Collagenolytic Activity Is Associated with Scar Resolution in Zebrafish Hearts after Cryoinjury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Procedure
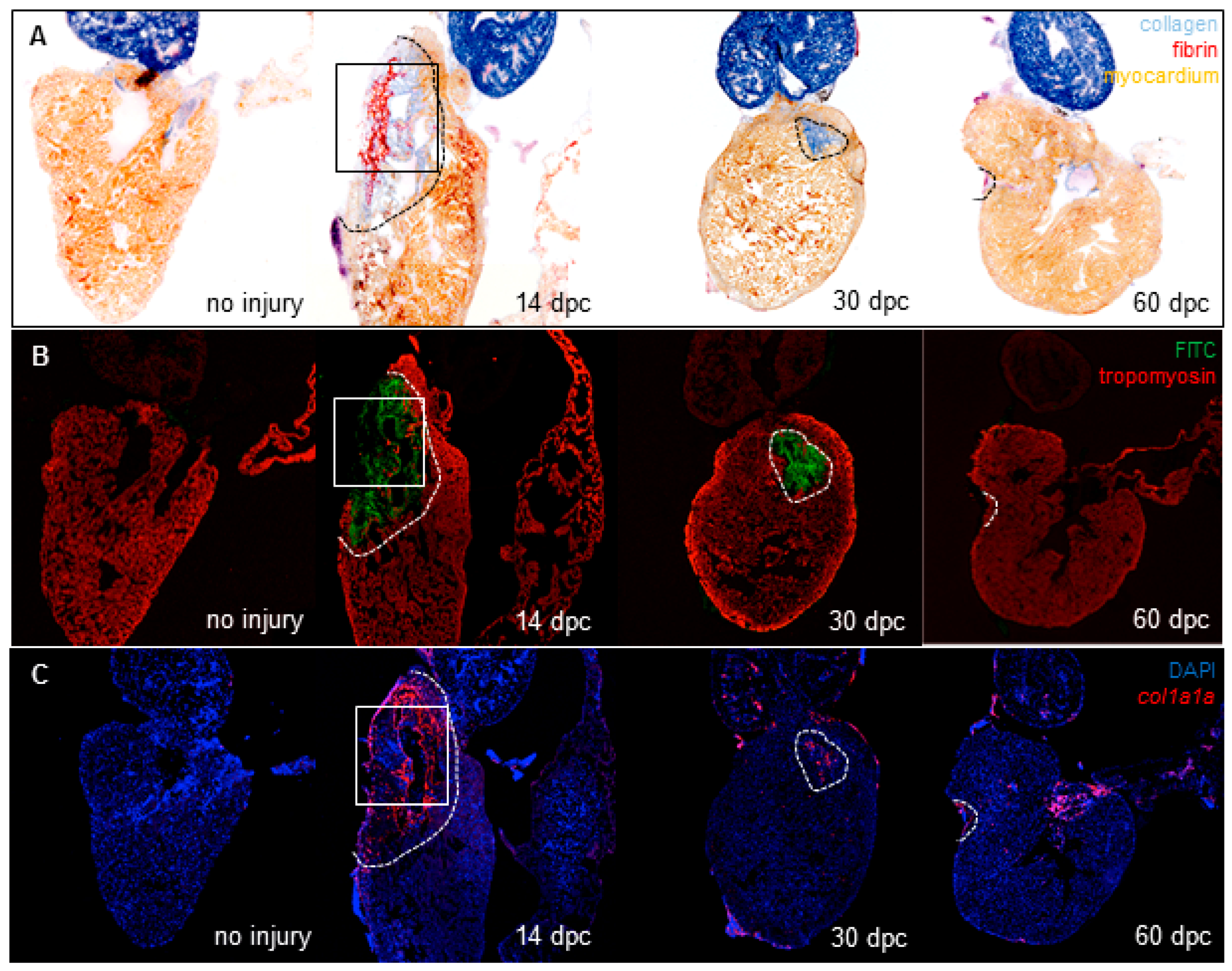
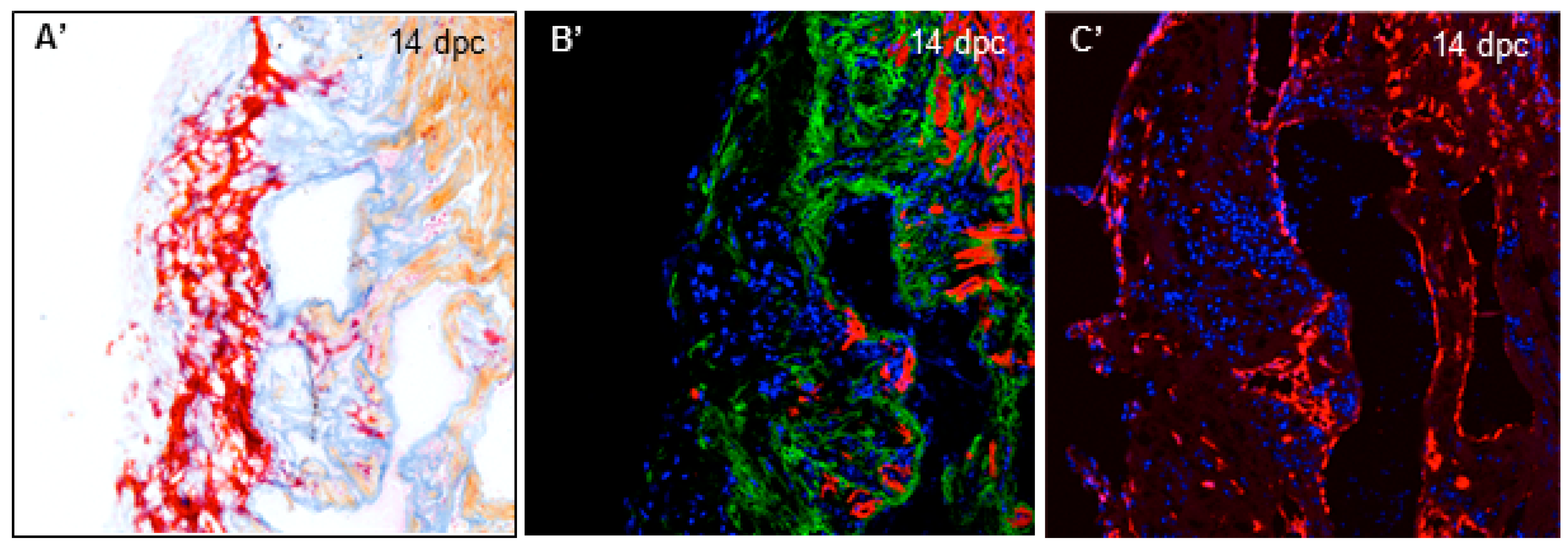
2.2. In Situ Collagen Zymography and Immunostaining
2.3. Acid Fuschin Orange G (AFOG) Staining
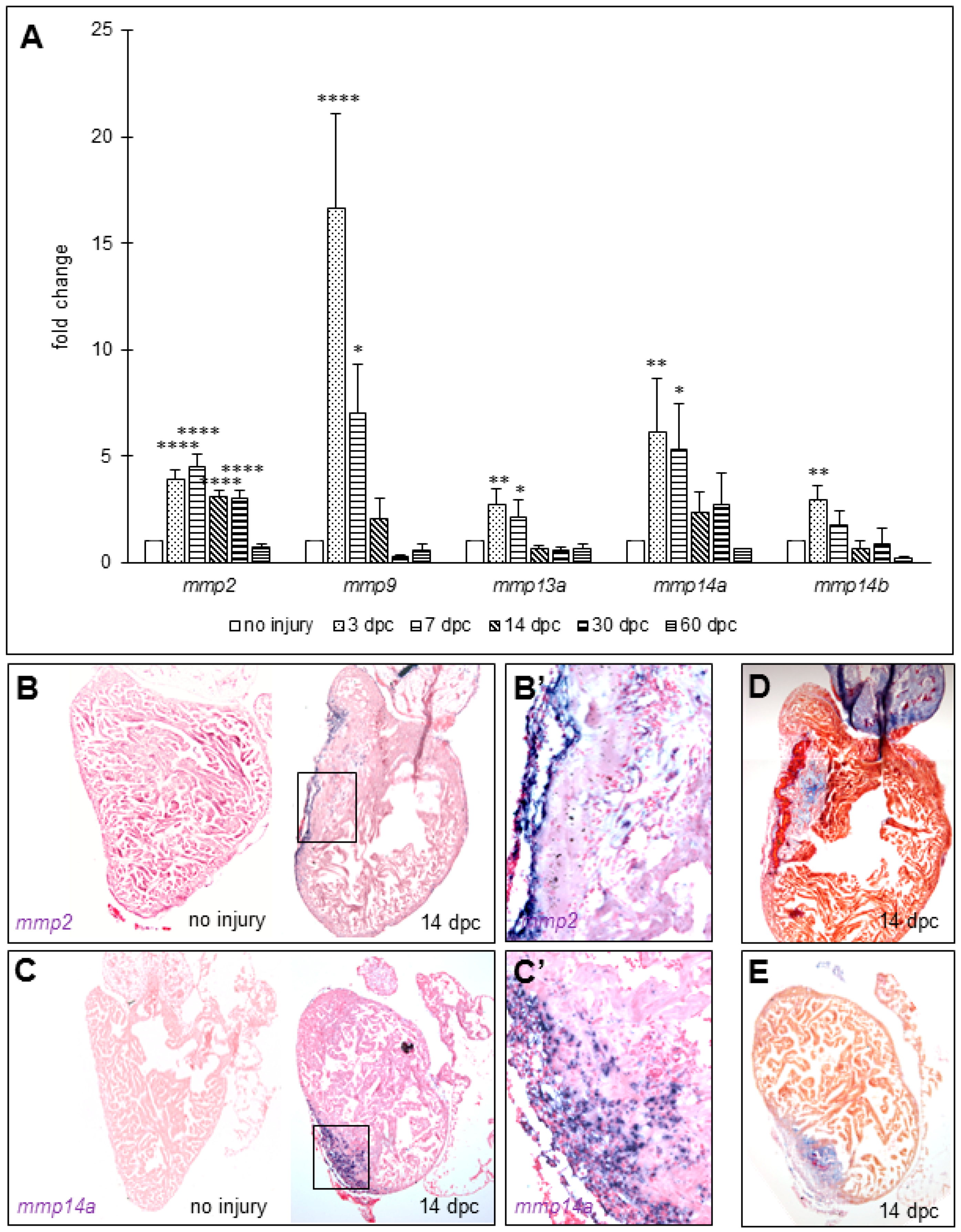
2.4. In Situ Hybridization
2.5. RT-Quantitative PCR
- mmp2 (forward CCTCACCCATCATAAAGTTCC, reverse TTTCTTCAGCGTGTCCTTCAA)
- mmp9 (forward CACCGTTGATGCCATGA, reverse TCCATGTCTGGCGAATAG)
- mmp13a (forward GAGGCTCAAGGAGATGC and reverse GTTGAGTAGGCCTTGATGT)
- mmp13b (forward GCAGAAGATCAGAGAGATGC and reverse AGAATCCTGAAGGTCACG)
- mmp14a (forward CGGGACCAGTGACAAAG, reverse CGAGATAGCGGAGTTGATAGA)
- mmp14b (forward ACAGTAACAAAGTGGTGTCA, reverse ATACTGCTGTAGCCATGC)
- eef1a1a (forward CTACAAATGCGGTGGAATC, reverse AATTTCCAGAGAGCAATGTCA)
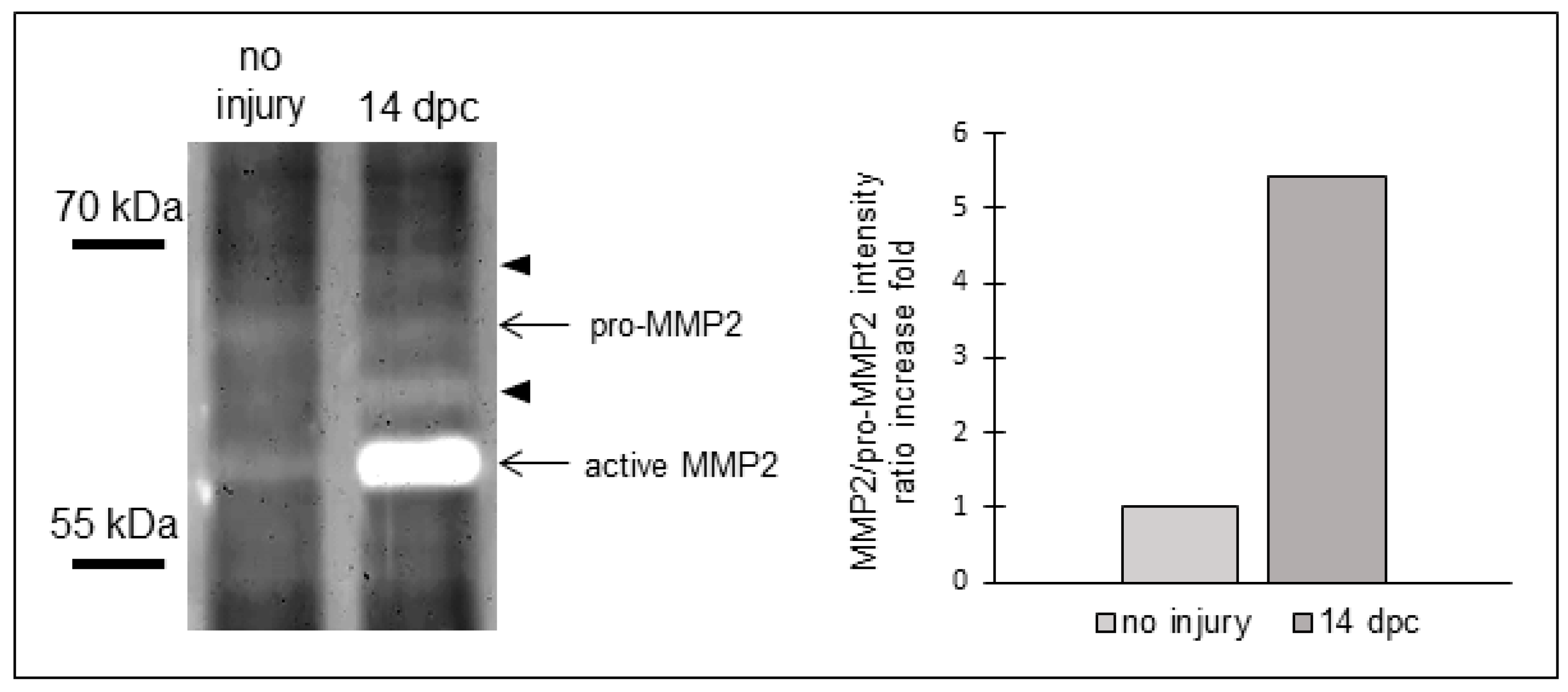
2.6. SDS-PAGE Gelatin Zymography
2.7. Confocal Imaging of Heart Sections
3. Results
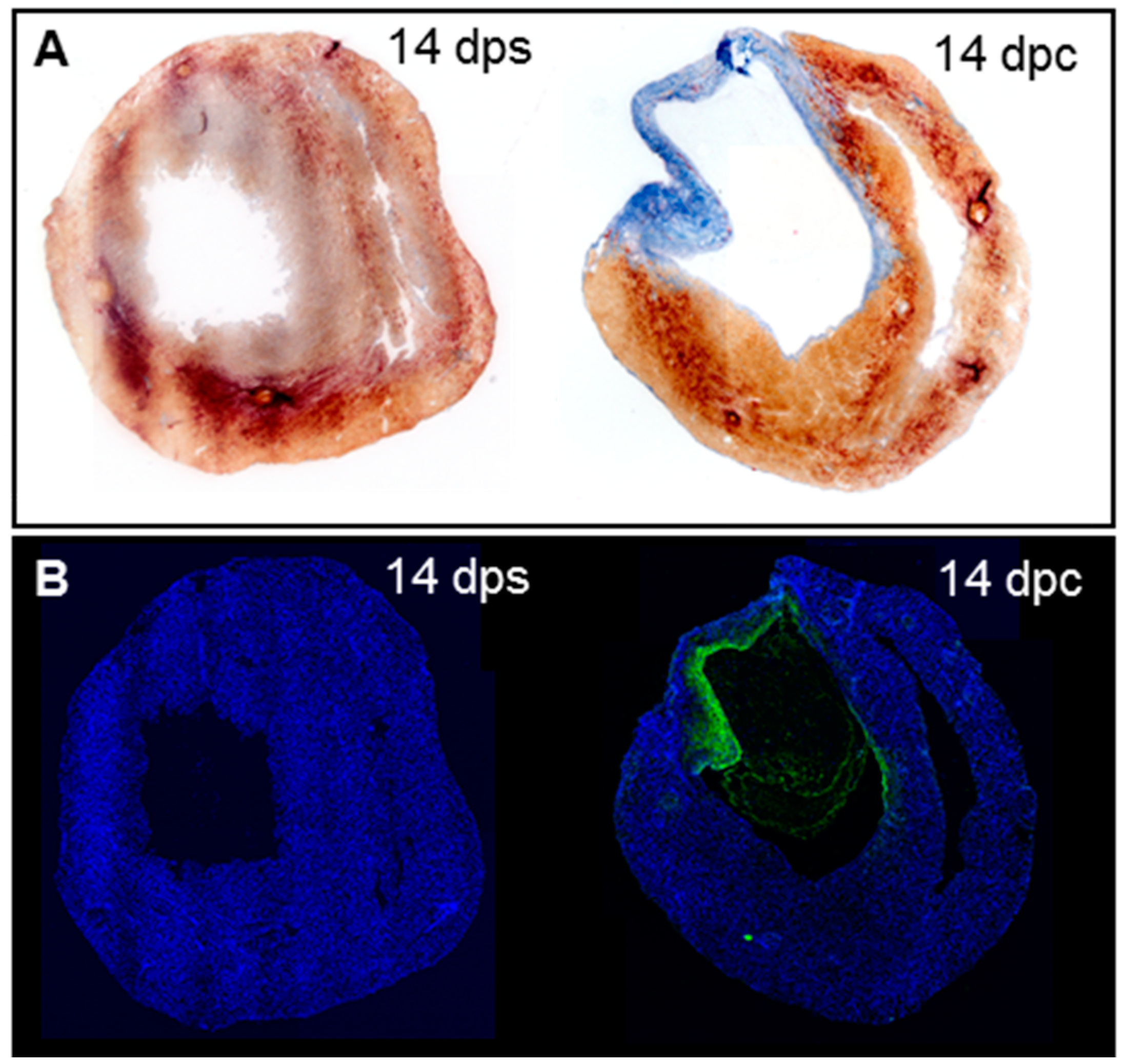
3.1. Cryoinjury Induces Extensive Collagenolytic Activity and col1a1a Expression in the Wound Area in Zebrafish Hearts
3.2. Matrix Metalloproteinases Transcripts Increase as well as MMP2 Activity Following Cryoinjury in Zebrafish Hearts
3.3. Collagenolytic Activity Is Present in the Scar Area of Neonatal Mouse Hearts Following Cryoinjury
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Izpisúa Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 2011, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Jazwinska, A. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development 2012, 139, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish, A Practical Approach; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Darehzereshki, A.; Rubin, N.; Gamba, L.; Kim, J.; Fraser, J.; Huang, Y.; Billings, J.; Mohammadzadeh, R.; Wood, J.; Warburton, D.; et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 2015, 399, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Thummel, R.; Godwin, A.R.; Nagase, H.; Itoh, Y.; Li, L.; Evans, R.; McDermott, J.; Seiki, M.; Sarras, M.P., Jr. Matrix metalloproteinase expression and function during fin regeneration in zebrafish: Analysis of MT1-MMP, MMP2 and TIMP2. Matrix Biol. 2005, 24, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.L.; Schebesta, M.; Makino, S.; Weber, G.J.; Keating, M.T. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006, 4, e260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Liu, Y.H.; Yang, X.P.; Xu, J.; Kapke, A.; Carretero, O.A. Myocardial infarction and cardiac remodelling in mice. Exp. Physiol. 2002, 87, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.; Csukasi, F.; Taylor, S.P.; Krakow, D.; Becerra, J.; Bombarely, A.; Marí-Beffa, M. Collagen duplicate genes of bone and cartilage participate during regeneration of zebrafish fin skeleton. Gene Expr. Patterns 2015, 19, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Huxley-Jones, J.; Clarke, T.K.; Beck, C.; Toubaris, G.; Robertson, D.L.; Boot-Handford, R.P. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Kleiner, D.E.; Unsworth, E.J.; Stetler-Stevenson, W.G. Cellular activation of the 72 kDa type IV procollagenase/TIMP-2 complex. Kidney Int. 1993, 43, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, E.; Sharif, F.; Metz, J.R.; Flik, G.; Richardson, M.K. Matrix metalloproteinases in osteoclasts of ontogenetic and regenerating zebrafish scales. Bone 2011, 48, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.E. Regulation of cardiovascular collagen deposition by mechanical forces. Mol. Med. Today 1998, 4, 69–75. [Google Scholar] [CrossRef]
- Van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 2016, 93, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.; Sharpe, N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Mukherjee, R.; Zavadzkas, J.A.; Koval, C.N.; Bouges, S.; Stroud, R.E.; Dobrucki, L.W.; Sinusas, A.J. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J. Biol. Chem. 2010, 285, 30316–30327. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Janicki, J.S.; Zile, M.R. Membrane-associated matrix proteolysis and heart failure. Circ. Res. 2013, 112, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, D.; González-Rosa, J.M.; Guzmán-Martínez, G.; Gutiérrez-Gutiérrez, Ó.; Aguado, T.; Sánchez-Ferrer, C.; Marques, I.J.; Galardi-Castilla, M.; de Diego, I.; Gómez, M.J.; et al. Telomerase Is Essential for Zebrafish Heart Regeneration. Cell Rep. 2015, 12, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [PubMed]
- Patterson, M.L.; Atkinson, S.J.; Knäuper, V.; Murphy, G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001, 503, 158–162. [Google Scholar] [CrossRef]
- Detry, B.; Erpicum, C.; Paupert, J.; Blacher, S.; Maillard, C.; Bruyère, F.; Pendeville, H.; Remacle, T.; Lambert, V.; Balsat, C.; et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 2012, 119, 5048–5056. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370, 61–65. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M.; Peralta, M.; Mercader, N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev. Biol. 2012, 370, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamba, L.; Amin-Javaheri, A.; Kim, J.; Warburton, D.; Lien, C.-L. Collagenolytic Activity Is Associated with Scar Resolution in Zebrafish Hearts after Cryoinjury. J. Cardiovasc. Dev. Dis. 2017, 4, 2. https://doi.org/10.3390/jcdd4010002
Gamba L, Amin-Javaheri A, Kim J, Warburton D, Lien C-L. Collagenolytic Activity Is Associated with Scar Resolution in Zebrafish Hearts after Cryoinjury. Journal of Cardiovascular Development and Disease. 2017; 4(1):2. https://doi.org/10.3390/jcdd4010002
Chicago/Turabian StyleGamba, Laurent, Armaan Amin-Javaheri, Jieun Kim, David Warburton, and Ching-Ling Lien. 2017. "Collagenolytic Activity Is Associated with Scar Resolution in Zebrafish Hearts after Cryoinjury" Journal of Cardiovascular Development and Disease 4, no. 1: 2. https://doi.org/10.3390/jcdd4010002




