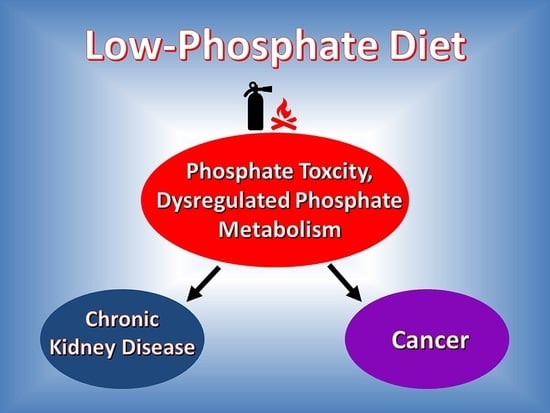
Can a Low-Phosphate Diet for Chronic Kidney Disease Treat Cancer? An Interdisciplinary Literature Review
Abstract
:1. Introduction
2. Diet and Cancer
3. Pi and Molecular Mechanisms in Tumorigenesis
4. Dietary Recommendations to Reduce Cancer Risk
5. Diet Therapy for Hyperphosphatemia
Vitamin D and Hyperphosphatemia
6. Cancer Cachexia, Protein, and Dietary Phosphate
7. Low-Phosphate Diet for Cancer Treatment
7.1. Fasting for Cancer Treatment
7.2. Tumor Dynamics Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castuera, I. Why the War on Cancer Failed. Am. J. Econ. Sociol. 2022, 81, 671–700. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Press Release No 224—Global Battle against Cancer Won’t Be Won with Treatment Alone Effective Prevention Measures Urgently Needed to Prevent Cancer Crisis. 2014. Available online: https://www.iarc.who.int/pressrelease/#year:2014 (accessed on 28 June 2023).
- Abudu, R.; Bouche, G.; Bourougaa, K.; Davies, L.; Duncan, K.; Estaquio, C.; Font, A.D.; Hurlbert, M.S.; Jackson, P.; Kroeskop-Bossenbroek, L.; et al. Trends in International Cancer Research Investment 2006–2018. JCO Glob. Oncol. 2021, 7, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit? Int. J. Mol. Sci. 2022, 23, 13217. [Google Scholar] [CrossRef] [PubMed]
- Salas-Vega, S.; Iliopoulos, O.; Mossialos, E. Assessment of Overall Survival, Quality of Life, and Safety Benefits Associated With New Cancer Medicines. JAMA Oncol. 2017, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, A.G.; Stern, P.L. The failure of radical treatments to cure cancer: Can less deliver more? Ther. Adv. Vaccines Immunother. 2018, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fojo, T. Journeys to Failure that Litter the Path to Developing New Cancer Therapeutics. JAMA Netw. Open 2023, 6, e2324949. [Google Scholar] [CrossRef]
- Sullivan, R.; Pramesh, C.S.; Booth, C.M. Cancer patients need better care, not just more technology. Nature 2017, 549, 325–328. [Google Scholar] [CrossRef]
- Gilligan, A.M.; Alberts, D.S.; Roe, D.J.; Skrepnek, G.H. Death or Debt? National Estimates of Financial Toxicity in Persons with Newly-Diagnosed Cancer. Am. J. Med. 2018, 131, 1187–1199.e1185. [Google Scholar] [CrossRef]
- Cagan, R.; Meyer, P. Rethinking cancer: Current challenges and opportunities in cancer research. Dis. Models Mech. 2017, 10, 349–352. [Google Scholar] [CrossRef]
- Bush, V. Science, the Endless Frontier; National Science Foundation: Washington, DC, USA, 1947. [Google Scholar]
- Thomas, L. Report of Panel on the National Cancer Program Plan. In National Cancer Act of 1974: Hearing Before the Subcommittee on Health of the U.S. Senate Committee on Labor and Public Welfare; U.S. Government Printing Office: Washington, DC, USA, 1974. [Google Scholar]
- National Academy of Sciences; National Academy of Engineering; Institute of Medicine. Facilitating Interdisciplinary Research; The National Academies Press: Washington, DC, USA, 2005; p. 332. [Google Scholar]
- Brown, R.B.; Razzaque, M.S. Chapter 31—Endocrine Regulation of Phosphate Homeostasis. In Textbook of Nephro-Endocrinology, 2nd ed.; Singh, A.K., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 539–548. [Google Scholar]
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. BoneKEy Rep. 2015, 4, 705. [Google Scholar] [CrossRef] [PubMed]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- Gacche, R.N. Dietary Research and Cancer; Springer: Singapore, 2021. [Google Scholar]
- Hosseini, F.; Alavi, N.M.; Mohammadi, E.; Sadat, Z. Motivation for Healing in Cancer Patients: A Qualitative Study. Iran J. Nurs. Midwifery Res. 2021, 26, 555–561. [Google Scholar] [PubMed]
- Zick, S.M.; Snyder, D.; Abrams, D.I. Pros and Cons of Dietary Strategies Popular Among Cancer Patients. Oncology 2018, 32, 542–547. [Google Scholar]
- McGee, E.E.; Kiblawi, R.; Playdon, M.C.; Eliassen, A.H. Nutritional Metabolomics in Cancer Epidemiology: Current Trends, Challenges, and Future Directions. Curr. Nutr. Rep. 2019, 8, 187–201. [Google Scholar] [CrossRef]
- Vahid, F.; Hajizadeghan, K.; Khodabakhshi, A. Nutritional Metabolomics in Diet-Breast Cancer Relations: Current Research, Challenges, and Future Directions-A Review. Biomedicines 2023, 11, 1845. [Google Scholar] [CrossRef]
- Arkady, M. Inorganic Phosphate: The Backbone of Life. In Functional Phosphate Materials and Their Applications; Sadia, A., Mohammad Shaheer, A., Hyung-Shik, S., Eds.; IntechOpen: Rijeka, Croatia, 2023; pp. 1–37. [Google Scholar]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017, 7, 41233. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, J.; Liang, Q.-R.; Luo, R.-G.; Fan, G.-Q.; Tang, Q. Intratumoral inorganic phosphate deprivation: A new anticancer strategy? Med. Hypotheses 2020, 135, 109497. [Google Scholar] [CrossRef]
- Lv, Y.F.; Deng, Z.Q.; Bi, Q.C.; Tang, J.J.; Chen, H.; Xie, C.S.; Liang, Q.R.; Xu, Y.H.; Luo, R.G.; Tang, Q. Intratumoral Pi deprivation benefits chemoembolization therapy via increased accumulation of intracellular doxorubicin. Drug Deliv. 2022, 29, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.-C.; Luo, R.-G.; Li, Y.-S.; Zhao, J.; Fu, X.; Chen, H.; Lv, Y.-F.; Liu, Z.-X.; Liang, Q.-R.; Tang, Q. Low Inorganic Phosphate Stress Inhibits Liver Cancer Progression: From In Vivo to In Vitro. Adv. Ther. 2022, 5, 2100224. [Google Scholar] [CrossRef]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate. Obesities 2022, 2, 64–75. [Google Scholar] [CrossRef]
- Tzenios, N.; Tazanios, M.E.; Chahine, M. The impact of body mass index on prostate cancer: An updated systematic review and meta-analysis. Medicine 2022, 101, e30191. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Ye, D.; Chen, J.; Qian, Y.; Fu, A.N.; Song, J.; Yang, H.; Liu, B.; Sun, X.; Du, L.; et al. Circulating phosphorus concentration and risk of prostate cancer: A Mendelian randomization study. Am. J. Clin. Nutr. 2022, 115, 534–543. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, C.; Hu, B.; Yuan, D.; Chen, W.; Wang, W.; Su, J.; Liu, Z.; Jiao, K.; Chen, X.; et al. Dietary phosphorus intake and serum prostate-specific antigen in non-prostate cancer American adults: A secondary analysis of the National Health and Nutrition Examination Survey (NHANES), 2003–2010. Asia Pac. J. Clin. Nutr. 2020, 29, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Neiterman, E. Breast cancer, alcohol, and phosphate toxicity. J. Appl. Toxicol. 2024, 44, 17–27. [Google Scholar] [CrossRef]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Mielke, J.G. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutrients 2023, 15, 3735. [Google Scholar] [CrossRef]
- Brown, R.B.; Bigelow, P.; Dubin, J.A. Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity. Cancers 2023, 15, 5093. [Google Scholar] [CrossRef]
- Hoyt, M.; Song, Y.; Gao, S.; O’Palka, J.; Zhang, J. Intake of Calcium, Magnesium, and Phosphorus and Risk of Pancreatic Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J. Am. Nutr. Assoc. 2022, 41, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy of Sciences: Washington, DC, USA, 1997. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Hernández, A.; Concepción, M.T.; Rodríguez, M.; Salido, E.; Torres, A. High phosphorus diet increases preproPTH mRNA independent of calcium and calcitriol in normal rats. Kidney Int. 1996, 50, 1872–1878. [Google Scholar] [CrossRef]
- Ward, D.N.; Griffin, A.C. Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Cancer Res. 1955, 15, 456–461. [Google Scholar]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 2, e1028. [Google Scholar] [CrossRef]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef]
- Banerjee, S.; Drapkin, R.; Richardson, D.L.; Birrer, M. Targeting NaPi2b in ovarian cancer. Cancer Treat. Rev. 2023, 112, 102489. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Cosentino-Gomes, D.; Nascimento, M.T.C.; Carvalho-Kelly, L.F.; Gomes, T.; Rodrigues, M.F.; König, S.; Rumjanek, F.D.; Monteiro, R.Q.; et al. H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2180–2188. [Google Scholar] [CrossRef]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Casarrubios, M.; Sierra-Rodero, B.; Provencio, M. The Role of Metabolism in Tumor Immune Evasion: Novel Approaches to Improve Immunotherapy. Biomedicines 2021, 9, 361. [Google Scholar] [CrossRef]
- Brown, R.B. Cancer Cachexia and Dysregulated Phosphate Metabolism: Insights from Mutant p53 and Mutant Klotho Mouse Models. Metabolites 2022, 12, 1284. [Google Scholar] [CrossRef]
- Adeyinka, A.; Bashir, K. Tumor Lysis Syndrome. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518985/ (accessed on 14 January 2024).
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Ghasemi, K.; Nasiri, M. Association between Hyperphosphatemia and Inflammation in Patients with End-Stage Renal Diseases Undergoing Hemodialysis. J. Adv. Pharm. Educ. Res. 2020, 10, 117. [Google Scholar]
- Brown, R.B. Potential interaction of inflammatory hyperemia and hyperphosphatemia in tumorigenesis. Future Oncol. 2019, 15, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.Y.; Xu, C.M.; Xia, M.; Zhu, H.Q.; Chen, Y.Q. Relationship between Gut Microbiota and Phosphorus Metabolism in Hemodialysis Patients: A Preliminary Exploration. Chin. Med. J. 2018, 131, 2792–2799. [Google Scholar] [PubMed]
- Rahbar Saadat, Y.; Niknafs, B.; Hosseiniyan Khatibi, S.M.; Ardalan, M.; Majdi, H.; Bahmanpoor, Z.; Abediazar, S.; Zununi Vahed, S. Gut microbiota; an overlooked effect of phosphate binders. Eur. J. Pharmacol. 2020, 868, 172892. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaum, E.; Nüssler, V. Cancer Prevention with Nutrition and Lifestyle. Visc. Med. 2019, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report 2018; World Cancer Research Fund International: London, UK, 2018; Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 17 October 2023).
- Dale, G.; Fleetwood, J.; Weddell, A.; Ellis, R.; Sainsbury, J. Fitness, unfitness, and phosphate. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 939. [Google Scholar] [CrossRef] [PubMed]
- Orcy, R.; Antunes, M.F.; Schiller, T.; Seus, T.; Böhlke, M. Aerobic exercise increases phosphate removal during hemodialysis: A controlled trial. Hemodial Int. 2014, 18, 450–458. [Google Scholar] [CrossRef]
- Schroeder, J. Phosphorus Impacts from Meat-, Dairy-, and Plant-Based Diets. Consilience 2018, 18, 17–35. [Google Scholar]
- Calvo, M.S.; Dunford, E.K.; Uribarri, J. Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk? Nutrients 2023, 15, 3510. [Google Scholar] [CrossRef]
- nih.gov. Phosphorus—Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/ (accessed on 16 October 2023).
- Brown, R.B. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine 2019, 65, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Sauve, R.S. Whole cow’s milk in infancy. Paediatr. Child Health 2003, 8, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.J.; Inoue-Choi, M.; Lazovich, D.; Robien, K. WCRF/AICR recommendation adherence and breast cancer incidence among postmenopausal women with and without non-modifiable risk factors. Int. J. Cancer 2016, 138, 2602–2615. [Google Scholar] [CrossRef]
- Hastert, T.A.; Beresford, S.A.; Patterson, R.E.; Kristal, A.R.; White, E. Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1498–1508. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and breast cancer risk. Int. J. Cancer 2016, 138, 2657–2664. [Google Scholar] [CrossRef]
- Makarem, N.; Lin, Y.; Bandera, E.V.; Jacques, P.F.; Parekh, N. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991–2008). Cancer Causes Control. 2015, 26, 277–286. [Google Scholar] [CrossRef]
- Catsburg, C.; Miller, A.B.; Rohan, T.E. Adherence to cancer prevention guidelines and risk of breast cancer. Int. J. Cancer 2014, 135, 2444–2452. [Google Scholar] [CrossRef]
- Adhikari, S.; Mamlouk, O.; Rondon-Berrios, H.; Workeneh, B.T. Hypophosphatemia in cancer patients. Clin. Kidney J. 2021, 14, 2304–2315. [Google Scholar] [CrossRef]
- Osuka, S.; Razzaque, M.S. Can features of phosphate toxicity appear in normophosphatemia? J. Bone Miner. Metab. 2012, 30, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dukmak, O.N.; Ayyad, M.; Albandak, M.; Hamadah, A.; Gharaibeh, K. Tumor Genesis Syndrome Presenting as Severe Hypophosphatemia in a Patient With T-Cell Acute Lymphoblastic Leukemia. Cureus 2023, 15, e38815. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Wu, H.Y.; Chiu, Y.L.; Yang, J.Y.; Pai, M.F.; Wu, Y.R.; Lin, W.Y.; Hung, K.Y.; Chien, K.L.; Hsu, S.P.; et al. Acute effects of dietary phosphorus intake on markers of mineral metabolism in hemodialysis patients: Post hoc analysis of a randomized crossover trial. Ren. Fail. 2021, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Hsu, S.-P.; Chiu, Y.-L.; Wu, H.-Y.; Luan, C.-C.; Yang, J.-Y.; Pai, M.-F.; Lin, C.-J.; Lin, W.-Y.; Sun, W.-H.; et al. Short-Term Effects of a Therapeutic Diet on Biochemical Parameters in Hemodialysis Patients: A Randomized Crossover Trial. J. Ren. Nutr. 2023, 33, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Rhee, C.M. Dietary Therapy for Managing Hyperphosphatemia. Clin. J. Am. Soc. Nephrol. 2020, 16, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Brauer, A.; Waheed, S.; Singh, T.; Maursetter, L. Improvement in Hyperphosphatemia Using Phosphate Education and Planning Talks. J. Ren. Nutr. 2019, 29, 156–162. [Google Scholar] [CrossRef]
- Tsai, W.C.; Yang, J.Y.; Luan, C.C.; Wang, Y.J.; Lai, Y.C.; Liu, L.C.; Peng, Y.S. Additional benefit of dietitian involvement in dialysis staffs-led diet education on uncontrolled hyperphosphatemia in hemodialysis patients. Clin. Exp. Nephrol. 2016, 20, 815–821. [Google Scholar] [CrossRef]
- Karavetian, M.; Rizk, R. Patient education for hyperphosphatemia management: Improving outcomes while decreasing costs? Kidney Res. Clin. Pract. 2018, 37, 4–7. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Rozga, M.R.; Handu, D.; Carrero, J.J. Effect of Phosphate-Specific Diet Therapy on Phosphate Levels in Adults Undergoing Maintenance Hemodialysis: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2020, 16, 107–120. [Google Scholar] [CrossRef]
- Milazi, M.; Bonner, A.; Douglas, C. Effectiveness of educational or behavioral interventions on adherence to phosphate control in adults receiving hemodialysis: A systematic review. JBI Database System Rev. Implement. Rep. 2017, 15, 971–1010. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; Yamasaki, M.; Matsuura, S.; Morinishi, M.; Nakagawa, T.; Matsuno, M.; Katsumoto, M.; Nii, S.; Fushitani, Y.; Sugihara, K.; et al. Phosphatemic Index Is a Novel Evaluation Tool for Dietary Phosphorus Load: A Whole-Foods Approach. J. Ren. Nutr. 2020, 30, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.S.; Choi, H.J.; Kim, H.; et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. eLife 2020, 9, 52709. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Joe, D.; Shah, A.D. Forget the phosphorus: A case of hypervitaminosis D-induced symptomatic hypercalcemia. Clin. Nephrol. Case Stud. 2021, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Katicic, D.; Gulin, T.; Josipovic, J. Vitamin d in the patients with chronic kidney disease: When, to whom and in which form. Mater. Sociomed. 2015, 27, 122–124. [Google Scholar] [CrossRef]
- Trump, D.L. Calcitriol and cancer therapy: A missed opportunity. Bone Rep. 2018, 9, 110–119. [Google Scholar] [CrossRef]
- Ritter, C.S.; Slatopolsky, E. Phosphate Toxicity in CKD: The Killer among Us. Clin. J. Am. Soc. Nephrol. 2016, 11, 1088–1100. [Google Scholar] [CrossRef]
- Prado, C.M.; Anker, S.D.; Coats, A.J.S.; Laviano, A.; von Haehling, S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J. Cachexia Sarcopenia Muscle 2021, 12, 3–8. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kao, T.W.; Chou, C.W.; Wu, C.J.; Yang, H.F.; Lai, C.H.; Wu, L.W.; Chen, W.L. Exploring the Link between Serum Phosphate Levels and Low Muscle Strength, Dynapenia, and Sarcopenia. Sci. Rep. 2018, 8, 3573. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Valenzuela, P.L.; Naves-Díaz, M.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Dietary phosphate restriction prevents the appearance of sarcopenia signs in old mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1060–1074. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- van de Worp, W.R.P.H.; Schols, A.M.W.J.; Theys, J.; van Helvoort, A.; Langen, R.C.J. Nutritional Interventions in Cancer Cachexia: Evidence and Perspectives From Experimental Models. Front. Nutr. 2020, 7, 601329. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2013, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.K.; Kumar, L.S.; Kumar, S. Proteins. In Chemistry of Natural Products: Amino Acids, Peptides, Proteins and Enzymes; Ahluwalia, V.K., Kumar, L.S., Kumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 113–156. [Google Scholar]
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 676–682. [Google Scholar]
- Batalha, I.L.; Lowe, C.R.; Roque, A.C.A. Platforms for enrichment of phosphorylated proteins and peptides in proteomics. Trends Biotechnol. 2012, 30, 100–110. [Google Scholar] [CrossRef] [PubMed]
- DeClercq, V.; Nearing, J.T.; Sweeney, E. Plant-Based Diets and Cancer Risk: What is the Evidence? Curr. Nutr. Rep. 2022, 11, 354–369. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Jagannathan, R.; Gutekunst, L.; Kalantar-Zadeh, K.; Sevick, M.A. Examining the Proportion of Dietary Phosphorus From Plants, Animals, and Food Additives Excreted in Urine. J. Ren. Nutr. 2017, 27, 78–83. [Google Scholar] [CrossRef]
- Hagihara, K.; Kajimoto, K.; Osaga, S.; Nagai, N.; Shimosegawa, E.; Nakata, H.; Saito, H.; Nakano, M.; Takeuchi, M.; Kanki, H.; et al. Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients 2020, 12, 1473. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Phosphate and oxysterols may mediate an inverse relationship between atherosclerosis and cancer. Eur. Med. J.-Oncol. 2020, 8, 114–121. [Google Scholar] [CrossRef]
- Kundu, S.; Hossain, K.S.; Moni, A.; Zahan, M.S.; Rahman, M.M.; Uddin, M.J. Potentials of ketogenic diet against chronic kidney diseases: Pharmacological insights and therapeutic prospects. Mol. Biol. Rep. 2022, 49, 9749–9758. [Google Scholar] [CrossRef]
- Ferrer, M.; Mourikis, N.; Davidson, E.E.; Kleeman, S.O.; Zaccaria, M.; Habel, J.; Rubino, R.; Gao, Q.; Flint, T.R.; Young, L.; et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 2023, 35, 1147–1162.e1147. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminazdeh-Gohari, S.; Kofler, B. Ketogenic diet in cancer therapy. Aging 2018, 10, 164–165. [Google Scholar] [CrossRef]
- Sahoo, B.; Srivastava, M.; Katiyar, A.; Ecelbarger, C.; Tiwari, S. Liver or kidney: Who has the oar in the gluconeogenesis boat and when? World J. Diabetes 2023, 14, 1049–1056. [Google Scholar] [CrossRef]
- Bozzetti, F.; Zupec-Kania, B. Toward a cancer-specific diet. Clin. Nutr. 2016, 35, 1188–1195. [Google Scholar] [CrossRef]
- Nelson, M. Ketogenic Diet and Cancer Treatment. Available online: https://www.aicr.org/resources/blog/the-ketogenic-diet-and-cancer-treatment-what-patients-should-know/ (accessed on 9 January 2024).
- Tiwari, S.; Sapkota, N.; Han, Z. Effect of fasting on cancer: A narrative review of scientific evidence. Cancer Sci. 2022, 113, 3291–3302. [Google Scholar] [CrossRef]
- Kalam, F.; James, D.L.; Li, Y.R.; Coleman, M.F.; Kiesel, V.A.; Cespedes Feliciano, E.M.; Hursting, S.D.; Sears, D.D.; Kleckner, A.S. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. JNCI Monogr. 2023, 2023, 84–103. [Google Scholar] [CrossRef]
- Myers, T.R.; Zittel, M.; Goldhamer, A.C. Follow-up of water-only fasting and an exclusively plant food diet in the management of stage IIIa, low-grade follicular lymphoma. BMJ Case Rep. 2018, 2018, bcr–2018-225520. [Google Scholar] [CrossRef]
- Grassilli, E.; Cerrito, M.G. “Ironing out” fasting-induced persister cancer cells to render chemotherapy effective: Is this the solution? eBioMedicine 2023, 90, 104542. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.; Turley, E.A.; Baum, M. A new biological framework for cancer research. The Lancet 1996, 348, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Nagy, J.D.; Elser, J.J. Biological stoichiometry of tumor dynamics: Mathematical models and analysis. Discret. Contin. Dyn. Syst. Ser. B 2004, 4, 221–240. [Google Scholar]
- National Cancer Institute. Diet. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet (accessed on 19 August 2023).

| WCRF/AICR Recommendations for Cancer Prevention | Additional Associations with Phosphate Toxicity |
|---|---|
| “Be a healthy weight—Keep your weight within the healthy range and avoid weight gain in adult life” | Obesity is linked to higher dietary phosphate intake and increased risk of certain cancers [30]. |
| “Be physically active—Be physically active as part of everyday life—walk more and sit less” | During exercise, phosphorus shifts from intracellular areas to extracellular fluid [60] for removal by the kidneys, and phosphate removal from blood during hemodialysis is increased with aerobic exercise [61]. |
| “Eat a diet rich in wholegrains, vegetables, fruit and beans—Make wholegrains, vegetables, fruit and pulses (legumes) such as beans and lentils a major part of your usual daily diet” | Plant-based diets are generally lower in phosphorus compared to meat-based and dairy-based diets [62]. |
| “Limit consumption of ‘fast foods’ and other processed foods high in fat, starches or sugars—Limiting these foods helps control calorie intake and maintain a healthy weight” | Ultra-processed food consumption with highly absorbable phosphate food additives is increasing [63]. |
| “Limit consumption of red and processed meat—Eat no more than moderate amounts of red meat, such as beef, pork and lamb. Eat little, if any, processed meat” | Meat is naturally high in phosphorus [62]. |
| “Limit consumption of sugar sweetened drinks—Drink mostly water and unsweetened drinks” | Phosphoric acid is a common additive in sugar-sweetened beverages like colas [63]. |
| “Limit alcohol consumption—For cancer prevention, it’s best not to drink alcohol” | Non-traumatic rhabdomyolysis from alcohol intake increases serum Pi [35]. |
| “Do not use supplements for cancer prevention—Aim to meet nutritional needs through diet alone” | Phosphorus is added to some dietary supplements [64]. Vitamin D supplements also do not lower cancer risk associated with high dietary phosphate [65]. |
| “For mothers: breastfeed your baby, if you can—Breastfeeding is good for both mother and baby” | Breast milk is six-times lower in phosphorus compared to cow’s milk [66], and increased need for phosphate during pregnancy and lactation may mitigate effects of excessive dietary phosphate intake [35]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B.; Bigelow, P. Can a Low-Phosphate Diet for Chronic Kidney Disease Treat Cancer? An Interdisciplinary Literature Review. Medicines 2024, 11, 5. https://doi.org/10.3390/medicines11020005
Brown RB, Bigelow P. Can a Low-Phosphate Diet for Chronic Kidney Disease Treat Cancer? An Interdisciplinary Literature Review. Medicines. 2024; 11(2):5. https://doi.org/10.3390/medicines11020005
Chicago/Turabian StyleBrown, Ronald B., and Philip Bigelow. 2024. "Can a Low-Phosphate Diet for Chronic Kidney Disease Treat Cancer? An Interdisciplinary Literature Review" Medicines 11, no. 2: 5. https://doi.org/10.3390/medicines11020005