Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now?
Abstract
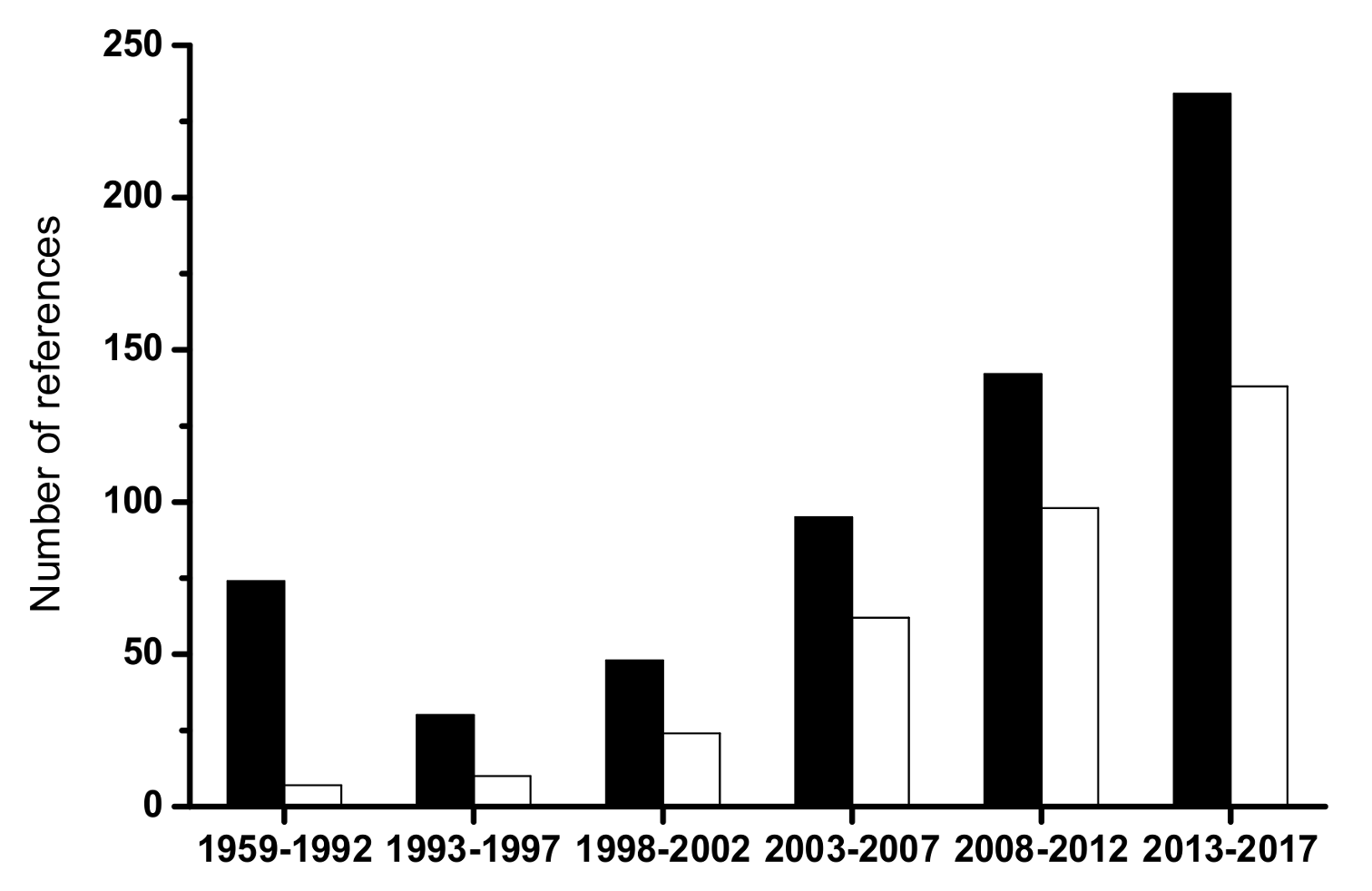
:1. Introduction
2. Volatile Phenols (VPs): Their Incidence and Origin
3. Brett Ecology: Infection Routes in the Winery
Old Wooden Vats: A Well-Known Ecological Niche
4. Brett Behavior and Tolerance in Wines
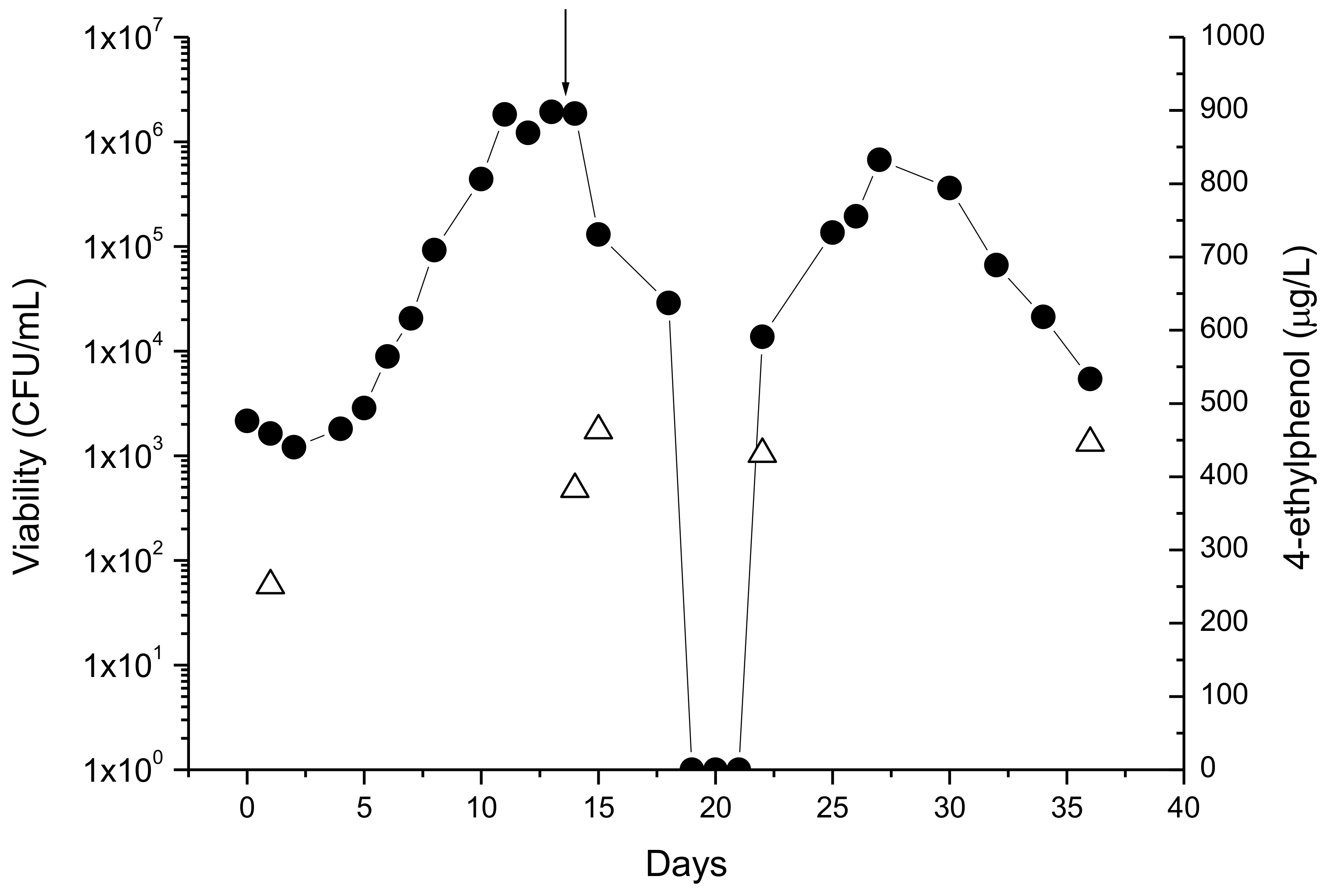
4.1. Attention to the Unnoticed Presence of Brett
4.2. Resistance to Antimicrobials
4.3. The Hurdle Concept in Food and Wine Preservation
5. Brett Prevention: How to Monitor Contaminations
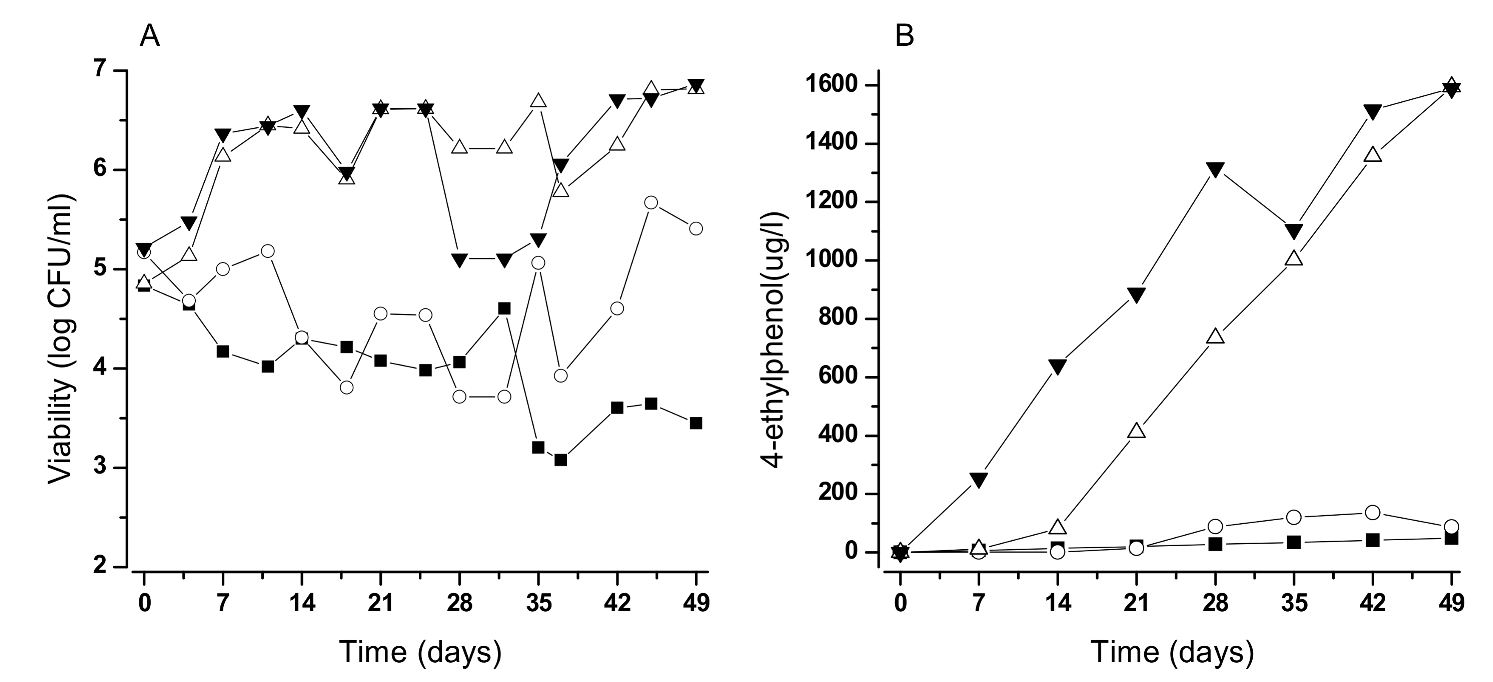
5.1. To Know Where They Are and How Fit They Are
5.2. Microbial Guidelines
5.3. The Question of Real Time PCR
6. Brett Prevention: How to Control, Kill, and Cure
6.1. Reduction of Dissemination
6.2. Prevention of D. bruxellensis Growth and 4-EP Production
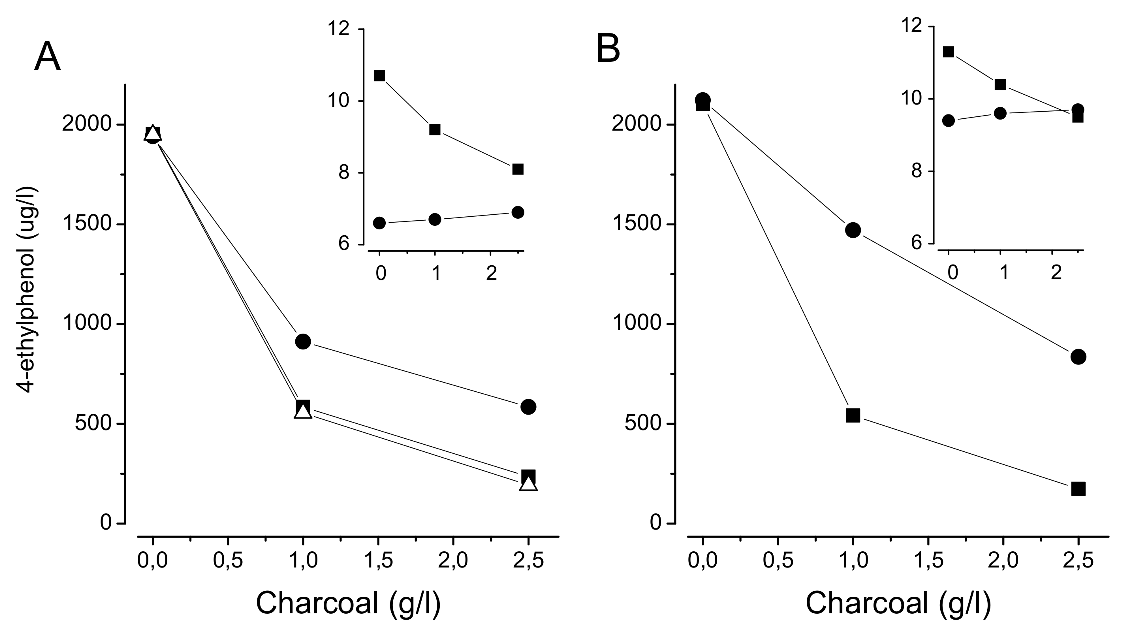
6.3. Curative Measures
7. The Brett Sequel: from “Terroir” to “Terror”
The Issue of Chemical Limits
8. Final Remarks
Acknowledgments
Conflicts of Interest
References
- Schifferdecker, A.J.; Dashko, S.; Ishchuk, O.P.; Piškur, J. The wine and beer yeast Dekkera bruxellensis. Yeast 2014, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, W.A.; Wikén, T.O. The Custers effect (negative Pasteur effect) as a diagnostic criterion for the genus Brettanomyces. Antonie Leeuwenhoek 1969, 35, A31–A32. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.-N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Curtin, C.; Varela, C.; Borneman, A. Harnessing improved understanding of Brettanomyces bruxellensis biology to mitigate the risk of wine spoilage. Aust. J. Grape Wine Res. 2015, 21, 680–692. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage activities of Dekkera/Brettanomyces spp. In Food Spoilage Microorganisms; Blackburn, C., Ed.; Woodhead Publishers: Cambridge, UK, 2006; Chapter 13; pp. 354–398. [Google Scholar]
- Renouf, V.; Lonvaud-Funel, A.; Coulon, J. The origin of Brettanomyces bruxellensis in wines: A review. J. Int. Sci. Vigne Vin 2007, 41, 161–173. [Google Scholar] [CrossRef]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Oelofse, A.; Pretorius, I.S.; du Toit, M. Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. S. Afr. J. Enol. Vitic. 2008, 29, 128–144. [Google Scholar] [CrossRef]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT Food Sci. Technol. 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Divol, B. Brettanomyces bruxellensis, a survivalist prepared for the wine apocalypse and other beverages. Food Microbiol. 2016, 59, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Agnolucci, M.; Tirelli, A.; Cocolin, L.; Toffanin, A. Brettanomyces bruxellensis yeasts: Impact on wine and winemaking. World J. Microbiol. Biotechnol. 2017, 33, 180. [Google Scholar] [CrossRef] [PubMed]
- Goode, J.; Harrop, S. Wine Faults and Their Prevalence: Data from the World’s Largest Blind Tasting. In Proceedings of the 20th Entretiens Scientifiques Lallemand, Horsens, Denmark, 15 May 2008. [Google Scholar]
- Schumaker, M.; Chandra, M.; Malfeito-Ferreira, M.; Ross, C. Influence of Brettanomyces ethylphenols on red wine aroma evaluated by consumers in the United States and Portugal. Food Res. Int. 2017, 100, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.; Hayasaka, Y.; Curtin, C.; Sefton, M.; Taylor, D. Hydroxycinnamoyl Glucose and Tartrate Esters and Their Role in the Formation of Ethylphenols in Wine. J. Agric. Food Chem. 2016, 64, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Schopp, L.; Lee, J.; Osborne, J.; Chescheir, S.; Edwards, C. Metabolism of Nonesterified and Esterified Hydroxycinnamic Acids in Red Wines by Brettanomyces bruxellensis. J. Agric. Food Chem. 2013, 61, 11610–11617. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.L.; Sleep, N.R.; Capone, D.L.; Elsey, G.M.; Curtin, C.D.; Sefton, M.A.; Taylor, D.K. Hydroxycinnamic Acid Ethyl Esters as Precursors to Ethylphenols in Wine. J. Agric. Food Chem. 2012, 60, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.J.; Torres, M.; Palma, V.; Alves, E.; Patão, R.; Costa Freitas, A.M. Impact of malolactic fermentation on low molecular weight phenolic compounds. Talanta 2008, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Chescheir, S.; Philbin, D.; Osborne, J.P. Impact of Oenococcus oeni on wine hydroxycinnamic acids and volatile phenol production by Brettanomyces bruxellensis. Am. J. Enol. Vitic. 2015, 66, 357–362. [Google Scholar] [CrossRef]
- Madsen, M.G.; Edwards, N.K.; Petersen, M.A.; Mokwena, L.; Swiegers, J.H.; Arneborg, N. Influence of Oenococcus oeni and Brettanomyces bruxellensis on hydroxycinnamic acids and volatile phenols of aged wine. Am. J. Enol. Vitic. 2017, 68, 23–29. [Google Scholar] [CrossRef]
- Dias, L.; Pereira-da-Silva, S.; Tavares, M.; Malfeito-Ferreira, M.; Loureiro, V. Factors affecting the production of 4-ethylphenol by the yeast Dekkera bruxellensis in enological conditions. Food Microbiol. 2003, 20, 377–384. [Google Scholar] [CrossRef]
- Chandra, M.; Madeira, I.; Coutinho, A.; Albergaria, M.; Malfeito-Ferreira, M. Growth and volatile phenol production by Brettanomyces bruxellensis in different grapevine varieties during fermentation and in finished wine. Eur. Food Res. Technol. 2015, 242, 487–494. [Google Scholar] [CrossRef]
- Barata, A.; Caldeira, J.; Botellheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Correia, P.; Nobre, A.; Malfeito-Ferreira, M.; Loureiro, V. Growth and 4-ethylphenol production by the yeast Pichia guilliermondii in grape juices. Am. J. Enol. Vitic. 2006, 57, 133–138. [Google Scholar]
- Dias, L.; Dias, S.; Sancho, T.; Stender, H.; Querol, A.; Malfeito-Ferreira, M.; Loureiro, V. Identification of yeasts isolated from wine related environments and capable of producing 4-ethylphenol. Food Microbiol. 2003, 20, 567–574. [Google Scholar] [CrossRef]
- Martorell, P.; Barata, A.; Malfeito-Ferreira, M.; Fernández-Espinar, M.; Loureiro, V.; Querol, A. Molecular typing of the yeast species Dekkera bruxellensis and Pichia guilliermondii recovered from wine related sources. Int. J. Food Microbiol. 2006, 106, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ison, R.; Gutteridge, C. Determination of the carbonation tolerance of yeasts. Lett. Appl. Microbiol. 1987, 5, 11–13. [Google Scholar] [CrossRef]
- Rodrigues, N.; Gonçalves, G.; Pereira-da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and use of a new medium to detect yeasts of the genera Dekkera/Brettanomyces spp. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Renouf, V.; Lonvaud-Funel, A. Development of an enrichment medium to detect Dekkera/Brettanomyces bruxellensis a spoilage yeast, on the surface of grape berries. Microbiol. Res. 2007, 162, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, M.E.; Marchetti, R. Analysis of yeast flora associated with grape sour rot and of the chemical disease markers. Appl. Environ. Microbiol. 1987, 53, 571–576. [Google Scholar] [PubMed]
- Connell, L.; Stender, H.; Edwards, C.G. Rapid detection and identification of Brettanomyces from winery air samples based on peptide nucleic acid analysis. Am. J. Enol. Vitic. 2002, 53, 322–324. [Google Scholar]
- Renouf, V.; Perello, M.-C.; De Revel, G.; Lonvaud-Funel, A. Survival of wine microorganisms in the bottle during storage. Am. J. Enol. Vitic. 2007, 58, 379–386. [Google Scholar]
- Malfeito-Ferreira, M.; Rodrigues, N.; Loureiro, V. The influence of oxygen on the “horse sweat taint” in red wines. Ital. Food Beverage Technol. 2001, 24, 34–38. [Google Scholar]
- Malfeito-Ferreira, M. Yeasts and wine off-flavours: A technological perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Capozzi, V.; Di Toro, M.R.; Grieco, F.; Michelotti, V.; Salma, M.; Lamontanara, A.; Russo, P.; Orrù, L.; Alexandre, H.; Spano, G. Viable But Not Culturable (VBNC) state of Brettanomyces bruxellensis in wine: New insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016, 59, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Bizzaro, D.; Comitini, F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016, 121, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. J. Int. Sci. Vigne Vin 2016, 50, 49–56. [Google Scholar] [CrossRef]
- Fabrizio, V.; Vigentini, I.; Parisi, N.; Picozzi, C.; Compagno, C.; Foschino, R. Heat inactivation of wine spoilage yeast Dekkera bruxellensis by hot water treatment. Lett. Appl. Microbiol. 2015, 61, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Delsart, C.; Grimi, N.; Boussetta, N.; Miot-Sertier, C.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M. Impact of pulsed-electric field and high-voltage electrical discharges on red wine microbial stabilization and quality characteristics. J. Appl. Microbiol. 2016, 120, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, S.; Silva, F.V. High pressure processing inactivation of Brettanomyces bruxellensis in seven different table wines. Food Control 2017, 81, 1–8. [Google Scholar] [CrossRef]
- Röder, C.; König, H.; Fröhlich, J. Species-specific identification of Dekkera/Brettanomyces yeasts by fluorescently labeled DNA probes targeting the 26S rRNA. FEMS Yeast Res. 2007, 7, 1013–1026. [Google Scholar]
- Agnolucci, M.; Scarano, S.; Rea, F.; Toffanin, A.; Nuti, M. Detection of Dekkera/Brettanomyces bruxellensis in pressed Sangiovese grapes by real time PCR. Ital. J. Food Sci. 2007, 19, 153–164. [Google Scholar]
- Longin, C.; Julliat, F.; Serpaggi, V.; Maupeu, J.; Bourbon, G.; Rousseaux, S.; Guilloux-Benatier, M.; Alexandre, H. Evaluation of three Brettanomyces qPCR commercial kits: Results from an interlaboratory study. J. Int. Sci. Vigne Vin 2016, 50, 223–230. [Google Scholar] [CrossRef]
- Vendrame, M.; Manzano, M.; Comi, G.; Bertrand, J.; Iacumin, L. Use of propidium monoazide for the enumeration of viable Brettanomyces bruxellensis in wine and beer by quantitative PCR. Food Microbiol. 2014, 42, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Lourenço, A.; Barata, A.; Brito, L.; Malfeito-Ferreira, M.; Loureiro, V. Susceptibility of wine spoilage yeasts and bacteria in the planktonic state and in biofilms to disinfectants. Ann. Microbiol. 2010, 60, 549–556. [Google Scholar] [CrossRef]
- Barata, A.; Laureano, P.; D’Antuono, I.; Martorell, P.; Stender, H.; Malfeito-Ferreira, M.; Querol, A.; Loureiro, V. Enumeration and identification of 4-ethylphenol producing yeasts recovered from the wood of wine ageing barriques after different sanitation treatments. J. Food Res. 2013, 2, 140–149. [Google Scholar] [CrossRef]
- Umiker, N.L.; Descenzo, R.A.; Lee, J.; Edwards, C.G. Removal of Brettanomyces bruxellensis from red wine using membrane filtration. J. Food Process. Preserv. 2013, 37, 799–805. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Barata, A.; Ferreira-Dias, S.; Malfeito-Ferreira, M.; Loureiro, V. A response surface methodology study on the role of factors affecting growth and volatile phenol production by Brettanomyces bruxellensis ISA 2211 in wine. Food Microbiol. 2014, 42, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Pagliara, D.; Piccininno, T.; Tarantino, F.; Ciardulli, W.; Malfeito-Ferreira, M.; Loureiro, V. The effect of sugar concentration and temperature on growth and volatile phenol production by Dekkera bruxellensis in wine. FEMS Yeast Res. 2008, 8, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F. A simple, cheap and reliable method for control of 4-ethylphenol and4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B 2017, 1041–1042, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Larcher, R.; Puecher, C.; Rohregger, S.; Malacarne, M.; Nicolini, G. 4-Ethylphenol and 4-ethylguaiacol depletion in wine using esterified cellulose. Food Chem. 2012, 132, 2126–2130. [Google Scholar] [CrossRef]
- Joseph, L.; Albino, E.; Bisson, L. Creation and Use of a Brettanomyces Aroma Wheel. Catalyst 2017, 1, 12–30. [Google Scholar]
- Smith, C. Postmodern Winemaking, Rethinking the Modern Science of an Ancient Craft; University California Press: Berkeley, CA, USA, 2013; ISBN 9780520282599. [Google Scholar]

| Species | 500 cells/mL | >104 cells/mL | Species | 500 cells/mL | >104 cells/mL |
|---|---|---|---|---|---|
| D. bruxellensis ISA 1791 | 100 | 300 | S. pombe ISA 1190 | 100 | >300 |
| P. guilliermondii ISA 2105 | 100 | 300 | Z. bailii ISA 1307 | 25 | 200 |
| S. cerevisiae ISA 1000 | 100 | 200 | Lactic acid bacteria | >300 | >300 |
| S. cerevisiae ISA 1026 | 100 | 200 | Acetic acid bacteria | >300 | >300 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfeito-Ferreira, M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now? Beverages 2018, 4, 32. https://doi.org/10.3390/beverages4020032
Malfeito-Ferreira M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now? Beverages. 2018; 4(2):32. https://doi.org/10.3390/beverages4020032
Chicago/Turabian StyleMalfeito-Ferreira, Manuel. 2018. "Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now?" Beverages 4, no. 2: 32. https://doi.org/10.3390/beverages4020032





