What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions
Abstract
:1. Introduction
2. Topographical versus Structural Heart
3. How Three Structural Components Cause Normal Functional Dynamics
4. Mitral Valve Opening
5. Isovolumic Relaxation Time
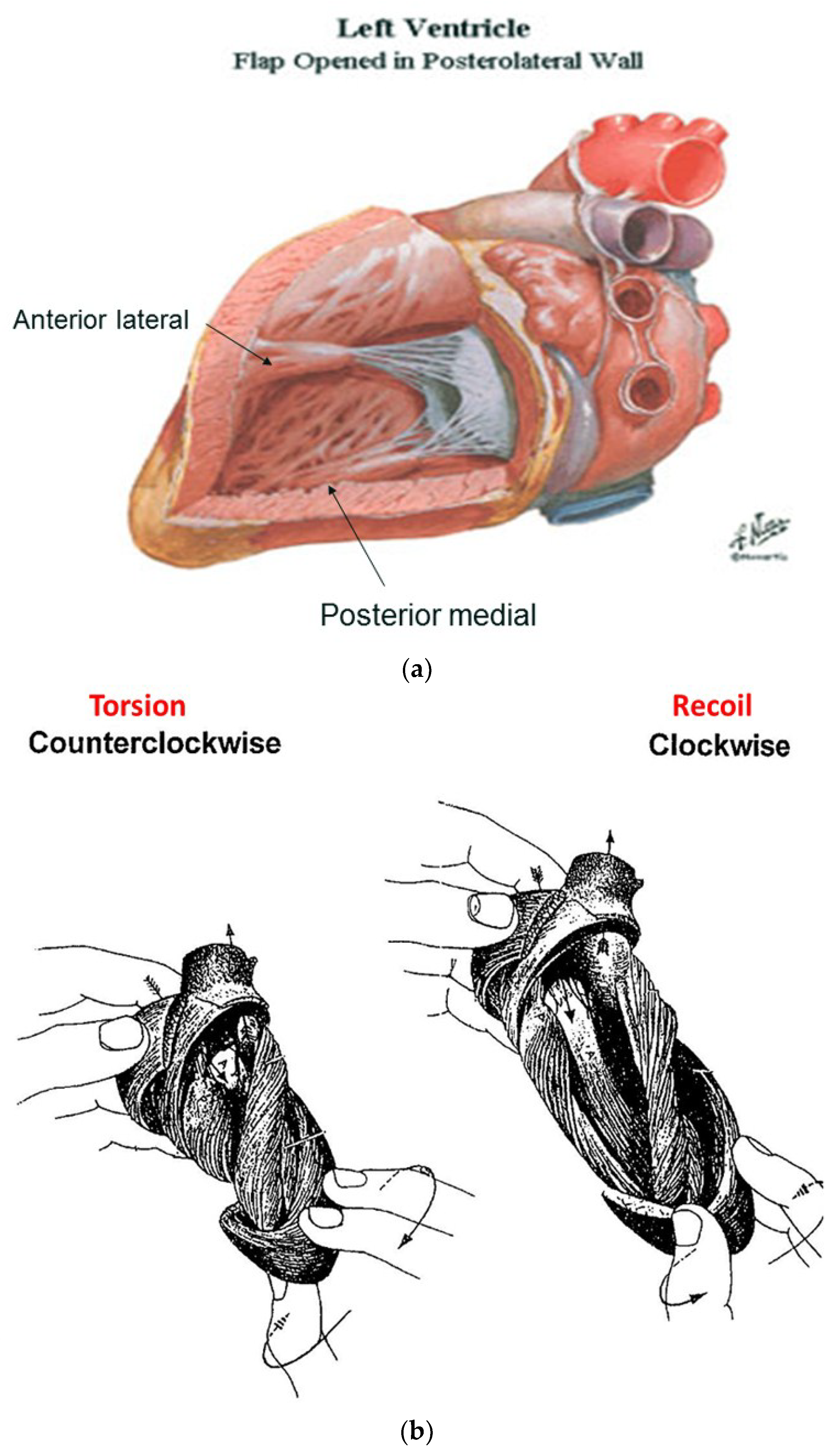
6. Twisting or Torsion
7. Untwisting
8. Longitudinal and Circumferential Strain
9. Regional Function versus HVMB
10. Resynchronization
11. Right Ventricular Function
12. Diastolic Dysfunction
13. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Lower, R. Tractus de corde 1669. In Early Science in Oxford; Gunther, R., Ed.; Sawsons: Oxford, UK, 1932. [Google Scholar]
- Senac, J.B. Traite de la Structure du Coeur; Vincent: Paris, France, 1749. [Google Scholar]
- Krehl, L. Kenntniss der fallung und entleerung des herzens. Abh. Math. Phys. 1891, 29, 341–362. [Google Scholar]
- Mall, F.P. On the muscular architecture of the ventricles of the human heart. Am. J. Anat. 1911, 11, 211–278. [Google Scholar] [CrossRef]
- Torrent-Guasp, F.; Buckberg, G.D.; Clemente, C.; Cox, J.L.; Coghlan, H.C.; Gharib, M. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I.; Mahajan, A.; Saleh, S.; Coghlan, C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation 2008, 118, 2571–2587. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I.; Nanda, N.C.; Coghlan, C.; Saleh, S.; Athanasuleas, C. Ventricular torsion and untwisting: Further insights into mechanics and timing interdependence: A viewpoint. Echocardiography 2011, 28, 782–804. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D.; Hoffman, J.I.; Coghlan, H.C.; Nanda, N.C. Ventricular structure-function relations in health and disease: Part I. The normal heart. Eur. J. Cardiothorac. Surg. 2015, 47, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.D. Anatomy: A Regional Atlas of the Human Body, 4th ed.; Williams & Wilkins: Philadelphia, PA, USA, 1997. [Google Scholar]
- Clemente, C.D. Anatomy: A Regional Atlas of the Human Body, 5th ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Moore, K.L.; Dalley, A.F.; Agur, A.M.R. Clinically Oriented Anatomy, 5th ed.; Lippincott & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Buckberg, G.D. Echogenic zone in mid-septum: Its structure/function relationship. Echocardiography 2016, 33, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Sallin, E.A. Fiber orientation and ejection fraction in the human ventricle. Biophys. J. 1969, 9, 954–964. [Google Scholar] [CrossRef]
- Borelli, G.A. History of Cardiology; Medical Life Press: New York, NY, USA, 1927. [Google Scholar]
- Harvey, W. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus; Sumptibus Guilielmi Fitzeri: UK, 1628. [Google Scholar]
- Robb, J.S.; Robb, R.C. The normal heart: Anatomy and physiology of the structural units. Am. Heart J. 1942, 23, 455–467. [Google Scholar] [CrossRef]
- Buckberg, G.D. Basic science review: The helix and the heart. J. Thorac. Cardiovasc. Surg. 2002, 124, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.P. Notes on the muscular architecture of the left ventricle. Circulation 1965, 32, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lev, M.; Simkins, C.S. Architecture of the human ventricular myocardium, technique for study using a modification of the mall-maccallum method. Lab. Investig. 1956, 8, 306–409. [Google Scholar]
- Anderson, R.H.; Siew, Y.H.; Sanchez-Quintana, D.; Redmann, K.; Lunkenheimer, P.P. Heuristic problems in defining the three-dimensional arrangement of the ventricular myocytes. Anat. Rec. 2006, 288A, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Randall, W.C. Electrical and mechanical activity of papillary muscle. Am. J. Physiol. 1970, 218, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- MacIver, D.H.; Partridge, J.B.; Agger, P.; Stephenson, R.S.; Boukens, B.J.D.; Omann, C.; Jarvis, J.C.; Zhang, H. The end of the unique myocardial band: Part II. Clinical and functional considerations. Eur. J. Cardiothorac. Surg. 2018, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- MacIver, D.H.; Stephenson, R.S.; Jensen, B.; Agger, P.; Sanchez-Quintana, D.; Jarvis, J.C.; Partridge, J.B.; Anderson, R.H. The end of the unique myocardial band: Part I. Anatomical considerations. Eur. J. Cardiothorac. Surg. 2018, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mekkaoui, C.; Porayette, P.; Jackowski, M.P.; Kostis, W.J.; Dai, G.; Sanders, S.; Sosnovik, D.E. Diffusion MRI tractography of the developing human fetal heart. PLoS ONE 2013, 8, e72795. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I. Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Kauer, F.; Michels, M.; Soliman, O.I.; Vletter, W.B.; van der Zwaan, H.B.; Ten Cate, F.J.; Geleijnse, M.L. Delayed left ventricular untwisting in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- LeGrice, I.J.; Smaill, B.H.; Chai, L.Z.; Edgar, S.G.; Gavin, J.B.; Hunter, P.J. Laminar structure of the heart: Ventricular myocyte arrangement and connective tissue architecture in the dog. Am. J. Physiol. 1995, 269, H571–H582. [Google Scholar] [CrossRef] [PubMed]
- LeGrice, I.J.; Takayama, Y.; Covell, J.W. Transverse shear along myocardial cleavage planes provides a mechanism for normal systolic wall thickening. Circ. Res. 1995, 77, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Kauer, F.; Vletter, W.B.; Soliman, O.I.; van der Zwaan, H.B.; Ten Cate, F.J.; Geleijnse, M.L. Influence of cardiac shape on left ventricular twist. J. Appl. Physiol. 2010, 108, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrent-Guasp, F.; Ballester, M.; Buckberg, G.D.; Carreras, F.; Flotats, A.; Carrio, I.; Ferreira, A.; Samuels, L.E.; Narula, J. Spatial orientation of the ventricular muscle band: Physiologic contribution and surgical implications. J. Thorac. Cardiovasc. Surg. 2001, 122, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streeter, D.D., Jr.; Spotnitz, H.M.; Patel, D.P.; Ross, J., Jr.; Sonnenblick, E.H. Fiber orientation in the canine left ventricle during diastole and systole. Circ. Res. 1969, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Keith, A. Harveian lecture on the functional anatomy of the heart. Br. Med. J. 1918, 1, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Korinek, J.; Belohlavek, M.; Narula, J.; Vannan, M.A.; Jahangir, A.; Khandheria, B.K. Left ventricular structure and function: Basic science for cardiac imaging. J. Am. Coll. Cardiol. 2006, 48, 1988–2001. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.; Vallabhajosyula, S.; Sengupta, P.P. Left ventricular twist and torsion: Research observations and clinical applications. Circ. Cardiovasc. Imaging 2015, 8, e003029. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Popovic, Z.B. Assessment of left ventricular function by cardiac ultrasound. J. Am. Coll. Cardiol. 2006, 48, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Schneider, B.; Markl, M.; Saurbier, B.; Geibel, A.; Hennig, J. Measurement of left ventricular velocities: Phase contrast MRI velocity mapping versus tissue-doppler-ultrasound in healthy volunteers. J. Cardiovasc. Magn. Reson. 2004, 6, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.H.; Pastorek, J.S.; Bundy, J.M. Delineation of normal human left ventricular twist throughout systole by tagged cine. J. Cardiovasc. Magn. Reson. 2000, 2, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Mahajan, A.; Saleh, S.; Hoffman, J.I.; Coghlan, C. Structure and function relationships of the helical ventricular myocardial band. J. Thorac. Cardiovasc. Surg. 2008, 136, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Lunkenheimer, P.P.; Muller, R.P.; Konermann, C.; Lunkenheimer, A.; Kohler, P. Architecture of the myocardium in computer-tomography. Investig. Radiol. 1984, 19, 271–278. [Google Scholar] [CrossRef]
- Agger, P.; Stephenson, R.; Dobrzynski, H.; Atkinson, A.; Iaizzo, P.; Anderson, R.; Jarvis, J.; Allan, S.; Partridge, J.; Zhao, J.; et al. Insights from echocardiography, magnetic resonance imaging, and micro-computed tomography relative to the mid-myocardial left ventricular echogenic zone. Echocardiography 2016, 33, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Hayabuchi, Y.; Sakata, M.; Kagami, S. Assessment of the helical ventricular myocardial band using standard echocardiography. Echocardiography 2015, 32, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Sosnovik, D.E.; Wang, R.; Dai, G.; Reese, T.G.; Wedeen, V.J. Diffusion MR tractography of the heart. J. Cardiovasc. Magn. Reson. 2009, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerts, L.; Bovendeerd, P.; Nicolay, K.; Arts, T. Characterization of the normal cardiac myofiber field in goat measured with MR-diffusion tensor imaging. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H139–H145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, S.K.; Liu, W.; McLean, M.; Allen, J.S.; Tan, J.; Wickline, S.A.; Yu, X. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H946–H954. [Google Scholar] [CrossRef] [PubMed]
- Castella, M.; Buckberg, G.D.; Saleh, S.; Gharib, M. Structure function interface with sequential shortening of basal and apical components of the myocardial band. Eur. J. Cardiothorac. Surg. 2005, 27, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingels, N.B., Jr.; Hansen, D.E.; Daughters, G.T.; Stinson, E.B.; Alderman, E.L.; Miller, D.C. Relation between longitudinal, circumferential, and oblique shortening and torsional deformation in the left ventricle of the transplanted human heart. Circ. Res. 1989, 64, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Beyar, R.; Shapiro, E.P.; Graves, W.L.; Rogers, W.J.; Guier, W.H.; Carey, G.A.; Soulen, R.L.; Zerhouni, E.A.; Weisfeldt, M.L.; Weiss, J.L. Quantification and validation of left ventricular wall thickening by a three-dimensional volume element magnetic resonance imaging approach. Circulation 1990, 81, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Markl, M.; Föll, D.; Hennig, J. Investigating myocardial motion by MRI using tissue phase mapping. Eur. J. Cardiothorac. Surg. 2006, 29, S150–S157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckberg, G.D.; Mahajan, A.; Jung, B.; Markl, M.; Hennig, J.; Ballester-Rodes, M. MRI myocardial motion and fiber tracking; a confirmation of knowledge from different imaging modalities. Eur. J. Cardiothorac. Surg. 2006, 29, S165–S177. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, H.; Coppola, B.A.; Hopenfeld, B.; Leifer, E.S.; McVeigh, E.R.; Omens, J.H. Transmural dispersion of myofiber mechanics—Implications for electrical heterogeneity in vivo. J. Am. Coll. Cardiol. 2007, 49, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.J.; Pohost, G.M.; Dinsmore, R.E.; Harthorne, J.W. The echocardiographic determination of mitral valve opening and closure. Correlation with hemodynamic studies in man. Circulation 1975, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Vancheri, F.; Josen, M.S.; Gibson, D.G. Discrepancies in the measurement of isovolumic relaxation time: A study comparing m mode and doppler echocardiography. Br. Heart J. 1990, 64, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Pohost, G.M.; Dinsmore, R.E.; Rubenstein, J.J.; O’Keefe, D.D.; Grantham, R.N.; Scully, H.E.; Beierholm, E.A.; Frederiksen, J.W.; Weisfeldt, M.L.; Daggett, W.M. The echocardiogram of the anterior leaflet of the mitral valve. Correlation with hemodynamic and cineroentgenographic studies in dogs. Circulation 1975, 51, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, A.G.; Gordon, D.A.; Padiyar, R.; Frechette, D. Relation of mitral valve opening and closure to left atrial and ventricular pressures in the intact dog. Am. J. Physiol. 1978, 234, H146–H151. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.J.; Hees, P.S.; Siu, C.O.; Weiss, J.L.; Shapiro, E.P. MRI assessment of LV relaxation by untwisting rate: A new isovolumic phase measure of tau. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2002–H2009. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D.; Hoffman, J.I.; Coghlan, H.C.; Nanda, N.C. Ventricular structure-function relations in health and disease: Part II. Clinical considerations. Eur. J. Cardiothorac. Surg. 2015, 47, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Karwatowski, S.P.; Brecker, S.J.; Yang, G.Z.; Firmin, D.N.; St John, S.M.; Underwood, S.R. A comparison of left ventricular myocardial velocity in diastole measured by magnetic resonance and left ventricular filling measured by doppler echocardiography. Eur. Heart J. 1996, 17, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Castella, M.; Buckberg, G.D.; Saleh, S. Diastolic dysfunction in stunned myocardium: A state of abnormal excitation-contraction coupling that is limited by Na+-H+ exchange inhibition. Eur. J. Cardiothorac. Surg. 2006, 29, S107–S114. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, C.J. The dynamics of the heart beat. In Physiology in Health and Disease, 5th ed.; Wiggers, C.J., Ed.; Henry Kimpton: London, UK, 1949; pp. 644–669. [Google Scholar]
- Stedman, T.L. Stedman’s Medical Dictionary, 28th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2005. [Google Scholar]
- Rademakers, F.E.; Buchalter, M.B.; Rogers, W.J.; Zerhouni, E.A.; Weisfeldt, M.L.; Weiss, J.L.; Shapiro, E.P. Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 1992, 85, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Stuber, M.; Scheidegger, M.B.; Fischer, S.E.; Nagel, E.; Steinemann, F.; Hess, O.M. Alterations in the local myocardial motion pattern in patients sufering from pressure overload due to aortic stenosis. Circulation 1999, 100, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Wenzelburger, F.W.; Sanderson, J.E.; Leyva, F. Exercise-induced torsional dyssynchrony relates to impaired functional capacity in patients with heart failure and normal ejection fraction. Heart 2013, 99, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, C.A.; Tyberg, J.V.; Beyar, R. Effects of ischemia on left ventricular apex rotation. An experimental study in anesthetized dogs. Circulation 1995, 92, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.A.; Yang, M.; Podszus, W.W. Mechanics of ventricular torsion. J. Biomech. 1996, 29, 745–752. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Tajik, A.J. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s rosetta stone. J. Am. Coll. Cardiol. 1997, 30, 8–18. [Google Scholar] [CrossRef]
- Maciver, D.H. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp. Clin. Cardiol. 2012, 17, 5–11. [Google Scholar] [PubMed]
- Helm, R.H.; Leclercq, C.; Faris, O.P.; Ozturk, C.; McVeigh, E.; Lardo, A.C.; Kass, D.A. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: Implications for assessing cardiac resynchronization. Circulation 2005, 111, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A. Heart’s Vortex: Intracardiac Blood Flow Phenomena, 1st ed.; People’s Medical Publishing House: Beijing, China, 2009. [Google Scholar]
- Rosenbaum, M.B.; Elizari, M.V.; Kretz, A.; Taratuto, A.L. Anatomical basis of AV conduction disturbances. Geriatrics 1970, 25, 132–144. [Google Scholar] [PubMed]
- Yue, A.M.; Betts, T.R.; Roberts, P.R.; Morgan, J.M. Global dynamic coupling of activation and repolarization in the human ventricle. Circulation 2005, 112, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Durrer, D.; van Dam, R.T.; Freud, G.E.; Janse, M.J.; Meijler, F.L.; Arzbaecher, R.C. Total excitation of the isolated human heart. Circulation 1970, 41, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Weidemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Riffel, J.H.; Keller, M.G.; Rost, F.; Arenja, N.; Andre, F.; Aus dem, S.F.; Fritz, T.; Ehlermann, P.; Taeger, T.; Frankenstein, L.; et al. Left ventricular long axis strain: A new prognosticator in non-ischemic dilated cardiomyopathy? J. Cardiovasc. Magn. Reson. 2016, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Athanasuleas, C.; Conte, J. Surgical ventricular restoration for the treatment of heart failure. Nat. Rev. Cardiol. 2012, 9, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Campana, M.; Brunelli, F.; Dalla, T.M.; Mhagna, Z.; Messina, A.; Villa, E.; Natalini, G.; Troise, G. Time series analysis of physiologic left ventricular reconstruction in ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2016, 152, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.L.; Verma, A.; Uno, H.; Shin, S.H.; Bourgoun, M.; Hassanein, A.H.; McMurray, J.J.; Velazquez, E.J.; Kober, L.; Pfeffer, M.A.; et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J. Am. Coll. Cardiol. 2010, 56, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Keyl, C.; Schneider, J.; Beyersdorf, F.; Ruile, P.; Siepe, M.; Pioch, K.; Schneider, R.; Jander, N. Right ventricular function after aortic valve replacement: A pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur. J. Cardiothorac. Surg. 2016, 49, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Popovic, Z.B.; Grimm, R.A.; Ahmad, A.; Agler, D.; Favia, M.; Dan, G.; Lim, P.; Casas, F.; Greenberg, N.L.; Thomas, J.D. Longitudinal rotation: An unrecognised motion pattern in patients with dilated cardiomyopathy. Heart 2008, 94, e11. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, H.; Grapsa, J.; Stefanadi, E.; Philippou, E.; Dawson, D.; Nihoyannopoulos, P. Is it only diastolic dysfunction? Segmental relaxation patterns and longitudinal systolic deformation in systemic hypertension. Eur. J. Echocardiogr. 2008, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Bertini, M.; Marsan, N.A.; Delgado, V.; van Bommel, R.J.; Nucifora, G.; Borleffs, C.J.; Boriani, G.; Biffi, M.; Holman, E.R.; van der Wall, E.E.; et al. Effects of cardiac resynchronization therapy on left ventricular twist. J. Am. Coll. Cardiol. 2009, 54, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Pastromas, S.; Manolis, A.S. Cardiac resynchronization therapy: Dire need for targeted left ventricular lead placement and optimal device programming. World J. Cardiol. 2014, 6, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fung, J.W.; Yip, G.W.; Chan, J.Y.; Lee, A.P.; Lam, Y.Y.; Wu, L.W.; Wu, E.B.; Yu, C.M. Improvement of left ventricular myocardial short-axis, but not long-axis function or torsion after cardiac resynchronisation therapy: An assessment by two-dimensional speckle tracking. Heart 2008, 94, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.W.; Chalil, S.; Khadjooi, K.; Irwin, N.; Smith, R.E.; Leyva, F. Left ventricular reverse remodelling, long-term clinical outcome, and mode of death after cardiac resynchronization therapy. Eur. J. Heart Fail. 2011, 13, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coghlan, H.C.; Coghlan, A.R.; Buckberg, G.D.; Gharib, M.; Cox, J.L. The structure and function of the helical heart and its buttress wrapping. III. The electric spiral of the heart: The hypothesis of the anisotropic conducting matrix. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, C.J. The muscular reactions of the mammalian ventricles to artificial surface stimuli. Am. J. Physiol. 1925, 73, 346–378. [Google Scholar] [CrossRef]
- Liakopoulos, O.; Tomioka, H.; Buckberg, G.D.; Tan, Z.; Hristov, N.; Trummer, G. Sequential deformation and physiological considerations in unipolar right or left ventricular pacing. Eur. J. Cardiothorac. Surg. 2006, 29, S188–S197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomioka, H.; Liakopoulos, O.J.; Buckberg, G.D.; Tan, Z.; Trummer, G.; Hristov, N. The effect of ventricular sequential contraction on helical heart during pacing. Eur. J. Cardiothorac. Surg. 2006, 29, S198–S206. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Barold, S.S. Direct his bundle and parahisian cardiac pacing. Ann. Noninvasive Electrocardiol. 2012, 17, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Svetlich, C.; Occhetta, E.; Catanzariti, D.; Cantu, F.; Padeletti, L.; Santini, M.; Senatore, G.; Comisso, J.; Varbaro, A.; et al. Safety and performance of a system specifically designed for selective site pacing. Pacing Clin. Electrophysiol. 2011, 34, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fontan, F.; Kirklin, J.W.; Fernandez, G.; Costa, F.; Naftel, D.C.; Tritto, F.; Blackstone, E.H. Outcome after a “perfect” fontan operation. Circulation 1990, 81, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Mery, C.M.; Peeler, B.B.; Kron, I.L.; Gangemi, J.J. Short and long-term outcomes for bidirectional glenn procedure performed with and without cardiopulmonary bypass. Ann. Thorac. Surg. 2012, 94, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H.; Lopez-Palop, R.; Bermejo, J.; Lopez-Sendon, J.L.; Delcan, J.L. In-hospital outcome of elderly patients with acute inferior myocardial infarction and right ventricular involvement. Circulation 1997, 96, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Rushmer, R.F.; Crystal, D.K.; Wagner, C. The functional anatomy of ventricular contraction. Circ. Res. 1953, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Raina, A.; Katz, D.; Szerlip, M.; Wiegers, S.E.; Forfia, P.R. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest 2011, 140, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Liakopoulos, O.J.; Buckberg, G.D. The septal motor of biventricular function. Eur. J. Cardiothorac. Surg. 2006, 29, S126–S138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawatani, S.; Mandell, C.; Kusaba, E. Ventricular performance following ablation and prosthetic replacement of right ventricular myocardium. Trans. Am. Artif. Intern. Organs 1974, 20, 629–636. [Google Scholar]
- Starr, I.; Jeffers, W.A.; Meade, R.H. The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am. Heart J. 1943, 26, 291–301. [Google Scholar] [CrossRef]
- Cox, J.L.; Bardy, G.H.; Damiano, R.J., Jr.; German, L.D.; Fedor, J.M.; Kisslo, J.A.; Packer, D.L.; Gallagher, J.J. Right ventricular isolation procedures for nonischemic ventricular tachycardia. J. Thorac. Cardiovasc. Surg. 1985, 90, 212–224. [Google Scholar] [PubMed]
- Donald, D.E.; Essex, H.E. Pressure studies after inactivation of the major portion of the canine right ventricle. Am. J. Physiol. 1954, 176, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, C.H.; Kwak, J.G.; Kim, S.H.; Shim, W.S.; Lee, S.Y.; Baek, J.S.; Jang, S.I.; Kim, Y.M. Does limited right ventriculotomy prevent right ventricular dilatation and dysfunction in patients who undergo transannular repair of tetralogy of fallot? Matched comparison of magnetic resonance imaging parameters with conventional right ventriculotomy long-term after repair. J. Thorac. Cardiovasc. Surg. 2014, 147, 889–895. [Google Scholar] [PubMed]
- Reynolds, H.R.; Tunick, P.A.; Grossi, E.A.; Dilmanian, H.; Colvin, S.B.; Kronzon, I. Paradoxical septal motion after cardiac surgery: A review of 3292 cases. Clin. Cardiol. 2007, 30, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Neragi-Miandoab, S.; Goldstein, D.; Bello, R.; Michler, R.; D’Alessandro, D. Right ventricular dysfunction following continuous flow left ventricular assist device placement in 51 patients: Predicators and outcomes. J. Cardiothorac. Surg. 2012, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and diastolic heart failure in the community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Notomi, Y.; Martin-Miklovic, M.G.; Oryszak, S.J.; Shiota, T.; Deserranno, D.; Popovic, Z.B.; Garcia, M.J.; Greenberg, N.L.; Thomas, J.D. Enhanced ventricular untwisting during exercise: A mechanistic manifestation of elastic recoil described by doppler tissue imaging. Circulation 2006, 113, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Torrent-Guasp, F.; Kocica, M.J.; Corno, A.; Komeda, M.; Cox, J.; Flotats, A.; Ballester-Rodes, M.; Carreras-Costa, F. Systolic ventricular filling. Eur. J. Cardiothorac. Surg. 2004, 25, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.M.; Zile, M.R. New molecular mechanism in diastolic heart failure. Circulation 2006, 113, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Torrent-Guasp, F. El Cicio Cardiaco; Espasa-Calpe: Madrid, Spain, 1954. [Google Scholar]
- Sabbah, H.N.; Stein, P.D. Pressure-diameter relations during early diastole in dogs. Incompatibility with the concept of passive left ventricular filling. Circ. Res. 1981, 48, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.N. The role played by the ventricular relaxation process in filling the ventricle. Am. J. Physiol. 1930, 95, 542–553. [Google Scholar] [CrossRef]
- Brecher, G.A. Experimental evidence of ventricular diastolic suction. Circ. Res. 1956, 4, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bloom, W.L.; Ferris, E.B. Elastic recoil of the heart as a factor in diastolic filling. Trans. Assoc. Am. Physicians 1956, 69, 200–206. [Google Scholar] [PubMed]
- Fowler, N.O.; Couves, C.; Bewick, J. Effect of inflow obstruction and rapid bleeding on ventricular diastolic pressure. J. Thorac. Surg. 1958, 35, 532–537. [Google Scholar] [PubMed]
- Wiggers, C.J. Studies on the consecutive phases of the cardiac cycle. Am. J. Physiol. 1921, 56, 415–459. [Google Scholar]
- Roberts, W.C.; Brownlee, W.J.; Jones, A.A.; Luke, J.L. Sucking action of the left ventricle: Demonstration of a physiologic principle by a gunshot wound penetrating only the right side of the heart. Am. J. Cardiol. 1979, 43, 1234–1237. [Google Scholar] [CrossRef]
- Van Dalen, B.M.; Soliman, O.I.; Vletter, W.B.; Ten Cate, F.J.; Geleijnse, M.L. Age-related changes in the biomechanics of left ventricular twist measured by speckle tracking echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1705–H1711. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Soliman, O.I.; Kauer, F.; Vletter, W.B.; Zwaan, H.B.; Cate, F.J.; Geleijnse, M.L. Alterations in left ventricular untwisting with ageing. Circ. J. 2010, 74, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Tzikas, A.; Soliman, O.I.; Kauer, F.; Heuvelman, H.J.; Vletter, W.B.; Ten Cate, F.J.; Geleijnse, M.L. Left ventricular twist and untwist in aortic stenosis. Int. J. Cardiol. 2011, 148, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Soliman, O.I.; Vletter, W.B.; Ten Cate, F.J.; Geleijnse, M.L. Left ventricular untwisting in restrictive and pseudorestrictive left ventricular filling: Novel insights into diastology. Echocardiography 2010, 27, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.P.; Rademakers, F.E. Importance of oblique fiber orientation for left ventricular wall deformation. Technol. Health Care 1997, 5, 21–28. [Google Scholar] [PubMed]
- Zile, M.R.; Brutsaert, D.L. New concepts in diastolic dysfunction and diastolic heart failure: Part I: Diagnosis, prognosis, and measurements of diastolic function. Circulation 2002, 105, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Heatlie, G.; Leyva, F.; Patel, K.; Frenneaux, M.; Sanderson, J.E. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J. Am. Coll. Cardiol. 2009, 54, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Pacileo, G.; Baldini, L.; Limongelli, G.; Di Salvo, G.; Iacomino, M.; Capogrosso, C.; Rea, A.; D’Andrea, A.; Russo, M.G.; Calabro, R. Prolonged left ventricular twist in cardiomyopathies: A potential link between systolic and diastolic dysfunction. Eur. J. Echocardiogr. 2011, 12, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Van Der, T.A.; Barenbrug, P.; Snoep, G.; Van Der Veen, F.H.; Delhaas, T.; Prinzen, F.W.; Maessen, J.; Arts, T. Transmural gradients of cardiac myofiber shortening in aortic valve stenosis patients using MRI tagging. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1609–H1615. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Athanasuleas, C.; Saleh, S. Septal myocardial protection during cardiac surgery for prevention of right ventricular dysfunction. Anadolu Kardiyol. Derg. 2008, 8, 108–116. [Google Scholar] [PubMed]
- Bhaya, M.; Sudhakar, S.; Sadat, K.; Beniwal, R.; Joshi, D.; George, J.F.; Nanda, N.C.; Buckberg, G.D.; Athanasuleas, C.L. Effects of antegrade versus integrated blood cardioplegia on left ventricular function evaluated by echocardiographic real-time 3-dimensional speckle tracking. J. Thorac. Cardiovasc. Surg. 2014, 149, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Djaiani, G.N.; McCreath, B.J.; Ti, L.K.; Mackensen, B.G.; Podgoreanu, M.; Phillips-Bute, B.; Mathew, J.P. Mitral flow propagation velocity identifies patients with abnormal diastolic function during coronary artery bypass graft surgery. Anesth. Analg. 2002, 95, 524–530. [Google Scholar] [PubMed]
- Lappas, D.G.; Skubas, N.J.; Lappas, G.D.; Ruocco, E.; Tambassis, E.; Pasque, M. Prevalence of left ventricular diastolic filling abnormalities in adult cardiac surgical patients: An intraoperative echocardiographic study. Semin. Thorac. Cardiovasc. Surg. 1999, 11, 125–133. [Google Scholar] [CrossRef]
- Apostolakis, E.E.; Baikoussis, N.G.; Parissis, H.; Siminelakis, S.N.; Papadopoulos, G.S. Left ventricular diastolic dysfunction of the cardiac surgery patient; a point of view for the cardiac surgeon and cardio-anesthesiologist. J. Cardiothorac. Surg. 2009, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Denault, A.Y.; Couture, P.; Butnaru, A.; Carrier, M.; Tardif, J.C. Biventricular diastolic filling patterns after coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2006, 131, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.; Marchandise, B.; Schoevaerdts, J.C.; Kremer, R. Paradoxical ventricular septal motion after cardiac surgery. Analysis of m-mode echocardiograms and follow-up in 324 patients. Acta Cardiol. 1985, 40, 315–324. [Google Scholar] [PubMed]














© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckberg, G.D.; Nanda, N.C.; Nguyen, C.; Kocica, M.J. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J. Cardiovasc. Dev. Dis. 2018, 5, 33. https://doi.org/10.3390/jcdd5020033
Buckberg GD, Nanda NC, Nguyen C, Kocica MJ. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. Journal of Cardiovascular Development and Disease. 2018; 5(2):33. https://doi.org/10.3390/jcdd5020033
Chicago/Turabian StyleBuckberg, Gerald D., Navin C. Nanda, Christopher Nguyen, and Mladen J. Kocica. 2018. "What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions" Journal of Cardiovascular Development and Disease 5, no. 2: 33. https://doi.org/10.3390/jcdd5020033






