Diversity of Arbuscular Mycorrhizal Fungi of the Rhizosphere of Lycium barbarum L. from Four Main Producing Areas in Northwest China and Their Effect on Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Locations and Soil Sampling
2.2. Sample Pretreatment
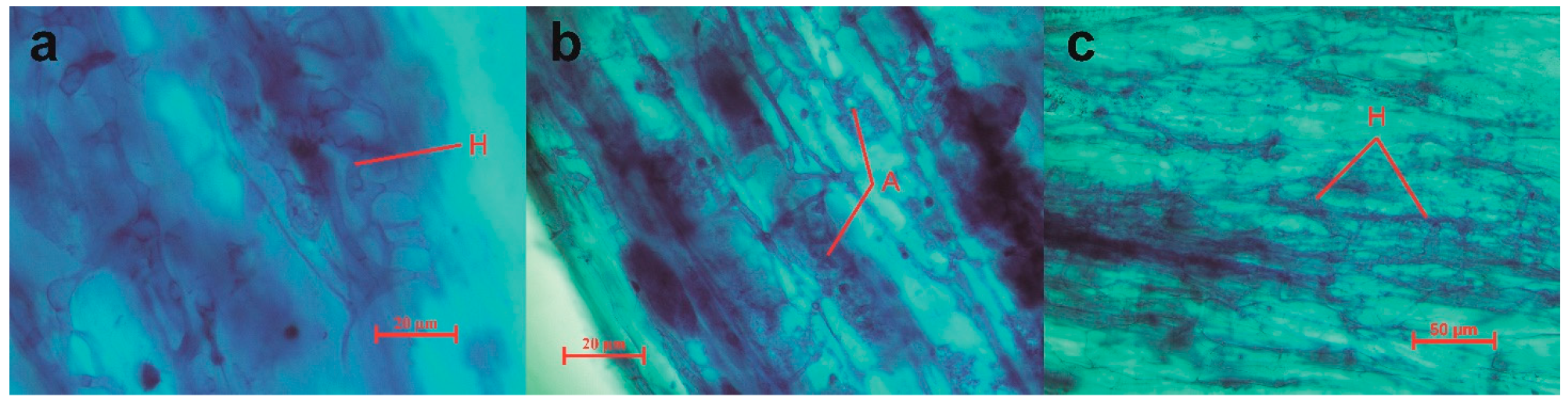
2.3. Quantification of AMF Root Colonization
2.4. Extraction of the AMF Spores
2.5. Morphological Identification of the AMF Spores
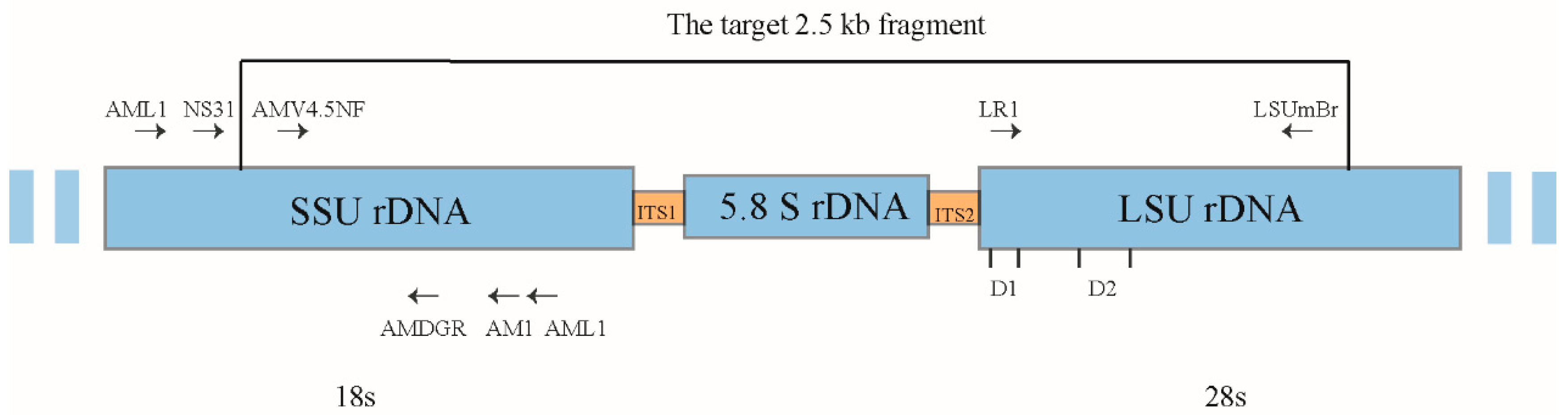
2.6. Molecular Identification of AMF
2.7. Multiplication of AMF Spores
2.8. Analysis of Effects by Inoculation on Plant Growth Parameters
2.9. Statistical Analysis
3. Results
3.1. Species Diversity of AMF in Roots of L. barbarum
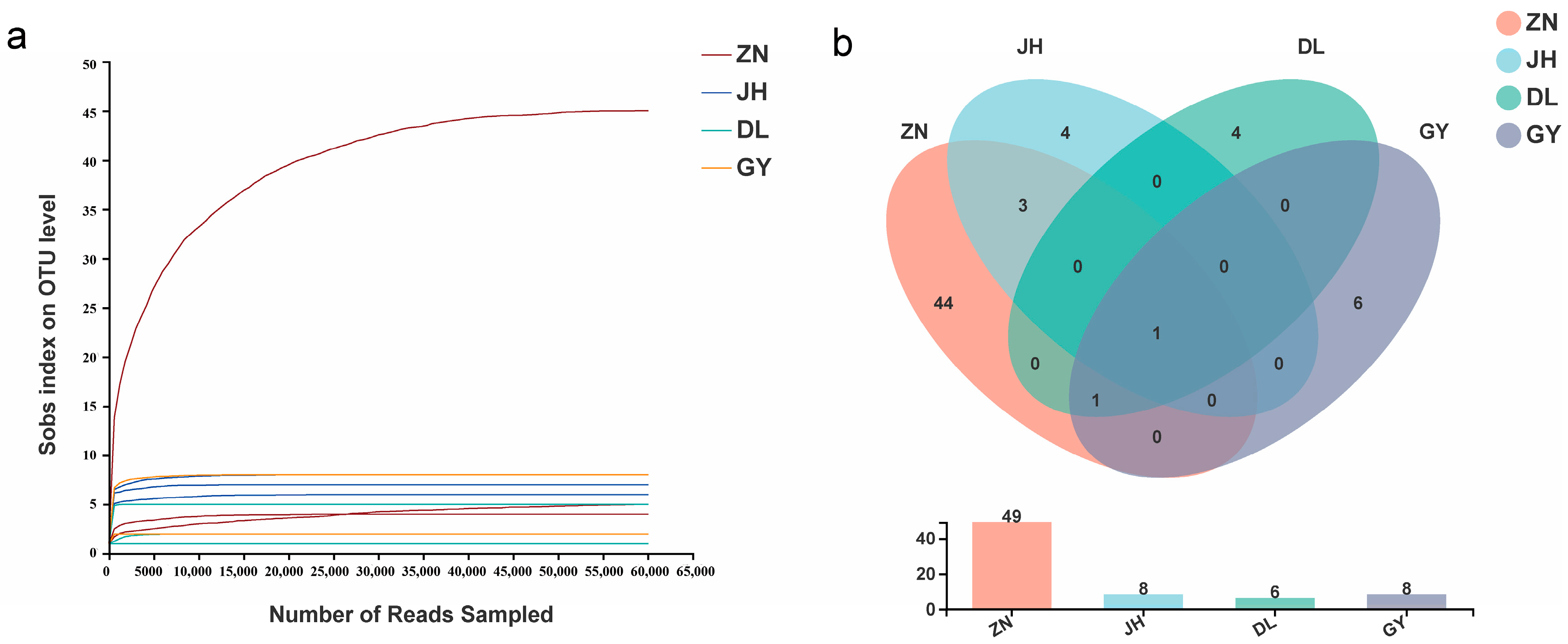
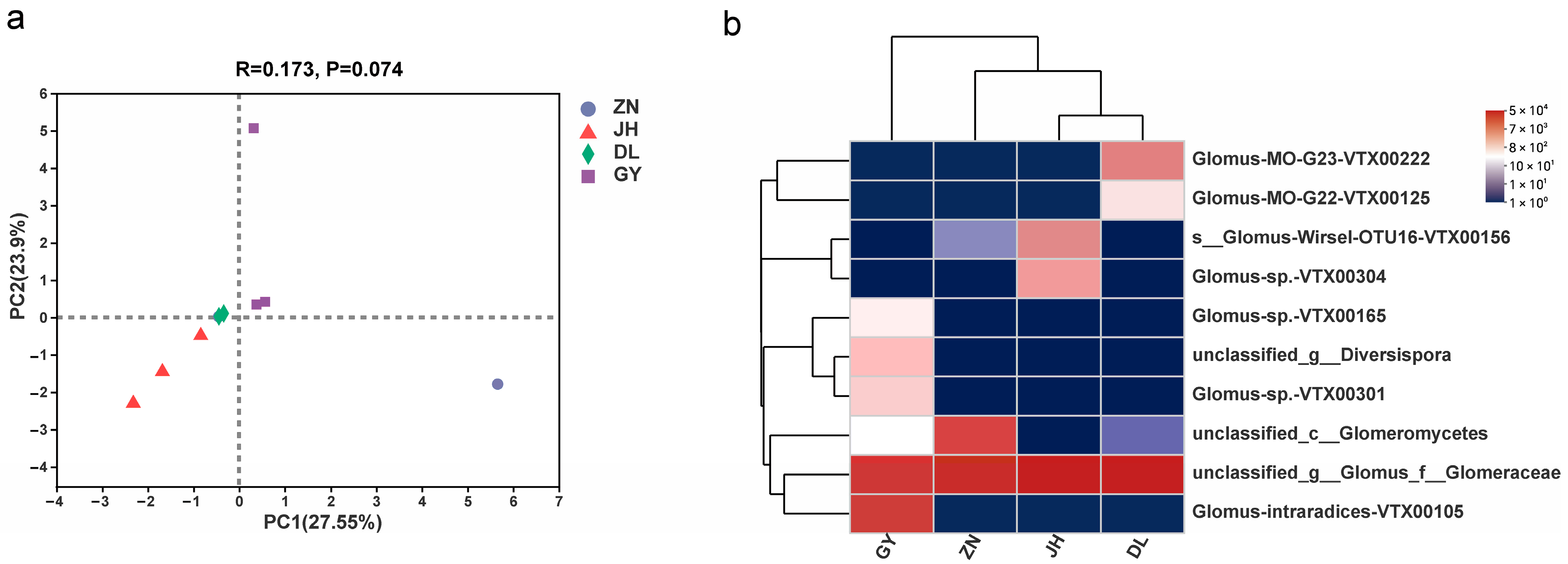
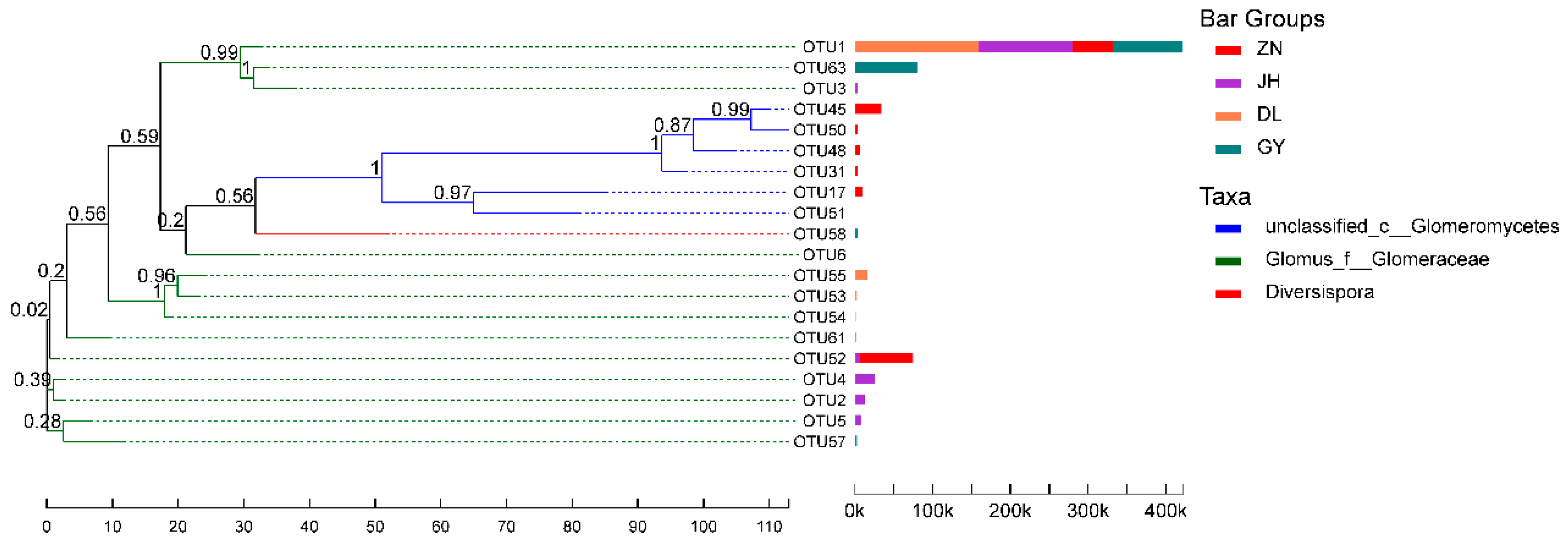
3.2. Species Diversity of Rhizosphere AMF in L. barbarum
3.3. Post-Expansion Result Test
3.4. Effect of Inoculation with AMF on the Biomass, Root, and Aboveground mass Element Uptake of L. barbarum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of characteristic aroma volatiles of Ningxia goji berries (Lycium barbarum L.) and their developmental changes. Int. J. Food Prop. 2017, 20, S214–S227. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lam, S.C.; Cheong, K.-L.; Wei, F.; Lin, P.-C.; Long, Z.-R.; Lv, X.; Zhao, J.; Ma, S.; Li, S.-P. Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016, 129, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-B.; Kang, Y.; Liu, S.-H.; Liu, S.-P. Alkaline phosphatase activity and its relationship to soil properties in a saline-sodic soil reclaimed by cropping wolfberry (Lycium barbarum L.) with drip irrigation. Paddy Water Environ. 2014, 12, 309–317. [Google Scholar] [CrossRef]
- Paulo, P.J.; Coutinho, M.B.; da Silva, M.d.C.S.; Reis, V.T.G.; Luiz, S.S.; Alves, F.R.B.; Sá, M.E.d.; Megumi, K.M.C. Agroecological coffee management increases arbuscular mycorrhizal fungi diversity. PLoS ONE 2019, 14, e0209093. [Google Scholar]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root metabolome of plant-arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef]
- Moreno Jiménez, E.; Ferrol, N.; Corradi, N.; Peñalosa, J.M.; Rillig, M.C. The potential of arbuscular mycorrhizal fungi to enhance metallic micronutrient uptake and mitigate food contamination in agriculture: Prospects and challenges. New Phytol. 2023. early view. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Sharma, M.P.; Maiti, D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr. Sci. 2015, 108, 1288–1293. [Google Scholar]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; Heijden, M.G.A.v.d. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Dierks, J.; Blaser-Hart, W.J.; Gamper, H.A.; Nyoka, I.B.; Barrios, E.; Six, J. Trees enhance abundance of arbuscular mycorrhizal fungi, soil structure, and nutrient retention in low-input maize cropping systems. Agric. Ecosyst. Environ. 2021, 318, 107487. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Han, G.; Wang, W.; Li, X.; Cheng, B. Identification and functional characterization of a maize phosphate transporter induced by mycorrhiza formation. Plant Cell Physiol. 2018, 59, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Powell, J.R.; Allsopp, P.G.; Sallam, N.; Johnson, S.N. Arbuscular mycorrhizal fungi promote silicon accumulation in plant roots, reducing the impacts of root herbivory. Plant Soil 2017, 419, 423–433. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Li, C.; Wang, L.; Liang, X.; Shi, J.; Zhan, F. Arbuscular Mycorrhizal Fungi Promote the Degradation of the Fore-Rotating Crop (Brassica napus L.) Straw, Improve the Growth of and Reduce the Cadmium and Lead Content in the Subsequent Maize. Agronomy 2023, 13, 767. [Google Scholar] [CrossRef]
- Correia, T.S.; Lara, T.S.; Santos, J.A.D.; Sousa, L.D.S.; Santana, M.D.F. Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits. Plants 2022, 11, 2731. [Google Scholar] [CrossRef]
- Liu, S.; Moora, M.; Vasar, M.; Zobel, M.; Öpik, M.; Koorem, K. Arbuscular mycorrhizal fungi promote small-scale vegetation recovery in the forest understorey. Oecologia 2021, 197, 685–697. [Google Scholar] [CrossRef]
- Opik, M.; Davison, J. Uniting species-and community-oriented approaches to understand arbuscular mycorrhizal fungal diversity. Fungal Ecol. 2016, 24, 106–113. [Google Scholar] [CrossRef]
- Vieira, L.C.; da Silva, D.K.A.; de Melo, M.A.C.; Escobar, I.E.C.; Oehl, F.; da Silva, G.A. Edaphic Factors Influence the Distribution of Arbuscular Mycorrhizal Fungi Along an Altitudinal Gradient of a Tropical Mountain. Microb. Ecol. 2019, 78, 904–913. [Google Scholar] [CrossRef]
- Kohout, P.; Sudová, R.; Janoušková, M.; Čtvrtlíková, M.; Hejda, M.; Pánková, H.; Slavíková, R.; Štajerová, K.; Vosátka, M.; Sýkorová, Z. Comparison of commonly used primer sets for evaluating arbuscular mycorrhizal fungal communities: Is there a universal solution? Soil Biol. Biochem. 2014, 68, 482–493. [Google Scholar] [CrossRef]
- Zuzana, K.; Renata, S.; Claudia, K.; Manuela, K.; Petr, K. PacBio sequencing of Glomeromycota rDNA: A novel amplicon covering all widely used ribosomal barcoding regions and its applicability in taxonomy and ecology of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 490–499. [Google Scholar]
- Vieira, C.K.; Marascalchi, M.N.; Rodrigues, A.V.; Armas, R.D.d.; Stürmer, S.L. Morphological and molecular diversity of arbuscular mycorrhizal fungi in revegetated iron-mining site has the same magnitude of adjacent pristine ecosystems. J. Environ. Sci. 2018, 67, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Feng, H.Y. Systematic classification and community research techniques of arbuscular mycorrhizal fungi: A review. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2010, 21, 1573–1580. [Google Scholar]
- Jiang, S.; Hu, X.; Kang, Y.; Xie, C.; An, X.; Dong, C.; Xu, Y.; Shen, Q. Arbuscular mycorrhizal fungal communities in the rhizospheric soil of litchi and mango orchards as affected by geographic distance, soil properties and manure input. Appl. Soil Ecol. 2020, 152, 103593. [Google Scholar] [CrossRef]
- Gabriele, C.; Erica, L.; Enrico, E.; Francesco, D.; Maria, G.; Annamaria, A.; Luigi, M.; Anna, F.; Marco, M. The abundance and diversity of arbuscular mycorrhizal fungi are linked to the soil chemistry of screes and to slope in the Alpic paleo-endemic Berardia subacaulis. PLoS ONE 2017, 12, e0171866. [Google Scholar]
- Zhao, F.; Feng, X.; Guo, Y.; Ren, C.; Wang, J.; Doughty, R. Elevation gradients affect the differences of arbuscular mycorrhizal fungi diversity between root and rhizosphere soil. Agric. For. Meteorol. 2020, 284, 107894. [Google Scholar] [CrossRef]
- Osborne, O.G.; De-Kayne, R.; Bidartondo, M.I.; Hutton, I.; Baker, W.J.; Turnbull, C.G.; Savolainen, V. Arbuscular mycorrhizal fungi promote coexistence and niche divergence of sympatric palm species on a remote oceanic island. New Phytol. 2018, 217, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhu, L.; Wang, J.; Liu, X.; Jia, Z.; Li, C.; Liu, J.; Zeng, J.; Zhang, J. Arbuscular Mycorrhizal Fungi Promote Gleditsia sinensis Lam. Root Growth under Salt Stress by Regulating Nutrient Uptake and Physiology. Forests 2022, 13, 688. [Google Scholar]
- Edmundo, B.; Barbara, G.H.; Abram, B.; Emma, S.; Ronnie, B.; Soren, M.; Caterina, B.; Pablo, T. The 10 Elements of Agroecology: Enabling transitions towards sustainable agriculture and food systems through visual narratives. Ecosyst. People 2020, 16, 230–247. [Google Scholar]
- Wang, S.Y.; Wei, H.; Chen, K.Y.; Dong, Q.; Ji, J.M.; Zhang, J. Practical methods for arbuscular mycorrhizal fungal spore density, hyphal density and colonization rate of AMF. Bio 2021, 101, e2104253. [Google Scholar]
- Berza, B.; Pagano, M.C.; Prabavathy, V.R.; Belay, Z.; Assefa, F. Arbuscular mycorrhizal status of Erythrina brucei in different land use types in Ethiopia. Appl. Soil Ecol. 2021, 165, 104018. [Google Scholar] [CrossRef]
- Morton, J.B. INVAM Newsletters; West Virginia University: Morgantown, WV, USA, 1991; Volume 1–5. [Google Scholar]
- Sato, H.; Wada, Y.; Itabashi, T.; Nakamura, M.; Kawamura, M.; Tamai, M. Mutations in the Pre-mRNA Splicing Gene, PRPF31, in Japanese Families with Autosomal Dominant Retinitis Pigmentosa. Am. J. Ophthalmol. 2005, 140, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-Q.; Zheng, Z.-Y.; Xu, X.; Hu, Z.-H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Kaili, C.; Gang, H.; Yuekun, L.; Xinrui, Z.; Yonghui, L.; Yang, L.; Jie, X.; Yanfei, S. Illumina MiSeq Sequencing Reveals Correlations among Fruit Ingredients, Environmental Factors, and AMF Communities in Three Lycium barbarum Producing Regions of China. Microbiol. Spectr. 2022, 10, e0229321. [Google Scholar]
- Pánková, H.; Münzbergová, Z.; Rydlová, J.; Vosátka, M. Co-Adaptation of Plants and Communities of Arbuscular Mycorrhizal Fungi to Their Soil Conditions. Folia Geobot. 2014, 49, 521–540. [Google Scholar] [CrossRef]
- Chourasiya, D.; Gupta, M.M.; Sahni, S.; Oehl, F.; Agnihotri, R.; Buade, R.; Maheshwari, H.S.; Prakash, A.; Sharma, M.P. Unraveling the AM fungal community for understanding its ecosystem resilience to changed climate in agroecosystems. Symbiosis 2021, 84, 295–310. [Google Scholar] [CrossRef]
- Bauer, J.T.; Koziol, L.; Bever, J.D. Local adaptation of mycorrhizae communities changes plant community composition and increases aboveground productivity. Oecologia 2020, 192, 735–744. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Ramos, R.J.; Sturmer, S.L.; Bever, J.D. Environmental identification of arbuscular mycorrhizal fungi using the LSU rDNA gene region: An expanded database and improved pipeline. Mycorrhiza 2022, 32, 145–153. [Google Scholar] [CrossRef]
- Kryukov, A.A.; Gorbunova, A.O.; Machs, E.M.; Mikhaylova, Y.V.; Rodionov, A.V.; Zhurbenko, P.M.; Yurkov, A.P. Perspectives of using Illumina MiSeq for identification of arbuscular mycorrhizal fungi. Vavilov J. Genet. Breed. 2020, 24, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Maxaieie, V.Í.M.; Andrea, B.; Alberto, O.; Samuele, V.; Valeria, B.; Erica, L. High-Throughput DNA Sequence-Based Analysis of AMF Communities. Methods Mol. Biol. 2020, 2146, 99–116. [Google Scholar]
- Thomas, C.; Cyril, A.; Dirk, R.; Lucie, B.; Nicolas, C.; Clément, R.; Linda, G.; Yvon, C.; Hamid, A. New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci. Rep. 2017, 7, 14306. [Google Scholar]
- Sarra, O.; Erica, L.; Valeria, B.; Habib, K.; Mustapha, E. Diversity of Arbuscular Mycorrhizal Fungi in olive orchard soils in arid regions of Southern Tunisia. Arid Land Res. Manag. 2022, 36, 411–427. [Google Scholar]
- Yang, M.; Shi, Z.-y.; Mickan, B.S.; Zhang, M.; Cao, L.X. Alterations to arbuscular mycorrhizal fungal community composition is driven by warming at specific elevations. PeerJ 2021, 9, e11792. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.M.; Naqvi, N.S.; Singh, V.K. The state of arbuscular mycorrhizal fungal diversity in India: An analysis. Sydowia 2014, 66, 265–288. [Google Scholar]
- de Oliveira Freitas, R.; Buscardo, E.; Nagy, L.; dos Santos Maciel, A.B.; Carrenho, R.; Luizão, R.C. Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 2014, 24, 21–32. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, Z. Biodiversity of arbuscular mycorrhizal fungi in the hot-dry valley of the Jinsha River, southwest China. Appl. Soil Ecol. 2007, 37, 118–128. [Google Scholar]
- Stutz, J.C.; Copeman, R.; Martin, C.A.; Morton, J.B. Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can. J. Bot. 2000, 78, 237–245. [Google Scholar]
- Qin, S.; Feng, W.W.; Zhang, Y.J.; Wang, T.T.; Xiong, Y.W.; Xing, K. Diversity of Bacterial Microbiota of Coastal Halophyte Limonium sinense and Amelioration of Salinity Stress Damage by Symbiotic Plant Growth-Promoting Actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Xu, J.; Li, Z.; Tang, Z.; Wu, X. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. Int. 2022, 29, 32988–33001. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Z.; Zhang, J.; Wang, P.; Li, Y.; Ji, B. Host-Specific Effects of Arbuscular Mycorrhizal Fungi on Two Caragana Species in Desert Grassland. J. Fungi 2021, 7, 1077. [Google Scholar] [CrossRef] [PubMed]
- Hibilik, N.; Selmaoui, K.; Msairi, S.; Touati, J.; Chliyeh, M.; Mouria, A.; Artib, M.; Touhami, A.O.; Benkirane, R.; Douira, A. Study of Arbuscular Mycorrhizal Fungi Diversity and Its Effect on Growth and Development of Leek Plants (Allium porrum L.). Annu. Res. Rev. Biol. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Bai, J.; Qin, H.; Wang, J.; Wang, J.; Lin, X. Intercropping with sunflower and inoculation with arbuscular mycorrhizal fungi promotes growth of garlic chive in metal-contaminated soil at a WEEE-recycling site. Ecotoxicol. Environ. Saf. 2019, 167, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Carmen, B.; Amélia, C.; Lovato, P.E.; Cinta, C. On-farm reduced irrigation and fertilizer doses, and arbuscular mycorrhizal fungal inoculation improve water productivity in tomato production. Sci. Hortic. 2021, 288, 110337. [Google Scholar]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Schall, P.; Lödige, C.; Beck, M.; Ammer, C. Biomass allocation to roots and shoots is more sensitive to shade and drought in European beech than in Norway spruce seedlings. For. Ecol. Manag. 2011, 266, 246–253. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.; Li, X.; Tian, C.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar]
- Zhu, X.; Song, F.; Liu, S. Arbuscular mycorrhiza impacts on drought stress of maize plants by lipid peroxidation, proline content and activity of antioxidant system. J. Food Agric. Environ. 2011, 9, 583–587. [Google Scholar]
- Yang, W.Y.; Sun, L.Y.; Song, F.B.; Yang, X.Q.; Zhang, M.J.; Li, S.X.; Zhu, X.C. Research advances in species diversity of arbuscular mycorrhizal fungi in terrestrial agro-ecosystem. Chin. J. Appl. Ecol. 2019, 30, 3971–3979. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular Mycorrhizal Colonization Promotes the Tolerance to Salt Stress in Lettuce Plants through an Efficient Modification of Ionic Balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z.; et al. Transformation and Immobilization of Chromium by Arbuscular Mycorrhizal Fungi as Revealed by SEM-EDS, TEM-EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; de Melo, N.F.; Mendes, A.M.S.; de Araújo, F.P.; Maia, L.C.; Yano-Melo, A.M. Response of Passiflora setacea to Mycorrhization and Phosphate Fertilization in a Semiarid Region of Brazil. J. Plant Nutr. 2015, 38, 431–442. [Google Scholar] [CrossRef]



| Province | Location (County) | Soil Type | Acquisition Subjects | Sample Codes | Latitude (N) | Longitude (E) | Altitude (m) | Average Annual Precipitation (mm) | Average Annual Temperature | Soil Characteristics |
|---|---|---|---|---|---|---|---|---|---|---|
| Ningxia | Zhongning | Calcisols soil | Lycium barbarum (NingQi 1) | ZN1 ZN2 ZN3 | 37°16′26″ | 105°27′16″ | 1123 | 202.1 | 9.5 °C | Oasis soil |
| Ningxia | Guyuan | Chernozems Soil | Lycium barbarum (NingQi 1) | GY1 GY2 GY3 | 36°25′48″ | 106°9′ | 1429 | 516.7 | 8.3 °C | Red clay |
| Xinjiang | Jinghe | Kastanozems soil | Lycium barbarum (NingQi 1) | JH1 JH2 JH3 | 44°20′28″ | 82°32′7″ | 290 | 129.0 | 8.0 °C | Calcium palm fiber |
| Qinghai | Dulan | Leptosols soil | Lycium barbarum (NingQi 1) | DL1 DL2 DL3 | 36°13′37″ | 92°15′19″ | 2783 | 179.1 | 2.7 °C | Calcium palm fiber |
| Ecological Parameters | Explanation |
|---|---|
| Spore density (SD) | Refers to the total number of AMF spores contained in each 50 g of air-dried soil sample. |
| Species richness (SR) | The total number of AMF spore species per 50 g of soil sample |
| Isolation frequency (IF) | The proportion of a genus or species of AMF that was present in the overall sample. |
| Relative abundance (RA) | The proportion of a genus or species of AMF in the total number of spores at a sample site. |
| Importance value (IV) | Refers to the average of separation frequency and relative abundance. |
| Diversity index: Shannon–Wiener index (H) and Simpson’s diversity index (D) | and , where ; is the number of AMF spores of a certain species (genus) in a sample site, and N is the total number of AMF spores in this sample site. |
| Region | Hyphae | Vesicles | Arbuscules | Total Colonization Rates |
|---|---|---|---|---|
| ZN | 68.57% | 2.86% | 14.29% | 68.57% |
| GY | 60.50% | 36.83% | 16.17% | 60.83% |
| JH | 82.92% | 46.00% | 18.46% | 82.92% |
| DL | 98.13% | 37.88% | 36.13% | 98.13% |
| Phylum (1) Glomeromycota Class (1) Glomeromycetes Orders (5) | Families (5) | Genera (5) | Species (14) |
|---|---|---|---|
| unclassified_c_ Glomeromycetes | unclassified_c_ Glomeromycetes | unclassified_c_ Glomeromycetes | unclassified_c__Glomeromycetes |
| Glomerales | Glomeraceae | Glomus_f_ Glomeraceae | unclassified_g__Glomus_f__ Glomeraceae |
| Glomus-Wirsel-OTU16-VTX00156 | |||
| Glomus-sp.-VTX00304 | |||
| Glomus-viscosum-VTX00063 | |||
| Glomus-MO-G22-VTX00125 | |||
| Glomus-MO-G23-VTX00222 | |||
| Glomus-sp.-VTX00165 | |||
| Glomus-intraradices-VTX00105 | |||
| Glomus-sp.-VTX00301 | |||
| Diversisporales | Diversisporaceae | Diversispora | unclassified_g__Diversispora |
| Paraglomerales | Paraglomeraceae | Paraglomus | unclassified_g__Paraglomus |
| Paraglomus-Glom-1B.13-VTX00308 | |||
| Archaeosporales | unclassified_o_ Archaeosporales | unclassified_o_ Archaeosporales | unclassified_o__Archaeosporales |
| Phylum Glomeromycota Class Glomeromycetes Orders (4) | Families (6) | Genera (8) | Regions | IF | RA | IV | Dominance Rank | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ZN | GY | JH | DL | |||||||
| Glomerales | Glomeraceae | Glomus | + | + | + | + | 33.88% | 91.36% | 62.62% | Dominant genus |
| Rhizophagus | + | 1.22% | 3.16% | 2.19% | Rare genus | |||||
| Septoglomus | + | 0.82% | 2.11% | 1.46% | Rare genus | |||||
| Diversisporales | Gigasporaceae | Scutellospora | + | + | + | 6.12% | 19.97% | 13.05% | Common genus | |
| Acaulosporaceae | Acaulospora | + | + | + | 10.41% | 31.32% | 20.86% | Common genus | ||
| Diversisporales | Diversispora | + | 2.04% | 10.00% | 6.02% | Rare genus | ||||
| Paraglomerales | Paraglomeraceae | Paraglomus | + | 1.63% | 4.55% | 3.09% | Rare genus | |||
| Archaeosporales | Ambisporaceae | Ambispora | + | 6.94% | 19.32% | 13.13% | Common genus | |||
| Phylum Glomeromycota Class Glomeromycetes Orders (4) | Families (8) | Genera (8) | Frequency of Occurrence (%) | |||
|---|---|---|---|---|---|---|
| ZN | GY | JH | DL | |||
| Glomerales | Glomeraceae | Glomus | 6.52% | – | 2.17% | – |
| Claroideoglomeraceae | Claroideoglomus | 2.17% | 2.17% | – | – | |
| Diversisporales | Gigasporaceae | Scutellospora | 2.17% | – | 2.17% | – |
| Acaulosporaceae | Acaulospora | – | – | 2.17% | – | |
| Diversisporaceae | Diversispora | – | 2.17% | – | – | |
| Paraglomerales | Paraglomeraceae | Paraglomus | 15.22% | 15.22% | 21.74% | 19.57% |
| Archaeosporales | Ambisporaceae | Ambispora | – | – | – | 2.17% |
| Archaeosporaceae | Archaeospora | – | – | 4.35% | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Chen, K.; He, D.; He, Y.; Lei, Y.; Sun, Y. Diversity of Arbuscular Mycorrhizal Fungi of the Rhizosphere of Lycium barbarum L. from Four Main Producing Areas in Northwest China and Their Effect on Plant Growth. J. Fungi 2024, 10, 286. https://doi.org/10.3390/jof10040286
Cheng Y, Chen K, He D, He Y, Lei Y, Sun Y. Diversity of Arbuscular Mycorrhizal Fungi of the Rhizosphere of Lycium barbarum L. from Four Main Producing Areas in Northwest China and Their Effect on Plant Growth. Journal of Fungi. 2024; 10(4):286. https://doi.org/10.3390/jof10040286
Chicago/Turabian StyleCheng, Yuyao, Kaili Chen, Dalun He, Yaling He, Yonghui Lei, and Yanfei Sun. 2024. "Diversity of Arbuscular Mycorrhizal Fungi of the Rhizosphere of Lycium barbarum L. from Four Main Producing Areas in Northwest China and Their Effect on Plant Growth" Journal of Fungi 10, no. 4: 286. https://doi.org/10.3390/jof10040286





