, 111412. [Google Scholar] [CrossRef]
- Collard, H.R. The Age of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 771–772. [Google Scholar] [CrossRef]
- Venosa, A. Senescence in Pulmonary Fibrosis: Between Aging and Exposure. Front. Med. 2020, 7, 606462. [Google Scholar] [CrossRef]
- Ahmad, T.; Sundar, I.K.; Lerner, C.A.; Gerloff, J.; Tormos, A.M.; Yao, H.; Rahman, I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: Implications for chronic obstructive pulmonary disease. FASEB J. 2015, 29, 2912–2929. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.K.; Pant, A.B.; Kashyap, M.P.; Kumar, V.; Lohani, M.; Jonas, L.; Rahman, Q. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology 2011, 5, 195–207. [Google Scholar] [CrossRef]
- Lee, J.W.; Won, E.J.; Kang, H.M.; Hwang, D.S.; Kim, D.H.; Kim, R.K.; Lee, S.J.; Lee, J.S. Effects of multi-walled carbon nanotube (MWCNT) on antioxidant depletion, the ERK signaling pathway, and copper bioavailability in the copepod (Tigriopus japonicus). Aquat. Toxicol. 2016, 171, 9–19. [Google Scholar] [CrossRef]
- Macip, S.; Igarashi, M.; Fang, L.; Chen, A.; Pan, Z.-Q.; Lee, S.W.; Aaronson, S.A. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002, 21, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, S.Y.; Bae, Y.-S. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol. Cells 2014, 37, 620–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; Von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Pertot, A.; Shirole, N.H.; Yao, Z.; Anaparthy, N.; Garvin, T.; Cox, H.; Chang, K.; Rollins, F.; Kendall, J.; et al. TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24− cancer cells. eLife 2017, 6, e21615. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, H.; Liang, J.; Gu, X.; Zhou, J.; Xie, C.; Lv, X.; Wang, R.; Li, Q.; Mao, Z.; et al. TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp. Mol. Med. 2020, 52, 130–151. [Google Scholar] [CrossRef] [Green Version]
- Gauldie, J.; Bonniaud, P.; Sime, P.; Ask, K.; Kolb, M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem. Soc. Trans. 2007, 35, 661–664. [Google Scholar] [CrossRef]
- Wang, P.; Nie, X.; Wang, Y.; Li, Y.; Ge, C.; Zhang, L.; Wang, L.; Bai, R.; Chen, Z.; Zhao, Y.; et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-β/Smad signaling pathway. Small 2013, 9, 3799–3811. [Google Scholar] [CrossRef]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial–Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef] [Green Version]
- Kortlever, R.M.; Higgins, P.J.; Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006, 8, 877–884. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Nijwening, J.H.; Bernards, R. Transforming growth factor-beta requires its target plasminogen activator inhibitor-1 for cytostatic activity. J. Biol. Chem. 2008, 283, 24308–24313. [Google Scholar] [CrossRef] [Green Version]


| MWCNT Characteristics | |
| Length * | 10–30 µM |
| Outer Diameter * | <8 nm |
| Z-average | 146.1 nm |
| PdI | 0.419 |
| Zeta Potential | −10.7 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, J.H.; Wang, Q.; Muthumalage, T.; Rahman, I. Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells. Toxics 2021, 9, 144. https://doi.org/10.3390/toxics9060144
Lucas JH, Wang Q, Muthumalage T, Rahman I. Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells. Toxics. 2021; 9(6):144. https://doi.org/10.3390/toxics9060144
Chicago/Turabian StyleLucas, Joseph H., Qixin Wang, Thivanka Muthumalage, and Irfan Rahman. 2021. "Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells" Toxics 9, no. 6: 144. https://doi.org/10.3390/toxics9060144