Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Locale and Design
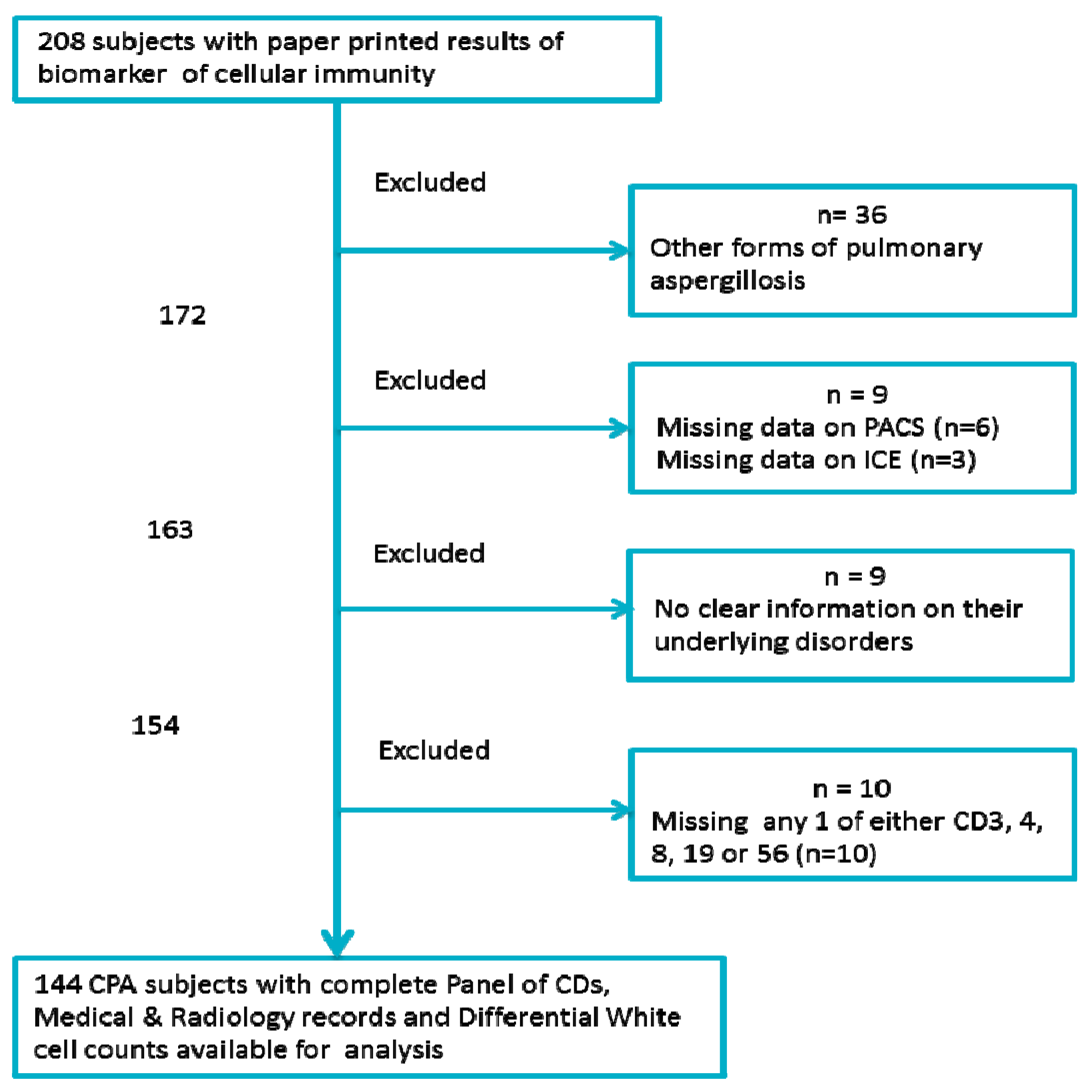
2.2. Patient Selection
2.3. Immunology Data
2.4. Medical Records
2.5. Radiology
2.6. Complete Blood Count
2.7. Data Management
2.8. Statistical Analysis
2.9. Ethical Consideration
3. Results
3.1. Demographics, Baseline Radiological and Clinical Characteristics
3.2. Expression of CDs, Total and Differential White Cell Counts
3.2.1. CD4:CD8 Ratio
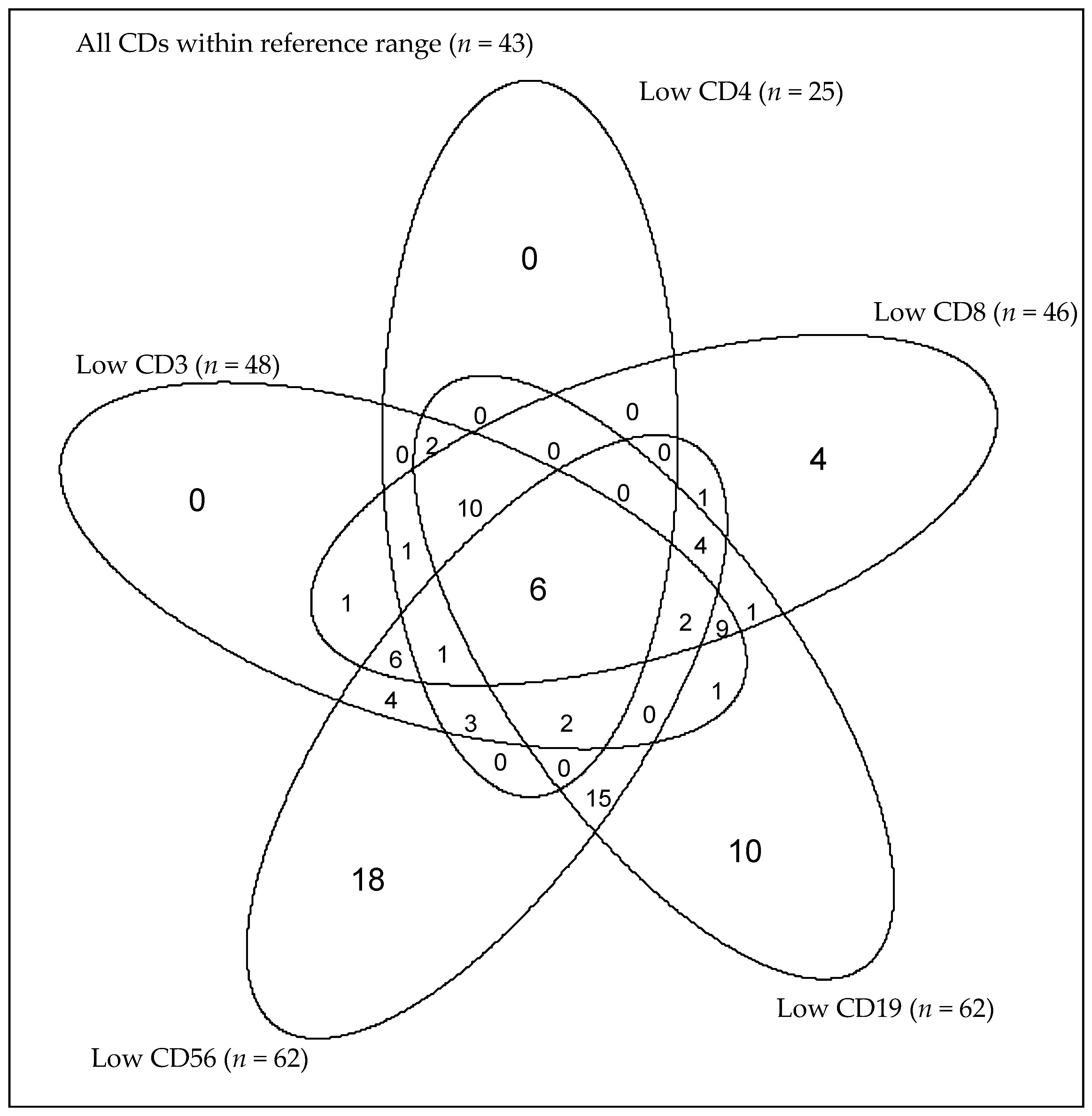
3.2.2. Combined and Isolated Lymphopenia
3.2.3. Correlation between CDs and CBC Parameters
3.3. Association between CDs and CBC Parameters, Underlying Disorders, Demographic and Radiological Characteristics
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus-What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Invasive Aspergillosis. Clin. Infect. Dis. 1998, 26, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Walsh, T.J.; Denning, D.W. The invasive and saprophytic syndromes due to Aspergillus spp. Med. Mycol. 2005, 43, S207–S238. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W.; ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Chronic forms of pulmonary aspergillosis. Clin. Microbiol. Infect. 2001, 7, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.C.; Strek, M.E. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest 2014, 146, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Camuset, J.; Lavole, A.; Wislez, M.; Khalil, A.; Bellocq, A.; Bazelly, B.; Mayaud, C.; Cadranel, J. Bronchopulmonary aspergillosis infections in the non-immunocompromised patient. Revue de Pneumologie Clinique 2007, 63, 155–166. [Google Scholar]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.E.; Novak-Frazer, L. Chronic Pulmonary Aspergillosis—Where Are We? and Where Are We Going? J. Fungi 2016, 2, 18. [Google Scholar] [CrossRef]
- Espinosa, V.; Rivera, A. First line of defense: Innate cell-mediated control of pulmonary Aspergillosis. Front. Microbiol. 2016, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Mehrad, B. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 2009, 22, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Beitzen-Heineke, A.; Bouzani, M.; Schmitt, A.L. Human Invariant Natural Killer T cells possess immune-modulating functions during Aspergillus infection. Medical 2016, 52, 169–176. [Google Scholar]
- Camargo, J.F.; Husain, S. Immune correlates of protection in human invasive aspergillosis. Clin. Infect. Dis. 2014, 59, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Travers, P.; Walport, M.; Janeway, C. Janeway’s Immuno Biology, 7th ed.; Garland Science: New York, NY, USA, 2008. [Google Scholar]
- Jolink, H.; De Boer, R.; Willems, L.N.A.; Van, D.J.T.; Falkenburg, J.H.F.; Heemskerk, M.H.M. T helper 2 response in allergic bronchopulmonary aspergillosis is not driven by specific Aspergillus antigens. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.; Singh, A.; Morris, J.; Smith, N.L.; Fraczek, M.G.; Moore, C.B.; Denning, D.W. Mannose-binding lectin genotype and serum levels in patients with chronic and allergic pulmonary aspergillosis. Int. J. Immunogenet. 2012, 39, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Koldehoff, M.; Beelen, D.W.; Elmaagacli, A.H. Increased susceptibility for aspergillosis and post-transplant immune deficiency in patients with gene variants of TLR4 after stem cell transplantation. Transpl. Infect. Dis. 2013, 15, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sainz, J.; Hassan, L.; Perez, E.; Romero, A.; Moratalla, A.; López-Fernández, E.; Oyonarte, S.; Jurado, M. Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis. Immunol. Lett. 2007, 109, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.D.; Denning, D.W. Clinical implications of interferon-γ genetic and epigenetic variants. Immunology 2014, 143, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, P.; Goodsall, A.; Mulgirigama, A.; Kunst, H.; Henderson, D.C.; Wilson, R.; Newman-Taylor, A.; Levin, M. Interferon-γ therapy in two patients with progressive chronic pulmonary aspergillosis. Eur. Respir. J. 2006, 27, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.D.; Hankinson, J.; Simpson, A.; Denning, D.W.; Bowyer, P. Reduced expression of TLR3, TLR10 and TREM1 by human macrophages in Chronic cavitary pulmonary aspergillosis, and novel associations of VEGFA, DENND1B and PLAT. Clin. Microbiol. Infect. 2014, 20, O960–O968. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Riniotis, K.; Dobrashian, R.; Sambatakou, H. Chronic Cavitary and Fibrosing Pulmonary and Pleural Aspergillosis: Case Series, Proposed Nomenclature Change, and Review. Clin. Infect. Dis. 2003, 37, S265–S280. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Romani, L.R. Invasive aspergillosis in chronic granulomatous disease. Med. Mycol. 2009, 47, S282–S290. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.; Poulsen, L.K.; Koch, C. Increased antigen-specific Th-2 response in allergic bronchopulmonary aspergillosis (ABPA) in patients with cystic fibrosis. Pediatr. Pulmonol. 1999, 27, 74–79. [Google Scholar] [CrossRef]
- Sweiss, N.J.; Salloum, R.; Ghandi, S.; Alegre, M.L.; Sawaqed, R.; Badaracco, M.; Pursell, K.; Pitrak, D.; Baughman, R.P.; Moller, D.R.; et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS ONE 2010, 5, e9088. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2013, 41, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Döffinger, R.; Harris, C.; Lear, S.; Newton, P.; Alachkar, H.; Dinakantha, S.; et al. Impaired Th1 and Th17 immunity in chronic pulmonary aspergillosis. In Proceedings of the 6th Advances against Aspergillosis; 6th Advances Against Aspergillosis: Madrid, Spain, 2014. [Google Scholar]
- Dorman, S.E.; Holland, S.M. Interferon-γ and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000, 11, 321–333. [Google Scholar] [CrossRef]
- Jayaraman, P.; Jacques, M.K.; Zhu, C.; Steblenko, K.M.; Stowell, B.L.; Madi, A.; Anderson, A.C.; Kuchroo, V.K.; Behar, S.M. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016, 12, e1005490. [Google Scholar] [CrossRef] [PubMed]
- Al-Aska, A.; Al-Anazi, A.R.; Al-Subaei, S.S.; Al-Hedaithy, M.A.; Barry, M.A.; Somily, A.M.; Buba, F.; Yusuf, U.; Al Anazi, N.A. CD4+ T-lymphopenia in HIV negative tuberculous patients at King Khalid University Hospital in Riyadh, Saudi Arabia. Eur. J. Med. Res. 2011, 16, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Cunha, C.; Iannitti, R.G.; De Luca, A.; Giovannini, G.; Bistoni, F.; Romani, L. Inflammation in aspergillosis: The good, the bad, and the therapeutic. Ann. N. Y. Acad. Sci. 2012, 1273, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hasenberg, M.; Behnsen, J.; Krappmann, S.; Brakhage, A.; Gunzer, M. Phagocyte responses towards Aspergillus fumigatus. Int. J. Med. Microbiol. 2011, 301, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.D.; Bromley, M.J.; Denning, D.W.; Simpson, A.; Bowyer, P. Elevated levels of the neutrophil chemoattractant pro-platelet basic protein in macrophages from individuals with chronic and allergic aspergillosis. J. Infect. Dis. 2015, 211, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Uffredi, M.L.; Mangiapan, G.; Cadranel, J.; Kac, G. Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Ohba, H.; Miwa, S.; Shirai, M.; Kanai, M.; Eifuku, T.; Suda, T.; Hayakawa, H.; Chida, K. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir. Med. 2012, 106, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Richardson, M.D.; Denning, D.W. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J. Infect. 2016, 72, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Powell, G.; Borrow, R.; Morris, J.; Alachkar, H.; Denning, D.W. Response to pneumococcal polysaccharide vaccination in patients with chronic and allergic aspergillosis. Vaccine 2015, 33, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Tubby, C.; Negm, O.H.; Harrison, T.; Tighe, P.J.; Todd, I.; Fairclough, L.C. Peripheral killer cells do not differentiate between asthma patients with or without fixed airway obstruction. J. Asthma 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Slade, J.D.; Hepburn, B. Prednisone-induced alterations of circulating human lymphocyte subsets. J. Lab. Clin. Meid. 1983, 101, 479–487. [Google Scholar]
| Characteristic | Number | Percent |
|---|---|---|
| Gender | ||
| Male | 85 | 59.0 |
| Female | 59 | 41.0 |
| Pleural thickening | ||
| Yes | 91 | 63.2 |
| No | 53 | 36.8 |
| Cavitation | ||
| Multicavitary | 91 | 63.2 |
| Single cavity or Aspergillus nodule | 53 | 36.8 |
| Aspergilloma | ||
| Yes | 71 | 49.3 |
| No | 73 | 50.7 |
| Lung involvement | ||
| Bilateral | 57 | 39.6 |
| Unilateral | 87 | 60.4 |
| Left Unilateral | 31 | 21.5 |
| Right Unilateral | 56 | 38.9 |
| Lobes involved | ||
| Upper lobes | 117 | 81.3 |
| Middle lobe | 11 | 7.6 |
| Lower lobe | 16 | 11.1 |
| Underlying Disorder | Number | Percent |
|---|---|---|
| Tuberculosis | 31 | 21.5 |
| Bronchiectasis | 25 | 17.4 |
| Asthma | 24 | 16.7 |
| Chronic obstructive pulmonary diseases | 23 | 16.0 |
| Non-tuberculous mycobacterial infection | 17 | 11.8 |
| Allergic bronchopulmonary aspergillosis | 17 | 11.8 |
| Previous lung surgery | 17 | 11.8 |
| Emphysema | 15 | 10.4 |
| Sarcoidosis | 13 | 9.0 |
| Pneumothorax | 13 | 9.0 |
| Gamma interferon deficiency | 12 | 8.3 |
| Mannose binding lectin-deficiency | 10 | 6.9 |
| Lung cancer survivors | 7 | 4.9 |
| Rheumatoid arthritis | 4 | 2.8 |
| Impaired interleukin-17 production | 2 | 1.4 |
| Total | 230 | 159.7% |
| Parameter (Reference Range *) | Mean or Median | Range | Standard Deviation | Below the Reference Range n (%) | Within the Reference Range n (%) | Above Reference Range n (%) |
|---|---|---|---|---|---|---|
| CD3 (700–2100) | 1019 | 73–2684 | 563 | 48 (33.3) | 90 (62.5) | 6 (4.2) |
| CD4 (300–1400) | 652 | 36–1815 | 361 | 25 (17.4) | 112 (77.8) | 7 (4.9) |
| CD4 <200 | - | - | - | 15 (10.4) | - | - |
| CD8 (200–900) | 312 | 25–1415 | - | 46 (31.9) | 89 (61.8) | 9 (6.3) |
| CD19 (100–500) | 114 | 11–1234 | - | 62 (43.1) | 79 (54.2) | 4 (2.8) |
| CD56 (90–600) | 113 | 2–556 | - | 62 (43.1) | 82 (56.9) | 0 (0.0) |
| CD4:CD8 Ratio (0.9–1.9) | 2.1 | 0.4–6.9 | 1.2 | 15 (10.4) | 61 (42.4) | 68 (47.2) |
| White cell count (4–11) | 8.2 | 3.9–22.2 | - | 1 (0.7) | 115 (79.9) | 28 (19.4) |
| Neutrophils (2.0–7.5) | 5.4 | 2.1–19.8 | - | 0 (0.0) | 106 (73.6) | 38 (26.4) |
| Lymphocytes (1.5–4.0) | 1.5 | 0.2–3.2 | 0.30 | 84 (58.3) | 60 (41.7) | 0 (0.0) |
| Monocytes (0.2–0.8) | 0.62 | 0.13–1.67 | - | 2 (1.4) | 99 (68.8) | 43 (29.9) |
| Biomarker # | N (%) | Mean CDs (Range) | Mean Lymphocytes (Range) | Mean Monocytes (Range) |
|---|---|---|---|---|
| CD56 | 18 (12.5) | 61 (21–86) | 2.0 (1.35–2.98) | 0.66 (0.33–1.04) |
| CD19 | 10 (6.9) | 76 (25–96) | 1.63 (0.98–2.51) | 0.82 (0.21–1.67) |
| CD56 and CD19 | 15 (10.4) | – | 1.6 (1.01–2.73) | 0.69 (0.43–1.35) |
| CD8 | 4 (2.8) | 155 (111–178) | 1.21 (1.12–1.27) | 0.55 (0.39–0.71) |
| CD4 | 0 | – | – | – |
| CD3 | 0 | – | – | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongomin, F.; Harris, C.; Foden, P.; Kosmidis, C.; Denning, D.W. Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis. J. Fungi 2017, 3, 26. https://doi.org/10.3390/jof3020026
Bongomin F, Harris C, Foden P, Kosmidis C, Denning DW. Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis. Journal of Fungi. 2017; 3(2):26. https://doi.org/10.3390/jof3020026
Chicago/Turabian StyleBongomin, Felix, Chris Harris, Philip Foden, Chris Kosmidis, and David W. Denning. 2017. "Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis" Journal of Fungi 3, no. 2: 26. https://doi.org/10.3390/jof3020026






