Virulence Factors as Targets for Anticryptococcal Therapy
Abstract
:1. Introduction
1.1. The Need for Antifungal Drugs
1.2. Cryptococcosis: A Systemic Fungal Infection with Alarming Mortality Indices
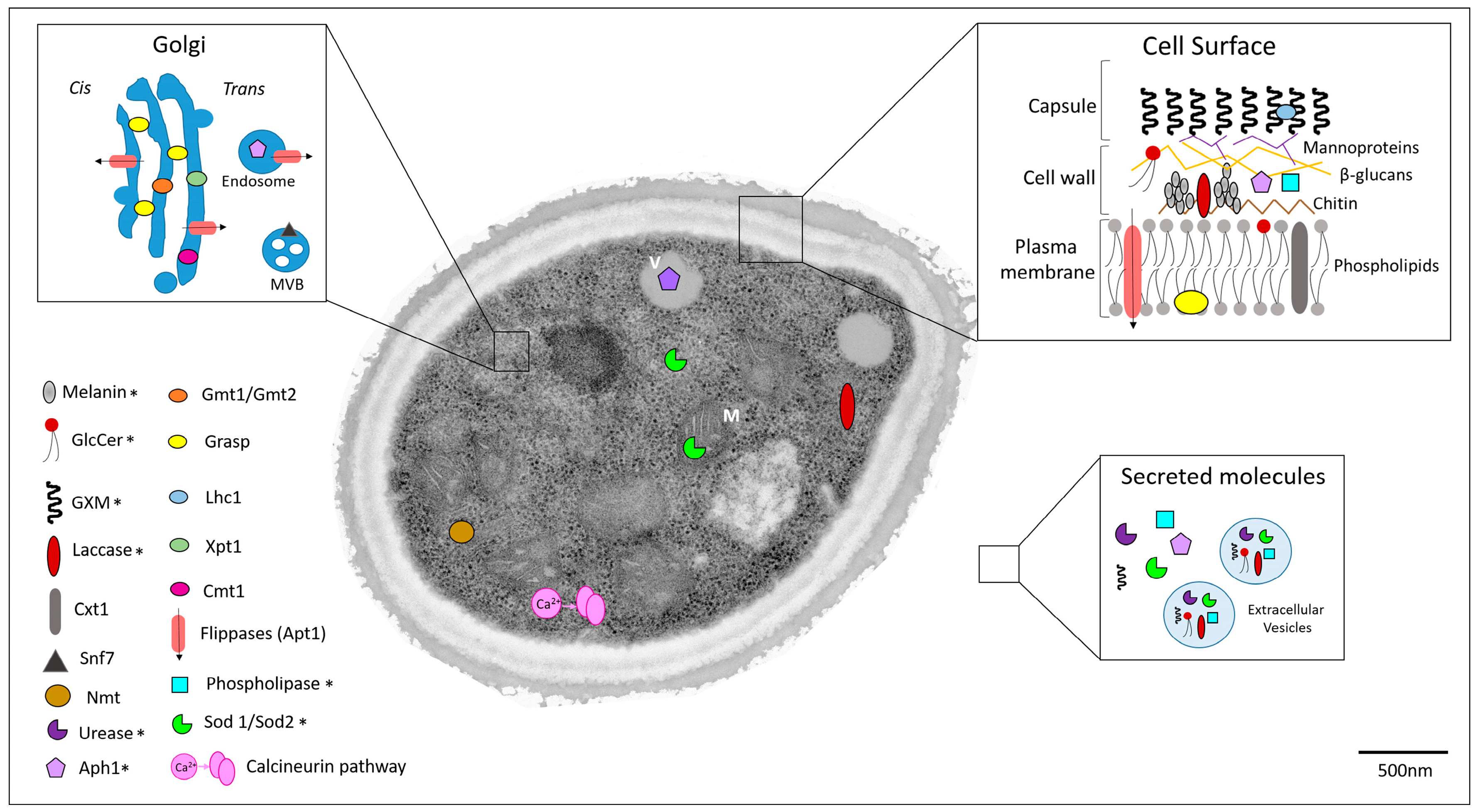
1.3. Potential Targets for Development of Novel Anticryptococcal Agents
1.3.1. Capsule Components
1.3.2. Melanin
1.3.3. Extracellular Enzymes
1.3.4. Regulators of Unconventional Secretory Pathways
1.3.5. Other Cryptococcal Targets
2. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond. Engl. 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C.; Association with the LIFE Program. The burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- WHO|Tuberculosis. Available online: http://www.who.int/mediacentre/factsheets/fs104/en/ (accessed on 30 May 2016).
- WHO|Malaria. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 30 May 2016).
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, R.O.; Teixeira, A.P.; Oliveira, W.A.; Lima, E.O.; Lima, I.O. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm. Biol. 2016, 54, 2594–2596. [Google Scholar] [CrossRef] [PubMed]
- Butts, A.; Krysan, D.J. Antifungal drug discovery: Something old and something new. PLoS Pathog. 2012, 8, e1002870. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Sullivan, D.J. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Butts, A.; DiDone, L.; Koselny, K.; Baxter, B.K.; Chabrier-Rosello, Y.; Wellington, M.; Krysan, D.J. A Repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot. Cell 2013, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- De Treviño-Rangel, R.J.; Villanueva-Lozano, H.; Hernández-Rodríguez, P.; Martínez-Reséndez, M.F.; García-Juárez, J.; Rodríguez-Rocha, H.; González, G.M. Activity of sertraline against Cryptococcus neoformans: In vitro and in vivo assays. Med. Mycol. 2016, 54, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Rhein, J.; Morawski, B.M.; Hullsiek, K.H.; Nabeta, H.W.; Kiggundu, R.; Tugume, L.; Musubire, A.; Akampurira, A.; Smith, K.D.; Alhadab, A.; et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: An open-label dose-ranging study. Lancet Infect. Dis. 2016, 16, 809–818. [Google Scholar] [CrossRef]
- Velagapudi, R.; Hsueh, Y.-P.; Geunes-Boyer, S.; Wright, J.R.; Heitman, J. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 2009, 77, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2006, 20, 507–544. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS Lond. Engl. 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: A sword and a shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Alviano, C.S.; Travassos, L.R. Pathogenicity of Cryptococcus neoformans: Virulence factors and immunological mechanisms. Microbes Infect. Inst. Pasteur 1999, 1, 293–301. [Google Scholar] [CrossRef]
- Williamson, P.R. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front. Biosci. J. Virtual Libr. 1997, 2, e99–e107. [Google Scholar] [CrossRef]
- Casadevall, A.; Rosas, A.L.; Nosanchuk, J.D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2000, 3, 354–358. [Google Scholar] [CrossRef]
- Casadevall, A.; Perfect, R.J. Cryptococcus neoformans; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [PubMed]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the immunological function of the Cryptococcus neoformans capsule. Future Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 1994, 14, 4912–4919. [Google Scholar] [CrossRef] [PubMed]
- Fromtling, R.A.; Shadomy, H.J.; Jacobson, E.S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 1982, 79, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Pontes, B.; Nimrichter, L.; Viana, N.B.; Rodrigues, M.L.; Casadevall, A. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Mukherjee, J.; Devi, S.J.; Schneerson, R.; Robbins, J.B.; Scharff, M.D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 1992, 165, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.A.; Rivera, J.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [PubMed]
- Shapiro, S.; Beenhouwer, D.O.; Feldmesser, M.; Taborda, C.; Carroll, M.C.; Casadevall, A.; Scharff, M.D. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect. Immun. 2002, 70, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.R.; Casadevall, A.; Oh, J.; Scharff, M.D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.A.; Pappas, P.G.; Perfect, J.; Aberg, J.A.; Casadevall, A.; Cloud, G.A.; James, R.; Filler, S.; Dismukes, W.E. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 2005, 49, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Klutts, J.S.; Levery, S.B.; Doering, T.L. A β-1,2-xylosyltransferase from Cryptococcus neoformans defines a new family of glycosyltransferases. J. Biol. Chem. 2007, 282, 17890–17899. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.A.; Owuor, E.A.; Thompson, S.H.; Garnsey, M.R.; Klutts, J.S.; Doering, T.L.; Levery, S.B. β1,2-xylosyltransferase Cxt1p is solely responsible for xylose incorporation into Cryptococcus neoformans glycosphingolipids. Eukaryot. Cell 2008, 7, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Klutts, J.S.; Doering, T.L. Cryptococcal xylosyltransferase 1 (Cxt1p) from Cryptococcus neoformans plays a direct role in the synthesis of capsule polysaccharides. J. Biol. Chem. 2008, 283, 14327–14334. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.C.; Levery, S.B.; Castle, S.A.; Klutts, J.S.; Doering, T.L. A novel xylosylphosphotransferase activity discovered in Cryptococcus neoformans. J. Biol. Chem. 2009, 284, 36118–36127. [Google Scholar] [CrossRef] [PubMed]
- Bencúr, P.; Steinkellner, H.; Svoboda, B.; Mucha, J.; Strasser, R.; Kolarich, D.; Hann, S.; Köllensperger, G.; Glössl, J.; Altmann, F.; et al. Arabidopsis thaliana β1,2-xylosyltransferase: An unusual glycosyltransferase with the potential to act at multiple stages of the plant N-glycosylation pathway. Biochem. J. 2005, 388, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Götting, C.; Kuhn, J.; Kleesiek, K. Human xylosyltransferases in health and disease. Cell. Mol. Life Sci. 2007, 64, 1498–1517. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.C.; Aoki, K.; Wang, Z.A.; Skowyra, M.L.; Williams, M.; Tiemeyer, M.; Doering, T.L. A xylosylphosphotransferase of Cryptococcus neoformans acts in protein O-glycan synthesis. J. Biol. Chem. 2011, 286, 26888–26899. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, T.R.; Griffith, C.L.; Liu, H.; Nenninger, A.A.; Doering, T.L. The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot. Cell 2007, 6, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.A.; Griffith, C.L.; Skowyra, M.L.; Salinas, N.; Williams, M.; Maier, E.J.; Gish, S.R.; Liu, H.; Brent, M.R.; Doering, T.L. Cryptococcus neoformans dual GDP-mannose transporters and their role in biology and virulence. Eukaryot. Cell 2014, 13, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Elbein, A.D. Glycosilation precursors. In Essentials of Glycobiology; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2009; pp. 47–61. [Google Scholar]
- Poster, J.B.; Dean, N. The yeast VRG4 gene is required for normal Golgi functions and defines a new family of related genes. J. Biol. Chem. 1996, 271, 3837–3845. [Google Scholar] [PubMed]
- Arakawa, K.; Abe, M.; Noda, Y.; Adachi, H.; Yoda, K. Molecular cloning and characterization of a Pichia pastoris ortholog of the yeast Golgi GDP-mannose transporter gene. J. Gen. Appl. Microbiol. 2006, 52, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.D.; Arentshorst, M.; Weenink, X.O.; Punt, P.J.; van den Hondel, C.A.; Ram, A.F. Functional YFP-tagging of the essential GDP-mannose transporter reveals an important role for the secretion related small GTPase SrgC protein in maintenance of Golgi bodies in Aspergillus niger. Fungal Biol. 2011, 115, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, A.; Poster, J.B.; Jigami, Y.; Dean, N. Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J. Bacteriol. 2002, 184, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Schmalhorst, P.S.; Routier, F.H. Biosynthesis of the fungal cell wall polysaccharide galactomannan requires intraluminal GDP-mannose. J. Biol. Chem. 2012, 287, 44418–44424. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, T.C.; Handford, M.G.; Yuseff, M.I.; Orellana, A.; Dupree, P. Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell 2001, 13, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Handford, M.G.; Sicilia, F.; Brandizzi, F.; Chung, J.H.; Dupree, P. Arabidopsis thaliana expresses multiple Golgi-localised nucleotide-sugar transporters related to GONST1. Mol. Genet. Genom. 2004, 272, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Russell, D.G.; Beverley, S.M.; Turco, S.J. Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J. Biol. Chem. 1997, 272, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.L. A unique alpha-1,3 mannosyltransferase of the pathogenic fungus Cryptococcus neoformans. J. Bacteriol. 1999, 181, 5482–5488. [Google Scholar] [PubMed]
- Sommer, U.; Liu, H.; Doering, T.L. An α-1,3-mannosyltransferase of Cryptococcus neoformans. J. Biol. Chem. 2003, 278, 47724–47730. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.C.; Cordero, R.J.B.; Fonseca, F.L.; da Silva, R.P.; Ramos, C.L.; Miranda, K.R.; Casadevall, A.; Puccia, R.; Nosanchuk, J.D.; Nimrichter, L.; et al. A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan. Fungal Genet. Biol. 2012, 49, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-D.; Shin, S.; Panepinto, J.; Ramos, J.; Qiu, J.; Frases, S.; Albuquerque, P.; Cordero, R.J.B.; Zhang, N.; Himmelreich, U.; et al. A role for LHC1 in higher order structure and complement binding of the Cryptococcus neoformans capsule. PLoS Pathog. 2014, 10, e1004037. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shinohara, M.; Sakoh, C.; Kataoka, M.; Shimizu, S. Lactone-ring-cleaving enzyme: Genetic analysis, novel RNA editing, and evolutionary implications. Proc. Natl. Acad. Sci. USA 1998, 95, 12787–12792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [PubMed]
- Van Duin, D.; Casadevall, A.; Nosanchuk, J.D. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 2002, 46, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Larsson, B.S. Interaction between chemicals and melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Casadevall, A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob. Agents Chemother. 1996, 40, 541–545. [Google Scholar] [PubMed]
- Eilam, Y.; Polacheck, I.; Ben-Gigi, G.; Chernichovsky, D. Activity of phenothiazines against medically important yeasts. Antimicrob. Agents Chemother. 1987, 31, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Ovalle, R.; Casadevall, A. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 2001, 183, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hasegawa, K.; Ito, S.; Wakamatsu, K.; Fujita, K. Antimelanoma activity of chloroquine, an antimalarial agent with high affinity for melanin. Pigment Cell Res. 1993, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Rosas, A.L.; Nosanchuk, J.D.; Casadevall, A. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 2001, 69, 3410–3412. [Google Scholar] [CrossRef] [PubMed]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Zoccarato, F.; Toscano, P.; Alexandre, A. Dopamine-derived dopaminochrome promotes H2O2 release at mitochondrial complex I: Stimulation by rotenone, control by Ca2+, and relevance to Parkinson disease. J. Biol. Chem. 2005, 280, 15587–15594. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [PubMed]
- Missall, T.A.; Moran, J.M.; Corbett, J.A.; Lodge, J.K. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot. Cell 2005, 4, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, I.; Hearing, V.J.; Kwon-Chung, K.J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J. Bacteriol. 1982, 150, 1212–1220. [Google Scholar] [PubMed]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Gerrald, Q.D.; Kraus, P.R.; Boily, M.-J.; Davis, M.J.; Giles, S.S.; Cox, G.M.; Heitman, J.; Alspaugh, J.A. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell 2005, 4, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Kronstad, J.; Saikia, S.; Nielson, E.D.; Kretschmer, M.; Jung, W.; Hu, G.; Geddes, J.M.H.; Griffiths, E.J.; Choi, J.; Cadieux, B.; et al. Adaptation of Cryptococcus neoformans to mammalian hosts: Integrated regulation of metabolism and virulence. Eukaryot. Cell 2012, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Wolf, J.M.; Casadevall, A. Virulence-associated enzymes of Cryptococcus neoformans. Eukaryot. Cell 2015, 14, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.M.; Mukherjee, J.; Cole, G.T.; Casadevall, A.; Perfect, J.R. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 2000, 68, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.A.; Noverr, M.C.; Chen, G.-H.; Toews, G.B.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004, 164, 1761–1771. [Google Scholar] [CrossRef]
- Casadevall, A. Cryptococci at the brain gate: Break and enter or use a Trojan horse? J. Clin. Investig. 2010, 120, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Collopy-Junior, I.; Esteves, F.F.; Nimrichter, L.; Rodrigues, M.L.; Alviano, C.S.; Meyer-Fernandes, J.R. An ectophosphatase activity in Cryptococcus neoformans. FEMS Yeast Res. 2006, 6, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Crossett, B.; Cha, S.Y.; Desmarini, D.; Li, C.; Chayakulkeeree, M.; Wilson, C.F.; Williamson, P.R.; Sorrell, T.C.; Djordjevic, J.T. Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans. mBio 2014, 5, e01649-14. [Google Scholar] [CrossRef] [PubMed]
- Rigden, D.J. The histidine phosphatase superfamily: Structure and function. Biochem. J. 2008, 409, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Wright, L.C.; Santangelo, R.T.; Muller, M.; Moran, V.R.; Kuchel, P.W.; Sorrell, T.C. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 1997, 65, 405–411. [Google Scholar] [PubMed]
- Cox, G.M.; McDade, H.C.; Chen, S.C.; Tucker, S.C.; Gottfredsson, M.; Wright, L.C.; Sorrell, T.C.; Leidich, S.D.; Casadevall, A.; Ghannoum, M.A.; et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 2001, 39, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 2003, 71, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.; Zoellner, H.; Sorrell, T.; Wilson, C.; Donald, C.; Djordjevic, J.; Shounan, Y.; Wright, L. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 2004, 72, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Siafakas, A.R.; Sorrell, T.C.; Wright, L.C.; Wilson, C.; Larsen, M.; Boadle, R.; Williamson, P.R.; Djordjevic, J.T. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J. Biol. Chem. 2007, 282, 37508–37514. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Du, H.; Hoffman, C.S.; Marcus, S. The phospholipase B homolog Plb1 is a mediator of osmotic stress response and of nutrient-dependent repression of sexual differentiation in the fission yeast Schizosaccharomyces pombe. Mol. Genet. Genom. 2003, 269, 116–125. [Google Scholar]
- Obando, D.; Pantarat, N.; Handke, R.; Koda, Y.; Widmer, F.; Djordjevic, J.T.; Ellis, D.H.; Sorrell, T.C.; Jolliffe, K.A. Synthesis, antifungal, haemolytic and cytotoxic activities of a series of bis(alkylpyridinium)alkanes. Bioorg. Med. Chem. 2009, 17, 6329–6339. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.T. Role of phospholipases in fungal fitness, pathogenicity, and drug development—Lessons from Cryptococcus neoformans. Front. Microbiol. 2010, 1, 125. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Sorrell, T.C.; Siafakas, A.R.; Wilson, C.F.; Pantarat, N.; Gerik, K.J.; Boadle, R.; Djordjevic, J.T. Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans. Mol. Microbiol. 2008, 69, 809–826. [Google Scholar] [PubMed]
- Lev, S.; Desmarini, D.; Li, C.; Chayakulkeeree, M.; Traven, A.; Sorrell, T.C.; Djordjevic, J.T. Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase. Infect. Immun. 2013, 81, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Flick, J.S.; Thorner, J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993, 13, 5861–5876. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.E.; McCreary, C.E.; Coleman, D.C. Genetic characterization of a phospholipase C gene from Candida albicans: Presence of homologous sequences in Candida species other than Candida albicans. Microbiol. Read. Engl. 1998, 144, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.M.; Harrison, T.S.; McDade, H.C.; Taborda, C.P.; Heinrich, G.; Casadevall, A.; Perfect, J.R. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 2003, 71, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Holdom, M.D. Biochemical comparison of the Cu,Zn superoxide dismutases of Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii. Infect. Immun. 1997, 65, 488–494. [Google Scholar] [PubMed]
- Narasipura, S.D.; Ault, J.G.; Behr, M.J.; Chaturvedi, V.; Chaturvedi, S. Characterization of Cu,Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: Role in biology and virulence. Mol. Microbiol. 2003, 47, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Narasipura, S.D.; Chaturvedi, V.; Chaturvedi, S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol. Microbiol. 2005, 55, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.S.; Jenkins, N.D.; Todd, J.M. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect. Immun. 1994, 62, 4085–4086. [Google Scholar] [PubMed]
- Giles, S.S.; Batinic-Haberle, I.; Perfect, J.R.; Cox, G.M. Cryptococcus neoformans mitochondrial superoxide dismutase: An essential link between antioxidant function and high-temperature growth. Eukaryot. Cell 2005, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Schekman, R.W. Regulation of membrane traffic in the secretory pathway. Harvey Lect. 1994, 90, 41–57. [Google Scholar] [PubMed]
- Kmetzsch, L.; Joffe, L.S.; Staats, C.C.; de Oliveira, D.L.; Fonseca, F.L.; Cordero, R.J.B.; Casadevall, A.; Nimrichter, L.; Schrank, A.; Vainstein, M.H.; et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol. Microbiol. 2011, 81, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Oliveira, D.L.; Joffe, L.S.; Hu, G.; Gazos-Lopes, F.; Fonseca, F.L.; Almeida, I.C.; Frases, S.; Kronstad, J.W.; Rodrigues, M.L. Role of the Apt1 protein in polysaccharide secretion by Cryptococcus neoformans. Eukaryot. Cell 2014, 13, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Godinho, R.M.; Crestani, J.; Kmetzsch, L.; Araujo, G.; Frases, S.; Staats, C.C.; Schrank, A.; Vainstein, M.H.; Rodrigues, M.L. The vacuolar-sorting protein Snf7 is required for export of virulence determinants in members of the Cryptococcus neoformans complex. Sci. Rep. 2014, 4, 6198. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Kirkman, E.; Lin, X. Secreted Acb1 contributes to the yeast-to-hypha transition in Cryptococcus neoformans. Appl. Environ. Microbiol. 2016, 82, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.F.S.; Garcia, A.F.; Kumagai, P.S.; de Morais, F.R.; Melo, F.A.; Kmetzsch, L.; Vainstein, M.H.; Rodrigues, M.L.; Costa-Filho, A.J. New structural insights into Golgi Reassembly and Stacking Protein (GRASP) in solution. Sci. Rep. 2016, 6, 29976. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Caza, M.; Cadieux, B.; Bakkeren, E.; Do, E.; Jung, W.H.; Kronstad, J.W. The endosomal sorting complex required for transport machinery influences haem uptake and capsule elaboration in Cryptococcus neoformans. Mol. Microbiol. 2015, 96, 973–992. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liao, G.; Baker, G.M.; Wang, Y.; Lau, R.; Paderu, P.; Perlin, D.S.; Xue, C. Lipid Flippase Subunit Cdc50 Mediates Drug Resistance and Virulence in Cryptococcus Neoformans. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, M.; Nimrichter, L.; Rodrigues, M.L.; Luberto, C. Synthesis and biological properties of fungal glucosylceramide. PLoS Pathog. 2014, 10, e1003832. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Travassos, L.R.; Miranda, K.R.; Franzen, A.J.; Rozental, S.; de Souza, W.; Alviano, C.S.; Barreto-Bergter, E. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 2000, 68, 7049–7060. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Shi, L.; Barreto-Bergter, E.; Nimrichter, L.; Farias, S.E.; Rodrigues, E.G.; Travassos, L.R.; Nosanchuk, J.D. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin. Vaccine Immunol. 2007, 14, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, P.C.; Kechichian, T.B.; Allegood, J.C.; Merrill, A.H.; Hennig, M.; Luberto, C.; Del Poeta, M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2006, 116, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Mor, V.; Rella, A.; Farnoud, A.M.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.B.; Ojima, I.; Bullesbach, E.; et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 2015, 6, e00647. [Google Scholar] [CrossRef] [PubMed]
- Burnie, J.P.; Carter, T.L.; Hodgetts, S.J.; Matthews, R.C. Fungal heat-shock proteins in human disease. FEMS Microbiol. Rev. 2006, 30, 53–88. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Evangelista, A.J.; Serpa, R.; Marques, F.J.; Melo, C.V.; Oliveira, J.S.; Franco, J.; Alencar, L.P.; Bandeira, T.; Brilhante, R.S.N.; et al. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiol. Read. Engl. 2016, 162, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Nooney, L.; Matthews, R.C.; Burnie, J.P. Evaluation of Mycograb, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn. Microbiol. Infect. Dis. 2005, 51, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Sia, R.A.; Olson, M.; Cox, G.M.; Heitman, J. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 2000, 68, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Odom, A.; Muir, S.; Lim, E.; Toffaletti, D.L.; Perfect, J.; Heitman, J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997, 16, 2576–2589. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Cryptococcus neoformans in organ transplant recipients: Impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 2007, 195, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, R.J.; Reedy, J.L.; Morales-Johansson, H.; Heitman, J.; Cardenas, M.E. Signaling cascades as drug targets in model and pathogenic fungi. Curr. Opin. Investig. Drugs Lond. Engl. 2008, 9, 856–864. [Google Scholar]
- Cruz, M.C.; Del Poeta, M.; Wang, P.; Wenger, R.; Zenke, G.; Quesniaux, V.F.; Movva, N.R.; Perfect, J.R.; Cardenas, M.E.; Heitman, J. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 2000, 44, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.K.; Jackson-Machelski, E.; Toffaletti, D.L.; Perfect, J.R.; Gordon, J.I. Targeted gene replacement demonstrates that myristoyl-CoA: Protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 1994, 91, 12008–12012. [Google Scholar] [CrossRef] [PubMed]
- Panethymitaki, C.; Bowyer, P.W.; Price, H.P.; Leatherbarrow, R.J.; Brown, K.A.; Smith, D.F. Characterization and selective inhibition of myristoyl-CoA:protein N-myristoyltransferase from Trypanosoma brucei and Leishmania major. Biochem. J. 2006, 396, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Langner, C.A.; Lodge, J.K.; Travis, S.J.; Caldwell, J.E.; Lu, T.; Li, Q.; Bryant, M.L.; Devadas, B.; Gokel, G.W.; Kobayashi, G.S. 4-oxatetradecanoic acid is fungicidal for Cryptococcus neoformans and inhibits replication of human immunodeficiency virus I. J. Biol. Chem. 1992, 267, 17159–17169. [Google Scholar] [PubMed]
- Georgopapadakou, N.H. Antifungals targeted to protein modification: Focus on protein N-myristoyltransferase. Expert Opin. Investig. Drugs 2002, 11, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, P.W.; Tate, E.W.; Leatherbarrow, R.J.; Holder, A.A.; Smith, D.F.; Brown, K.A. N-myristoyltransferase: A prospective drug target for protozoan parasites. ChemMedChem 2008, 3, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, S. Recent advances in the discovery of N-myristoyltransferase inhibitors. ChemMedChem 2014, 9, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.S.; Wood, D.C.; Mehta, P.P.; Wade, A.C.; Lu, T.; Gokel, G.W.; Gordon, J.I. Comparison of the acyl chain specificities of human myristoyl-CoA synthetase and human myristoyl-CoA:protein N-myristoyltransferase. J. Biol. Chem. 1993, 268, 4889–4902. [Google Scholar] [PubMed]
- Gunaratne, R.S.; Sajid, M.; Ling, I.T.; Tripathi, R.; Pachebat, J.A.; Holder, A.A. Characterization of N-myristoyltransferase from Plasmodium falciparum. Biochem. J. 2000, 348, 459–463. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, R.V.D.M.; Rizzo, J.; Rodrigues, M.L. Virulence Factors as Targets for Anticryptococcal Therapy. J. Fungi 2016, 2, 29. https://doi.org/10.3390/jof2040029
Azevedo RVDM, Rizzo J, Rodrigues ML. Virulence Factors as Targets for Anticryptococcal Therapy. Journal of Fungi. 2016; 2(4):29. https://doi.org/10.3390/jof2040029
Chicago/Turabian StyleAzevedo, Renata V. D. M., Juliana Rizzo, and Marcio L. Rodrigues. 2016. "Virulence Factors as Targets for Anticryptococcal Therapy" Journal of Fungi 2, no. 4: 29. https://doi.org/10.3390/jof2040029




