Intramuscular Adipose Tissue and the Functional Components of Sarcopenia in Hospitalized Geriatric Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurements
2.3. Statistics
2.4. Informed Consent
3. Results
3.1. Baseline Characteristics
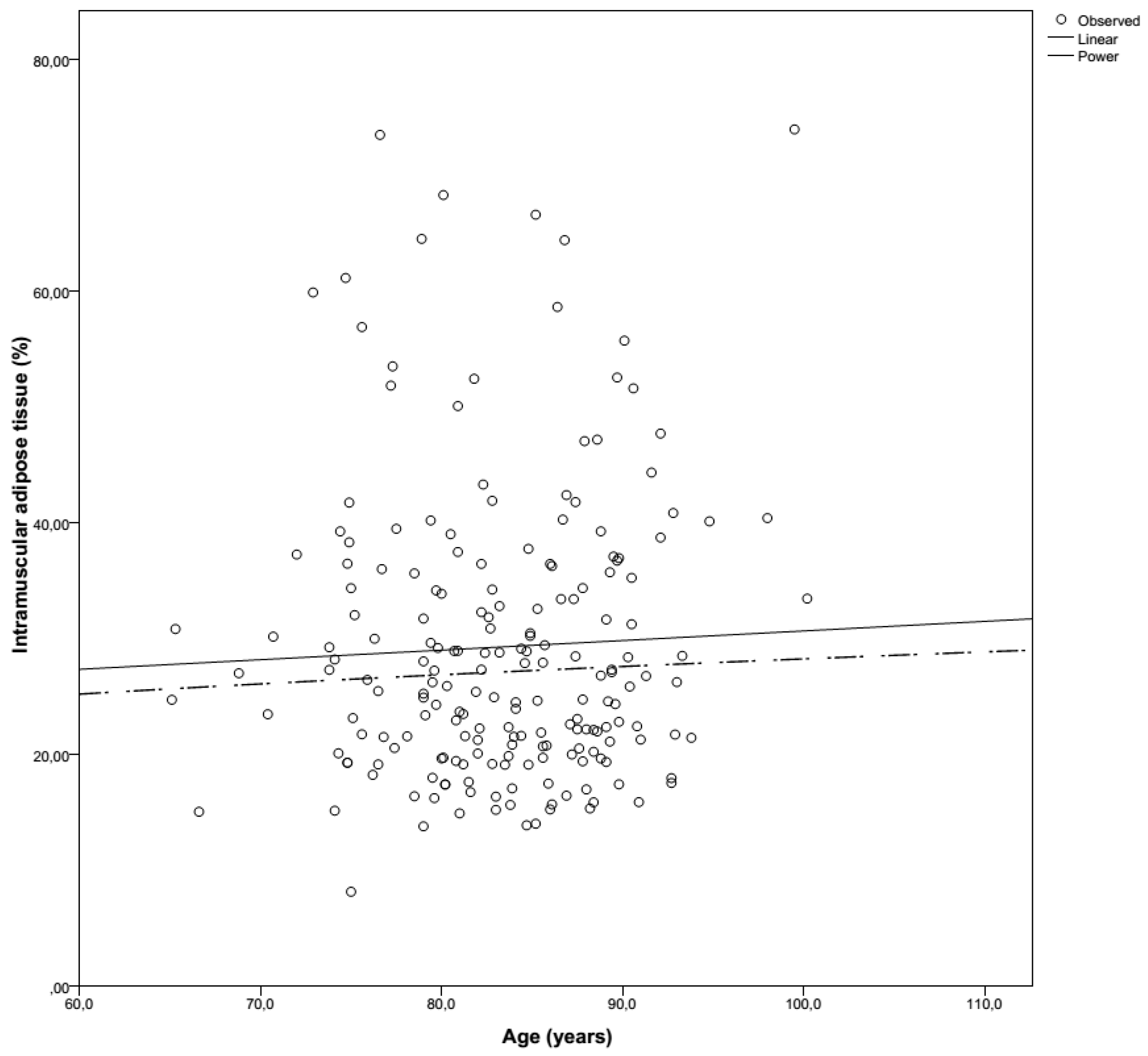
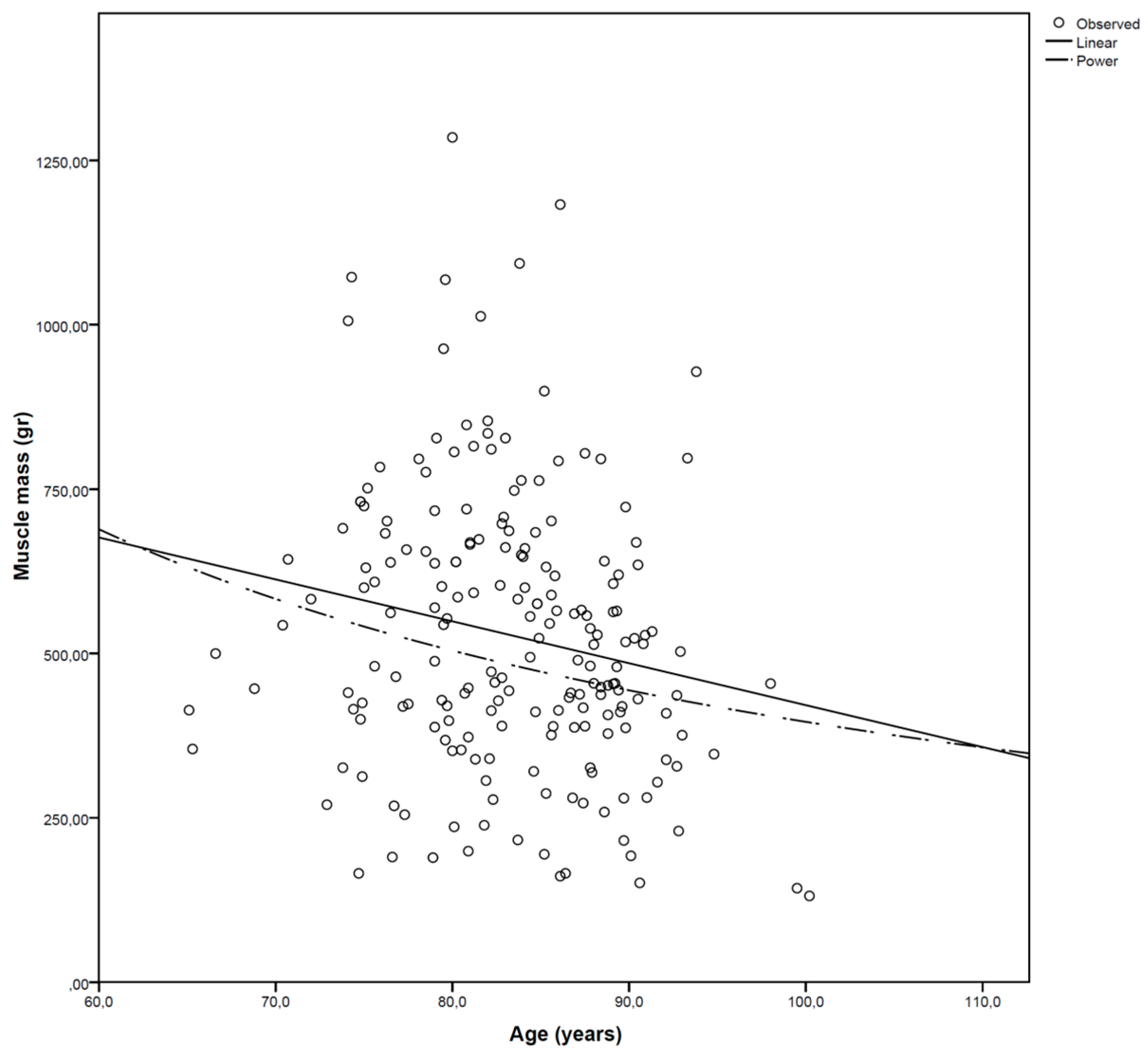
3.2. Muscle Mass
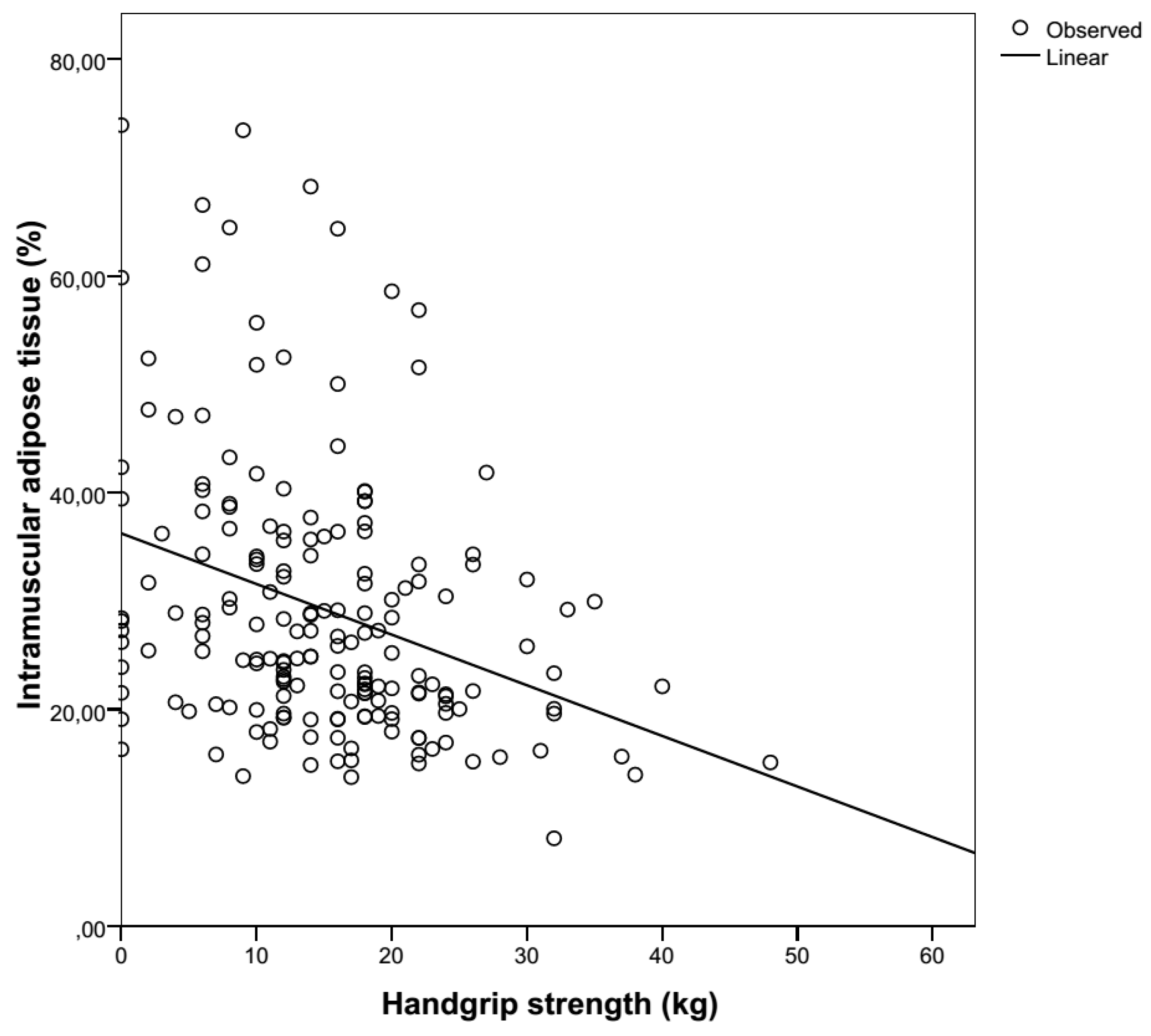
3.3. Muscle Strength
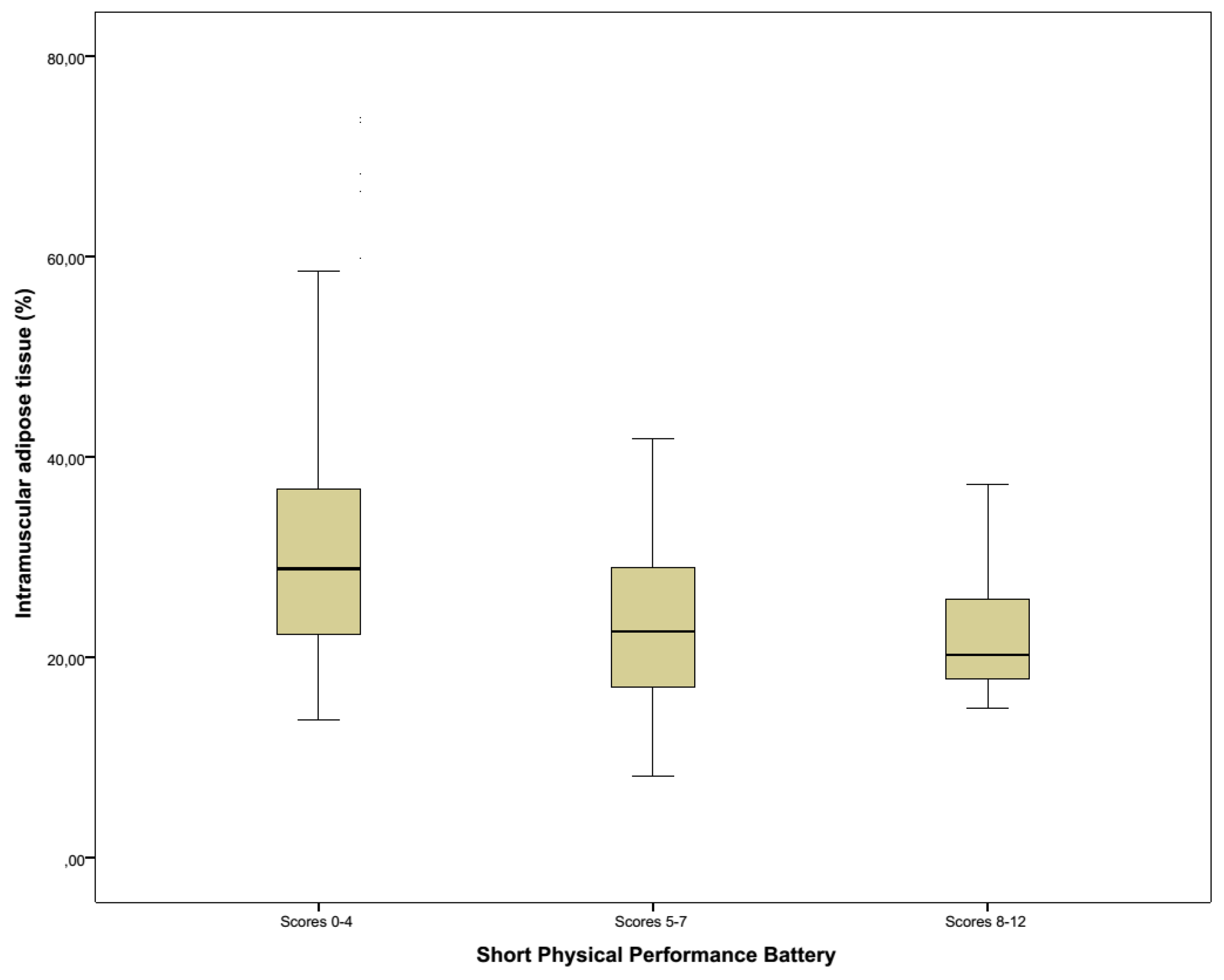
3.4. Functionality
3.5. Nutritional Risk
3.6. Other
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.; Watanabe, Y.; Fujiwara, Y.; Kim, H.; Edahiro, A.; Kawai, H.; Yoshida, H.; Obuchi, S.; Tanaka, Y.; Hirano, H. Association between the nutritional status and the severity of sarcopenia among community-dwelling elderly Japanese people. Nihon Ronen Igakkai zasshi Jpn. J. Geriatr. 2016, 53, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Lancha, A.H., Jr.; Zanella, R., Jr.; Tanabe, S.G.; Andriamihaja, M.; Blachier, F. Dietary protein supplementation in the elderly for limiting muscle mass loss. Amino Acids 2017, 49, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; De Cock, A.; Verhoeven, V.; Vandewoude, M. Physiological and architectural changes in the ageing muscle and their relation to strength and function in sarcopenia. Eur. Geriatr. Med. 2016, 7, 201–206. [Google Scholar] [CrossRef]
- Metter, E.J.; Lynch, N.; Conwit, R.; Lindle, R.; Tobin, J.; Hurley, B. Muscle quality and age: Cross-sectional and longitudinal comparisons. J. Gerontol. Ser. A 1999, 54, B207–B218. [Google Scholar] [CrossRef]
- Narici, M.V.; Reeves, N.D.; Morse, C.I.; Maganaris, C.N. Muscular adaptations to resistance exercise in the elderly. J. Musculoskelet. Neuronal Interact. 2004, 4, 161–164. [Google Scholar] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Kuipers, A.L.; Cauley, J.A.; Prasad, T.; Lee, C.G.; Ensrud, K.E.; Cawthon, P.M.; Hoffman, A.R.; Dam, T.T.; Gordon, C.L.; et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J. Gerontol. Ser. A 2015, 70, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Sinanan, A.C.; Buxton, P.G.; Lewis, M.P. Muscling in on stem cells. Biol. Cell 2006, 98, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Smith, S.R.; Heilbronn, L.K.; Kelley, D.E.; Ravussin, E.; Newcomer, B.R. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity 2006, 14, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.P.; Aragaki, A.K.; Gray, S.L.; Wactawski-Wende, J.; Cauley, J.A.; Cochrane, B.B.; Kooperberg, C.L.; Woods, N.F.; LaCroix, A.Z. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am. J. Med. 2009, 122, 947–954. [Google Scholar] [CrossRef]
- Beasley, J.M.; LaCroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the Women’s Health Initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Abboud, L.; Barry, M.; Reed, J.E.; Sheedy, P.F.; Jensen, M.D. Measuring leg muscle and fat mass in humans: Comparison of CT and dual-energy X-ray absorptiometry. J. Appl. Physiol. 2000, 88, 452–456. [Google Scholar] [PubMed]
- Yoshida, Y.; Marcus, R.L.; Lastayo, P.C. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve 2012, 46, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Masgrau, A.; Walrand, S.; Boirie, Y. Impaired protein metabolism: Interlinks between obesity, insulin resistance and inflammation. Obes. Rev. 2012, 13 (Suppl. S2), 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, L.J.; Sinacore, D.R.; Mueller, M.J. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res. 2012, 2012, 172957. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Murphy, R.A.; Brouwer, I.A.; Visser, M.; Launer, L.; Siggeirsdottir, K.; Eiriksdottir, G.; Gudnason, V.; Jonsson, P.V.; Lang, T.F.; et al. Muscle Quality and Myosteatosis: Novel Associations With Mortality Risk: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am. J. Epidemiol. 2016, 183, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zmuda, J.M.; Kuipers, A.L.; Jonnalagadda, P.; Bunker, C.H.; Patrick, A.L.; Youk, A.O.; Miljkovic, I. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing 2016, 45, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Shirai, H.; Fujimoto, Y.; Iida, T.; Yagi, S.; Taura, K.; Hatano, E.; et al. Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T.; et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Ishizaki, M.; Iida, H.; Matsui, K.; Sakaguchi, T.; Inoue, K.; Mizuta, T.; Ide, Y.; Iwasaka, J.; Kimura, Y.; et al. Effect of Intramuscular Adipose Tissue Content on Prognosis in Patients Undergoing Hepatocellular Carcinoma Resection. J. Gastrointest. Surg. 2015, 19, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Moynagh, M.R.; Multinu, F.; Cliby, W.A.; McGree, M.E.; Weaver, A.L.; Young, P.M.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Dowdy, S.C.; et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol. Oncol. 2016, 142, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.J.; Velleman, S.G.; Cowieson, A.J.; Singh, M.; Muir, W.I. Influence of hatch time and access to feed on intramuscular adipose tissue deposition in broilers. Poultry Sci. 2016, 95, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.R.; Lieber, R.L. Density and hydration of fresh and fixed human skeletal muscle. J. Biomech. 2005, 38, 2317–2320. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Goodpaster, B.H.; Newman, A.B.; Nevitt, M.; Stamm, E.; Harris, T.B. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J. Am. Geriatr. Soc. 2002, 50, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar] [PubMed]
- Ostir, G.V.; Volpato, S.; Fried, L.P.; Chaves, P.; Guralnik, J.M. Reliability and sensitivity to change assessed for a summary measure of lower body function: Results from the Women’s Health and Aging Study. J. Clin. Epidemiol. 2002, 55, 916–921. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.L.; Morley, J.E. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition 2000, 16, 131–140. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Chomentowski, P.; Ward, B.K.; Rossi, A.; Glynn, N.W.; Delmonico, M.J.; Kritchevsky, S.B.; Pahor, M.; Newman, A.B. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: A randomized controlled trial. J. Appl. Physiol. 2008, 105, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef]
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [PubMed]
- Perkisas, S.; Vandewoude, M. Where frailty meets diabetes. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 261–267. [Google Scholar] [CrossRef] [PubMed]
- Keevil, V.; Mazzuin Razali, R.; Chin, A.V.; Jameson, K.; Aihie Sayer, A.; Roberts, H. Grip strength in a cohort of older medical inpatients in Malaysia: A pilot study to describe the range, determinants and association with length of hospital stay. Arch. Gerontol. Geriatr. 2013, 56, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Syddall, H.E.; Westbury, L.D.; Dodds, R.M.; Cooper, C.; Aihie Sayer, A. Grip strength among community-dwelling older people predicts hospital admission during the following decade. Age Ageing 2015, 44, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Almurdhi, M.M.; Reeves, N.D.; Bowling, F.L.; Boulton, A.J.; Jeziorska, M.; Malik, R.A. Reduced Lower-Limb Muscle Strength and Volume in Patients with Type 2 Diabetes in Relation to Neuropathy, Intramuscular Fat, and Vitamin D Levels. Diabetes Care 2016, 39, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Maly, M.R.; Calder, K.M.; Macintyre, N.J.; Beattie, K.A. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res. 2013, 65, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; Hajj, E.l.; Fuleihan, G.; Josse, R.; Lips, P.; Morales, T.J.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2013, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Hardy, R. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Al Snih, S.; Markides, K. Lower body function as a predictor of mortality over 13 years of follow up: Findings from Hispanic Established Population for the Epidemiological Study of the Elderly. Geriatr. Gerontol. Int. 2016, 16, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Vandewoude, M.; Van Gossum, A. Nutritional screening strategy in nonagenarians: The value of the MNA-SF (mini nutritional assessment short form) in NutriAction. J. Nutr. Health Aging. 2013, 17, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [PubMed]
- Volkert, D. The role of nutrition in the prevention of sarcopenia. Wiener Med. Wochenschr. 2011, 161, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Vandewoude, M.F.; Alish, C.J.; Sauer, A.C.; Hegazi, R.A. Malnutrition-sarcopenia syndrome: Is this the future of nutrition screening and assessment for older adults? J. Aging Res. 2012, 2012, 651570. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Mean | Median | Range |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 83.0 ± 6.4 | 83 | 64–101 |
| Length (cm) | 163 ± 8 | 163 | 142–186 |
| Weight (kg) | 65.9 ± 15.4 | 64.0 | 33.2–154.5 |
| BMI (kg/m2) | 24.7 ± 5.3 | 23.9 | 14.2–47.4 |
| Laboratory | |||
| Pre-albumin (mg/dL) | 20.0 ± 7.1 | 20.0 | 5–43 |
| Albumin (g/L) | 31.3 ± 5.5 | 31.0 | 11–53 |
| CRP (mg/dL) | 2.6 ± 3.6 | 1.0 | 0–22 |
| Vitamin D (ng/dL) | 17.5 ± 12.2 | 14.0 | 3–69 |
| Transferrin (mg/dL) | 195 ± 47 | 190 | 91–384 |
| Total lymphocyte count | 1.63 ± 0.93 | 2.05 | 0–9 |
| Measurements | Mean | p-Value | Median | Range | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| Overall muscle mass | 522 ± 15 | 533 ± 16 | 0.080 | 484 | 518 | 114–1244 | 60–1325 |
| Men | 549 ± 215 | 565 ± 226 | 0.213 | 548 | 545 | 123–1197 | 97–1169 |
| Women | 511 ± 209 | 518 ± 216 | 0.213 | 470 | 472 | 114–1244 | 60–1325 |
| Overall IMAT (%) | 29.3 ± 13.4 | 29.1 ± 12.5 | 0.751 | 25.8 | 25.6 | 3.2–82.7 | 8.5–86.2 |
| Men | 27.2 ± 11.0 | 26.9 ± 11.9 | 0.731 | 23.8 | 22.9 | 13.5–56.7 | 14.0–63.0 |
| Women | 30.2 ± 14.3 | 30.0 ± 12.6 | 0.860 | 26.9 | 26.6 | 3.2–82.7 | 8.5–86.2 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Normative Data | Current Data | Normative Data | Current Data | |||
| Age | N | Hand | Mean ± SD (kg) | Mean ± SD (kg) | Mean ± SD (kg) | Mean ± SD (kg) |
| 65–69 | 3 | R | 41.3 ± 9.3 | 0.0 ± NA | 22.5 ± 4.4 | 5.5 ± 7.8 |
| L | 34.8 ± 9.0 | 22.0 ± 22.0 | 18.6 ± 3.7 | 4.0 ± 5.7 | ||
| 70–74 | 24 | R | 34.2 ± 9.8 | 14.3 ± 16.3 | 22.5 ± 5.3 | 16.4 ± 9.8 |
| L | 29.4 ± 8.2 | 10.3 ± 10.0 | 18.8 ± 4.6 | 13.0 ± 9.2 | ||
| 75+ | 246 | R | 29.8 ± 9.5 | 14.7 ± 9.2 | 19.3 ± 5.0 | 13.0 ± 8.8 |
| L | 24.9 ± 7.7 | 14.0 ± 8.9 | 17.1 ± 4.0 | 11.9 ± 8.3 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkisas, S.; De Cock, A.-M.; Verhoeven, V.; Vandewoude, M. Intramuscular Adipose Tissue and the Functional Components of Sarcopenia in Hospitalized Geriatric Patients. Geriatrics 2017, 2, 11. https://doi.org/10.3390/geriatrics2010011
Perkisas S, De Cock A-M, Verhoeven V, Vandewoude M. Intramuscular Adipose Tissue and the Functional Components of Sarcopenia in Hospitalized Geriatric Patients. Geriatrics. 2017; 2(1):11. https://doi.org/10.3390/geriatrics2010011
Chicago/Turabian StylePerkisas, Stany, Anne-Marie De Cock, Veronique Verhoeven, and Maurits Vandewoude. 2017. "Intramuscular Adipose Tissue and the Functional Components of Sarcopenia in Hospitalized Geriatric Patients" Geriatrics 2, no. 1: 11. https://doi.org/10.3390/geriatrics2010011






