Immunomodulatory and Antibacterial Properties of the Chumash Medicinal Plant Trichostema lanatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Plant Material and Generation of Extracts
2.3. Immunomodulatory Assays
2.4. Antibacterial Disc Diffusion Assay
2.5. Chemical Analysis of T. lanatum Extract
3. Results
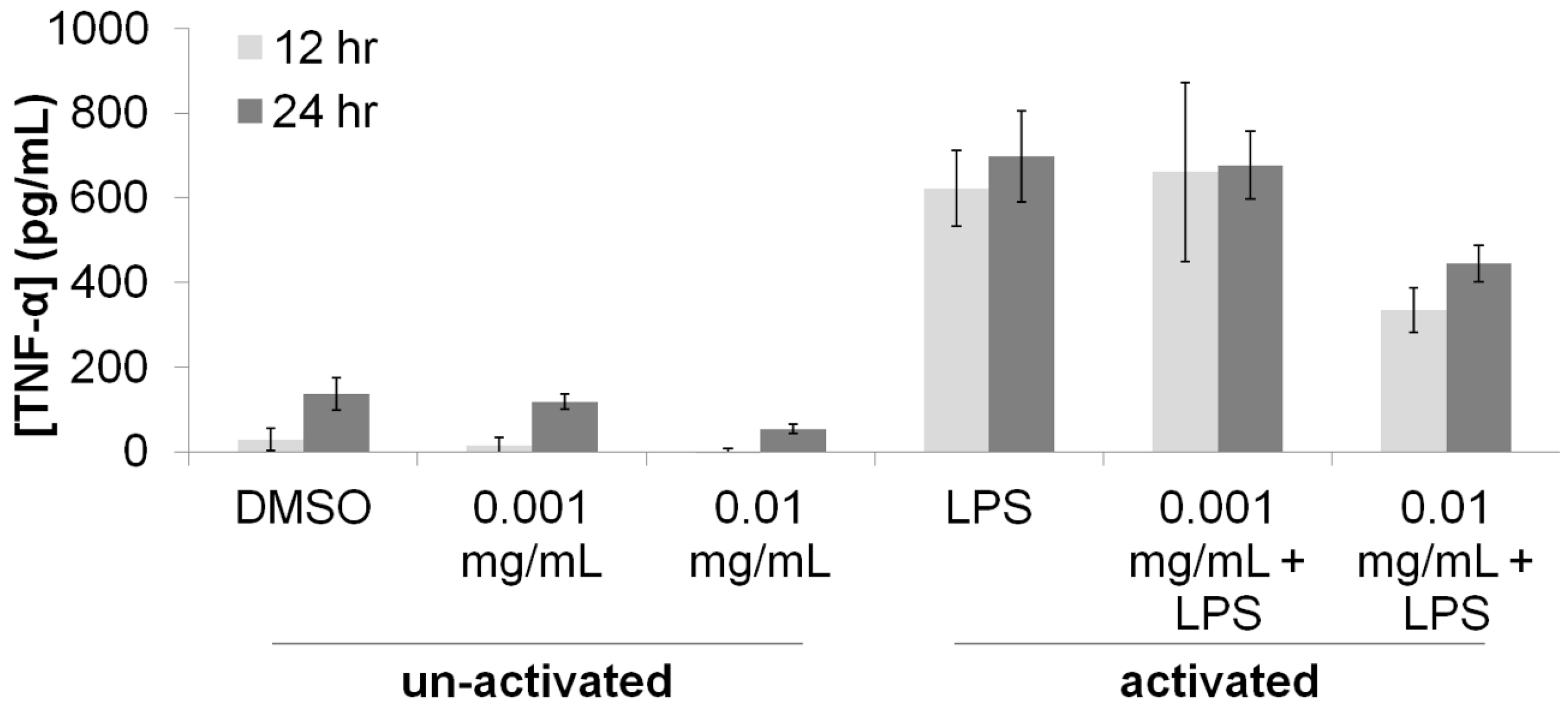
3.1. Immunomodulatory Properties of T. lanatum Extract
3.2. Antibacterial Properties of T. lanatum Extract
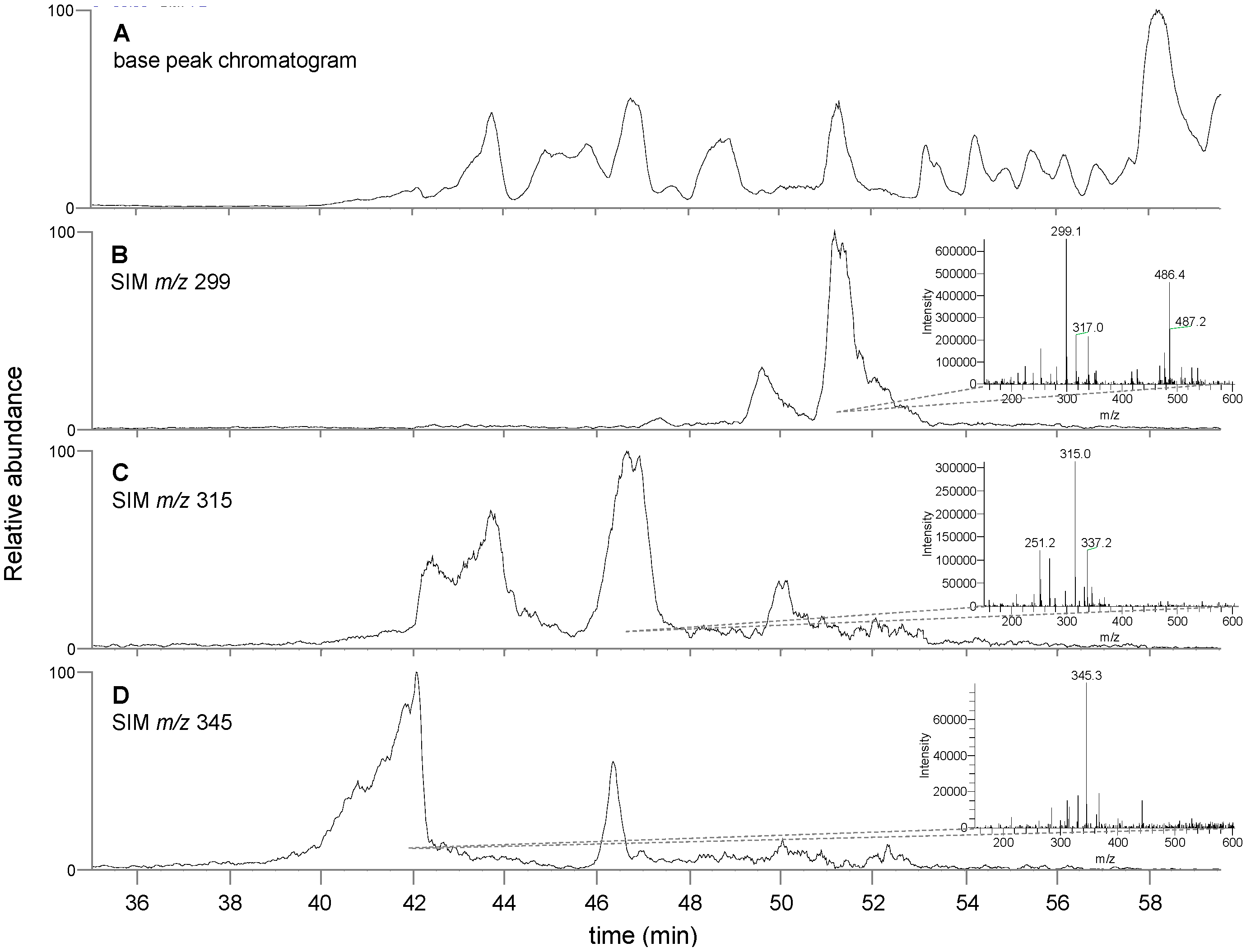
3.3. Chemical Analysis of T. lanatum Extract
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Timbrook, J. Chumash Ethnobotany: Plant Knowledge among the Chumash People of Southern California; Heyday Books: Berkley, CA, USA, 2007; ISBN 978-1-59714-048-5. [Google Scholar]
- Cutolo, M. Macrophages as Effectors of the Immunoendocrinologic Interactions in Autoimmune Rheumatic Diseasesa. Ann. N. Y. Acad. Sci. 1999, 876, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Laria, A.; Lurati, A.; Marrazza, M.; Mazzocchi, D.; Re, K.A.; Scarpellini, M. The macrophages in rheumatic diseases. J. Inflamm. Res. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R.S. Anti-Inflammatory Compounds of Plant Origin. Part I. Action on Arachidonic Acid Pathway, Nitric Oxide and Nuclear Factor κ B (NF-κB). Planta Med. 2003, 69, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Brancato, S.K.; Thomay, A.A.; Reichner, J.S.; Albina, J.E. The phenotype of murine wound macrophages. J. Leukoc. Biol. 2010, 87, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.H.; Black, D.R.; Mon, T.R.; Connolly, G.E. Vinegar weed volatile constituents. J. Agric. Food Chem. 1976, 24, 862–865. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J. The Essential Oil of Trichostema dichotomum L. J. Essent. Oil Res. 1990, 2, 149–150. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Rustaiyan, A.; Roitman, J.N.; Graven, E.H. Notes: Exudate Flavonoids of Some Salvia and a Trichostema Species. Z. Naturforsch. C 1992, 47, 782–784. [Google Scholar] [CrossRef]
- Jackowski, S.; Zhang, Y.-M.; Price, A.C.; White, S.W.; Rock, C.O. A Missense Mutation in the fabB (?-Ketoacyl-Acyl Carrier Protein Synthase I) Gene Confers Thiolactomycin Resistance to Escherichia coli. Antimicrob. Agents Chemother. 2002, 46, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Lewis, K. In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol. 2001, 3, 247–254. [Google Scholar] [PubMed]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- WHO|WHO Traditional Medicine Strategy: 2014–2023. Available online: http://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ (accessed on 1 September 2015).
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. 1), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Ligresti, A.; Longo, R.; Russo, A.; Borrelli, F.; Sautebin, L. The inhibitory effect of propolis and caffeic acid phenethyl ester on cyclooxygenase activity in J774 macrophages. Phytomedicine 2002, 9, 530–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]

| Bacterium | Gram (+/−) | Ampicillin (0.01 mg) | T. lanatum Extract | ||
|---|---|---|---|---|---|
| (1.25 mg) | (0.50 mg) | (0.20 mg) | |||
| Corynebacterium xerosis | + | 53.0 ± 9.0 | 19.6 ± 1.9 | 17.7 ± 1.2 | 14.0 ± 1.2 |
| Enterococcus faecalis | + | 25.3 ± 0.3 | 11.6 ± 0.2 | 9.0 ± 0.3 | no inhibition |
| Bacillus subtilis | + | 27.3 ± 0.7 | 11.4 ± 0.8 | 9.7 ± 0.6 | 6.1 ± 3.3 |
| Staphylococcus epidermidis | + | 16.0 ± 0.7 | 11.1 ± 0.7 | 3.7 ± 4.3 | no inhibition |
| Bacillus megaterium | + | 23.8 ± 1.1 | 10.9 ± 0.7 | 9.0 ± 1.2 | no inhibition |
| Staphylococcus aureus | + | 31.0 ± 1.0 | 9.2 ± 0.4 | no inhibition | no inhibition |
| Escherichia coli | − | 15.1 ± 0.5 | no inhibition | no inhibition | no inhibition |
| Escherichia coli ΔtolC | − | 16.3 ± 0.3 | 12.3 ± 1.2 | 2.7 ± 2.7 | no inhibition |
| Salmonella typhimurium | − | 25.1 ± 1.6 | no inhibition | no inhibition | no inhibition |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, M.C.; Hester, V.; Allison, B.J.; Foster, M.C.; Nofziger, D.; Joyner, P.M. Immunomodulatory and Antibacterial Properties of the Chumash Medicinal Plant Trichostema lanatum. Medicines 2018, 5, 25. https://doi.org/10.3390/medicines5020025
Fleming MC, Hester V, Allison BJ, Foster MC, Nofziger D, Joyner PM. Immunomodulatory and Antibacterial Properties of the Chumash Medicinal Plant Trichostema lanatum. Medicines. 2018; 5(2):25. https://doi.org/10.3390/medicines5020025
Chicago/Turabian StyleFleming, Matthew C., Victoria Hester, Brittany J. Allison, Majie C. Foster, Donna Nofziger, and P. Matthew Joyner. 2018. "Immunomodulatory and Antibacterial Properties of the Chumash Medicinal Plant Trichostema lanatum" Medicines 5, no. 2: 25. https://doi.org/10.3390/medicines5020025