Global Aspects of Triazole Resistance in Aspergillus fumigatus with Focus on Latin American Countries
Abstract
:1. An Overview of Aspergillosis in Contemporary Medicine
2. Methods
3. Global Scenario of Resistance to Azoles
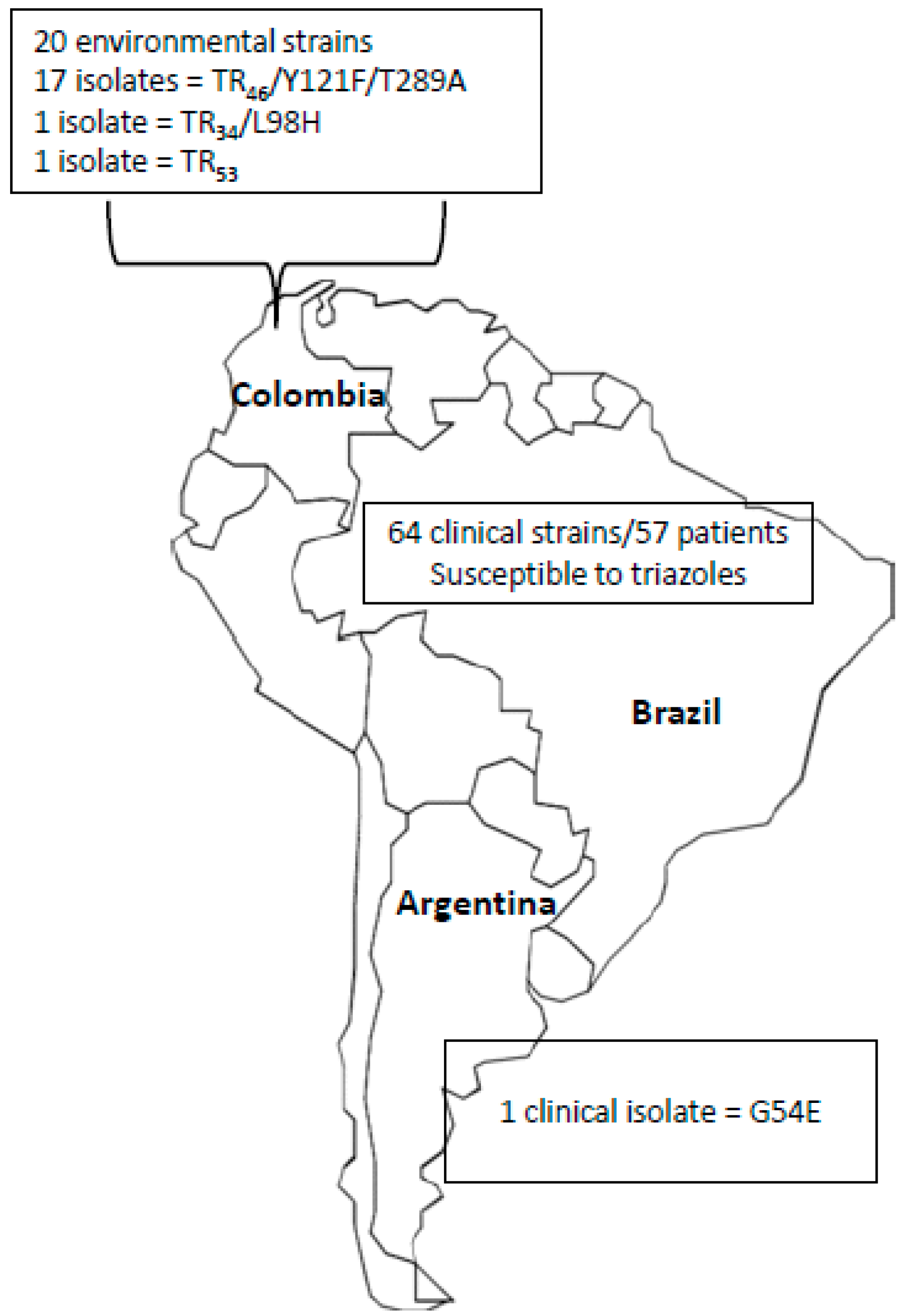
4. Epidemiology of Triazole Resistance in A. fumigatus in Latin American Countries
5. Clinical Implications and the Management of Azole-Resistant Aspergillosis
6. Final Considerations
Conflicts of Interest
References
- Nicolle, M.C.; Benet, T.; Vanhems, P. Aspergillosis: Nosocomial or community-acquired? Med. Mycol. 2011, 49, S24–S29. [Google Scholar] [CrossRef]
- Lamoth, F. Aspergillus fumigatus-related species in clinical practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Garnaud, C.; Brenier-Pinchart, M.P.; Thiebaut-Bertrand, A.; Saint-Raymond, C.; Camara, B.; Hamidfar, R.; Cognet, O.; Maubon, D.; Cornet, M.; et al. Direct molecular diagnosis of aspergillosis and CYP51A profiling from respiratory samples of french patients. Front. Microbiol. 2016, 7, 1164. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ashu, E.; Sharma, C.; Kathuria, S.; Chowdhary, A.; Xu, J. Diversity and origins of indian multi-triazole resistant strains of Aspergillus fumigatus. Mycoses 2016, 59, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.C. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 2009, 47, S261–S270. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Voss, A.; Verweij, P.E. Hospital sources of aspergillus: New routes of transmission? Rev. Iberoam. Micol. 2001, 18, 156–162. [Google Scholar] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Leonardelli, F.; Theill, L.; Nardin, M.E.; Macedo, D.; Dudiuk, C.; Mendez, E.; Gamarra, S.; Garcia-Effron, G. First itraconazole resistant Aspergillus fumigatus clinical isolate harbouring a G54E substitution in CYP51AP in South America. Rev. Iberoam. Micol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Tobon, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of opportunistic fungal infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A. Clinical aspergillosis for basic scientists. Med. Mycol. 2009, 47, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Martino, B.; Specchia, G.; Pastore, D.; Stanzani, M.; Cattaneo, C.; Fanci, R.; et al. Invasive aspergillosis in patients with acute myeloid leukemia: A seifem-2008 registry study. Haematologica 2010, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Cypowyj, S.; Picard, C.; Bustamante, J.; Lortholary, O.; Casanova, J.L.; Puel, A. Primary immunodeficiencies underlying fungal infections. Curr. Opin. Pediatr. 2013, 25, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Antachopoulos, C.; Walsh, T.J.; Roilides, E. Fungal infections in primary immunodeficiencies. Eur. J. Pediatr. 2007, 166, 1099–1117. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C.; Griff, K.; Mayr, A.; Petzer, A.; Gastl, G.; Bonatti, H.; Freund, M.; Kropshofer, G.; Dierich, M.P.; Nachbaur, D. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br. J. Haematol. 2005, 131, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Perlin, D.S. Azole resistance in aspergillus: A growing public health menace. Future Microbiol. 2011, 6, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Bruggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K.; et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 2015, 21–22, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Arendrup, M.C. Acquired antifungal drug resistance in Aspergillus fumigatus: Epidemiology and detection. Med. Mycol. 2011, 49, S90–S95. [Google Scholar] [CrossRef]
- Shi, J.Y.; Xu, Y.C.; Shi, Y.; Lu, H.X.; Liu, Y.; Zhao, W.S.; Chen, D.M.; Xi, L.Y.; Zhou, X.; Wang, H.; et al. In vitro susceptibility testing of aspergillus spp. Against voriconazole, itraconazole, posaconazole, amphotericin b and caspofungin. Chin. Med. J. 2010, 123, 2706–2709. [Google Scholar] [PubMed]
- Goncalves, S.S.; Souza, A.C.; Chowdhary, A.; Meis, J.F.; Colombo, A.L. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 2016. [Google Scholar] [CrossRef]
- Goncalves, S.S.; Stchigel, A.M.; Cano, J.; Guarro, J.; Colombo, A.L. In vitro antifungal susceptibility of clinically relevant species belonging to aspergillus section flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Koss, T.; Bagheri, B.; Zeana, C.; Romagnoli, M.F.; Grossman, M.E. Amphotericin B-resistant Aspergillus flavus infection successfully treated with caspofungin, a novel antifungal agent. J. Am. Acad. Dermatol. 2002, 46, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Azzola, A.; Passweg, J.R.; Habicht, J.M.; Bubendorf, L.; Tamm, M.; Gratwohl, A.; Eich, G. Use of lung resection and voriconazole for successful treatment of invasive pulmonary aspergillus ustus infection. J. Clin. Microbiol. 2004, 42, 4805–4808. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Marr, K.A.; Andes, D.R.; Walsh, T.J.; Kauffman, C.A.; Kontoyiannis, D.P.; Ito, J.I.; Balajee, S.A.; Pappas, P.G.; Moser, S.A. Patterns of susceptibility of aspergillus isolates recovered from patients enrolled in the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009, 47, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An emerging non-fumigatus aspergillus species of significance. Mycoses 2009, 52, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, J.W.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef] [Green Version]
- Bastos, V.R.; Santos, D.W.; Padovan, A.C.; Melo, A.S.; Mazzolin Mde, A.; Camargo, L.F.; Colombo, A.L. Early invasive pulmonary aspergillosis in a kidney transplant recipient caused by Aspergillus lentulus: First brazilian report. Mycopathologia 2015, 179, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.E.; Goncalves, S.S.; Xafranski, H.; Bergamasco, M.D.; Aquino, V.R.; Castro, P.T.; Colombo, A.L. Cryptic and rare Aspergillus species in Brazil: Prevalence in clinical samples and in vitro susceptibility to triazoles. J. Clin. Microbiol. 2014, 52, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.; Goldman, G.H.; Park, S.; Marras, S.A.; Delmas, G.; Oza, U.; Lolans, K.; Dudley, M.N.; Mann, P.A.; Perlin, D.S. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 2003, 47, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.M.; van der Linden, J.W.; Li, Y.; Kuijper, E.J.; van Dissel, J.T.; Verweij, P.E.; Melchers, W.J. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: A case study and review of the literature. Antimicrob. Agents Chemother. 2012, 56, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Snelders, E.; van der Lee, H.A.; Kuijpers, J.; Rijs, A.J.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.; Melchers, W.J.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Rhodes, J.; Beale, M.A.; Hagen, F.; Rogers, T.R.; Chowdhary, A.; Meis, J.F.; Armstrong-James, D.; Fisher, M.C. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 2015, 6, e00536. [Google Scholar] [PubMed]
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-α sterol demethylase-related genes (CYP51A and CYP51B) in Aspergillus fumigatus and other aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Moye-Rowley, W.S. Multiple mechanisms contribute to the development of clinically significant azole resistance in Aspergillus fumigatus. Front. Microbiol. 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, C.; Esposto, M.C.; Prigitano, A.; Cogliati, M.; De Lorenzis, G.; Tortorano, A.M. Azole resistance in Aspergillus fumigatus clinical isolates from an italian culture collection. Antimicrob. Agents Chemother. 2015, 60, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Bowyer, P. Voriconazole resistance in Aspergillus fumigatus: Should we be concerned? Clin. Infect. Dis. 2013, 57, 521–523. [Google Scholar] [CrossRef]
- Mellado, E.; Garcia-Effron, G.; Alcazar-Fuoli, L.; Melchers, W.J.; Verweij, P.E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of CYP51A alterations. Antimicrob. Agents Chemother. 2007, 51, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, J.W.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; Van Tiel, F.H.; Melchers, W.J.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, the netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; van der Lee, H.A.; Rijs, T.A.; Zoll, J.; Leenstra, T.; Melchers, W.J.; Verweij, P.E. Azole, polyene and echinocandin mic distributions for wild-type, TR34/L98H AND TR46/Y121F/T289A Aspergillus fumigatus isolates in The Netherlands. J. Antimicrob. Chemother. 2015, 70, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, P.; Lavergne, R.A.; Morio, F.; Alvarez-Moreno, C. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, colombia, 2015. Emerg. Infect. Dis. 2016, 22, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Fuhren, J.; Voskuil, W.S.; Boel, C.H.; Haas, P.J.; Hagen, F.; Meis, J.F.; Kusters, J.G. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J. Antimicrob. Chemother. 2015, 70, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Izumikawa, K.; Hirano, K.; Ide, S.; Mihara, T.; Hosogaya, N.; Takazono, T.; Morinaga, Y.; Nakamura, S.; Kurihara, S.; et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob. Agents Chemother. 2012, 56, 4870–4875. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; van Koningsbruggen-Rietschel, S.; Rietschel, E.; Vehreschild, M.J.; Wisplinghoff, H.; Kronke, M.; Hamprecht, A. Prevalence and molecular characterization of azole resistance in Aspergillus spp. Isolates from german cystic fibrosis patients. J. Antimicrob. Chemother. 2014, 69, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.; Jensen, R.H.; Hassan, T.M.; Mathiasen, E.G.; Thomsen, G.M.; Pedersen, U.G.; Christensen, M.; Hilberg, O.; Arendrup, M.C. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob. Agents Chemother. 2014, 58, 5096–5101. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother. 2014, 69, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Hagen, F.; Meis, J.F. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 2014, 9, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Lu, Z.; Li, P.; Zhang, Q.; Jia, T.; Zhao, J.; Tian, S.; Han, X.; Chen, F.; et al. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolate from a Chinese patient. Antimicrob. Agents Chemother. 2015, 59, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, T.; Monteiro, M.C.; Garcia-Rubio, R.; Bouza, E.; Gomez-Lopez, A.; Mellado, E. First detection of Aspergillus fumigatus azole-resistant strain due to CYP51A TR46/Y121F/T289A in an azole-naive patient in spain. New Microbes New Infect. 2015, 6, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Hamprecht, A.; Vehreschild, M.J.; Cornely, O.A.; Buchheidt, D.; Spiess, B.; Koldehoff, M.; Buer, J.; Meis, J.F.; Rath, P.M. Emergence of azole-resistant invasive aspergillosis in hsct recipients in germany. J. Antimicrob. Chemother. 2015, 70, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, R.A.; Morio, F.; Favennec, L.; Dominique, S.; Meis, J.F.; Gargala, G.; Verweij, P.E.; Le Pape, P. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in france. Antimicrob. Agents Chemother. 2015, 59, 4331–4335. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Pasqualotto, A.C.; Anderson, M.J.; Leatherbarrow, H.; Albarrag, A.M.; Harrison, E.; Gregson, L.; Bowyer, P.; Denning, D.W. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses 2013, 56, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Gregson, L.; Goodwin, J.; Johnson, A.; McEntee, L.; Moore, C.B.; Richardson, M.; Hope, W.W.; Howard, S.J. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: Correlation with itraconazole, voriconazole, and posaconazole. Antimicrob. Agents Chemother. 2013, 57, 5778–5780. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Marchillo, K.; Vanhecker, J.; Andes, D.R. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and CYP51 mutant isolates of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2013, 57, 6284–6289. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bruggemann, R.J.; Meis, J.F.; Melchers, W.J.; Verweij, P.E.; Mouton, J.W. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob. Agents Chemother. 2015, 59, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging threats in antifungal-resistant fungal pathogens. Front Med 2016, 3, 11. [Google Scholar] [CrossRef]
- Van der Linden, J.W.; Camps, S.M.; Kampinga, G.A.; Arends, J.P.; Debets-Ossenkopp, Y.J.; Haas, P.J.; Rijnders, B.J.; Kuijper, E.J.; van Tiel, F.H.; Varga, J.; et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 2013, 57, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.H.; Hagen, F.; Astvad, K.M.; Tyron, A.; Meis, J.F.; Arendrup, M.C. Azole-resistant Aspergillus fumigatus in Denmark: A laboratory-based study on resistance mechanisms and genotypes. Clin. Microbiol. Infect. 2016, 22, 570.e1–570.e9. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Lagrou, K.; Verweij, P.E. Azole resistance in Aspergillus fumigatus: A growing public health concern. Curr. Opin. Infect. Dis. 2013, 26, 493–500. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdiere, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Jensen, R.H.; Johansen, H.K.; Skov, M.; Pressler, T.; Howard, S.J.; Leatherbarrow, H.; Mellado, E.; Arendrup, M.C. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: A laboratory-based study with focus on Aspergillus fumigatus azole resistance. J. Clin. Microbiol. 2011, 49, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of aspergillosis: Clinical practice guidelines of the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole resistance in Aspergillus fumigatus: Can we retain the clinical use of mold-active antifungal azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Barnes, R.A.; Barton, R.C.; Cleverley, J.R.; Lucas, S.B.; Kibbler, C.C.; Denning, D.W.; on behalf of the British Society for Medical Mycology. British society for medical mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015, 15, 461–474. [Google Scholar] [CrossRef]
- Fraczek, M.G.; Kirwan, M.B.; Moore, C.B.; Morris, J.; Denning, D.W.; Richardson, M.D. Volume dependency for culture of fungi from respiratory secretions and increased sensitivity of aspergillus quantitative pcr. Mycoses 2014, 57, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, J.W.; Arendrup, M.C.; Melchers, W.J.; Verweij, P.E. Azole resistance of Aspergillus fumigatus in immunocompromised patients with invasive aspergillosis. Emerg. Infect. Dis. 2016, 22, 158–159. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, S.S. Global Aspects of Triazole Resistance in Aspergillus fumigatus with Focus on Latin American Countries. J. Fungi 2017, 3, 5. https://doi.org/10.3390/jof3010005
Gonçalves SS. Global Aspects of Triazole Resistance in Aspergillus fumigatus with Focus on Latin American Countries. Journal of Fungi. 2017; 3(1):5. https://doi.org/10.3390/jof3010005
Chicago/Turabian StyleGonçalves, Sarah Santos. 2017. "Global Aspects of Triazole Resistance in Aspergillus fumigatus with Focus on Latin American Countries" Journal of Fungi 3, no. 1: 5. https://doi.org/10.3390/jof3010005




