Monocyte Phenotype and IFN-γ-Inducible Cytokine Responses Are Associated with Cryptococcal Immune Reconstitution Inflammatory Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Peripheral Blood Mononuclear Cells (PBMCs) Stimulation
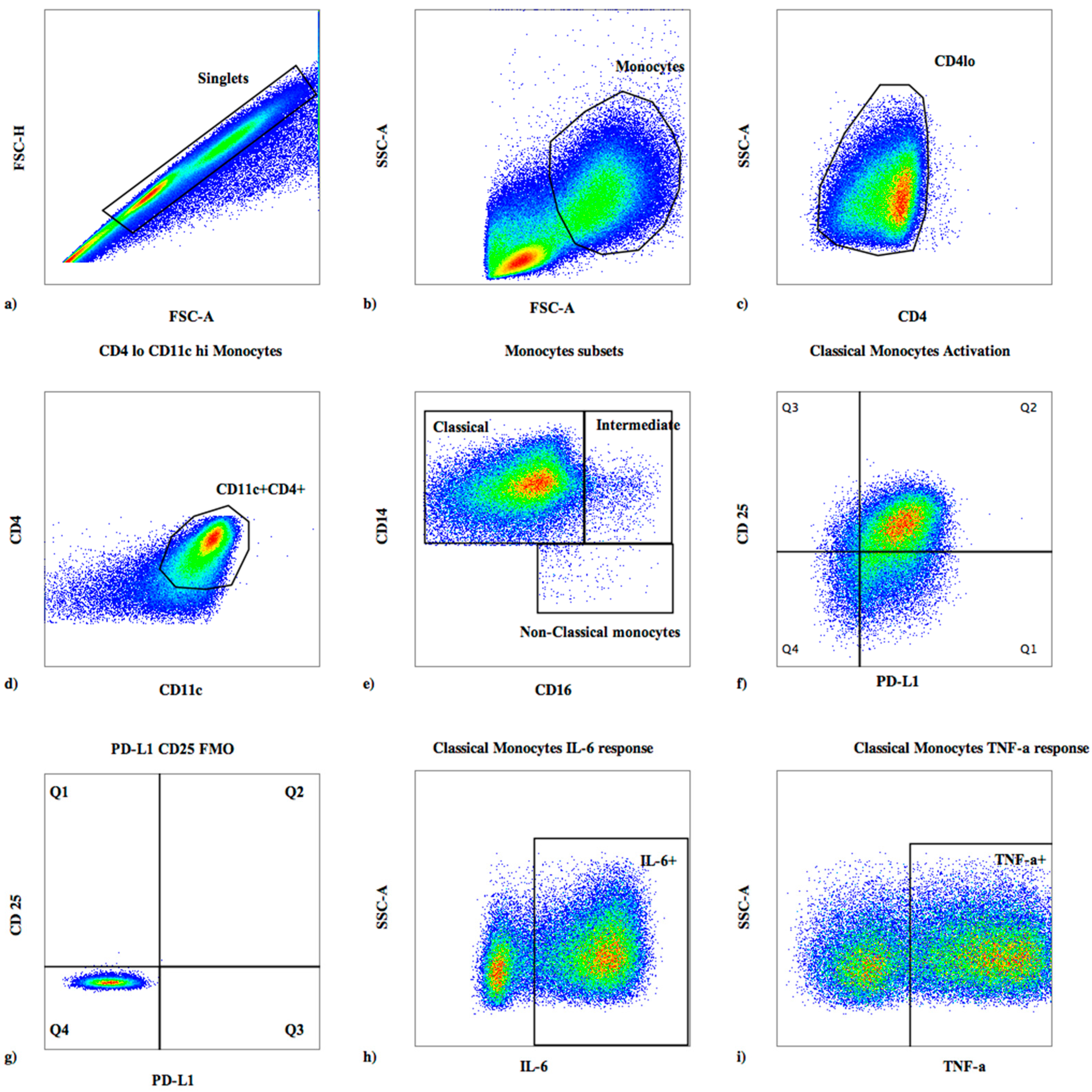
2.3. Surface Flow Cytometric Staining
2.4. Intracellular Cytokine Staining
2.5. Statistical Analysis
3. Results
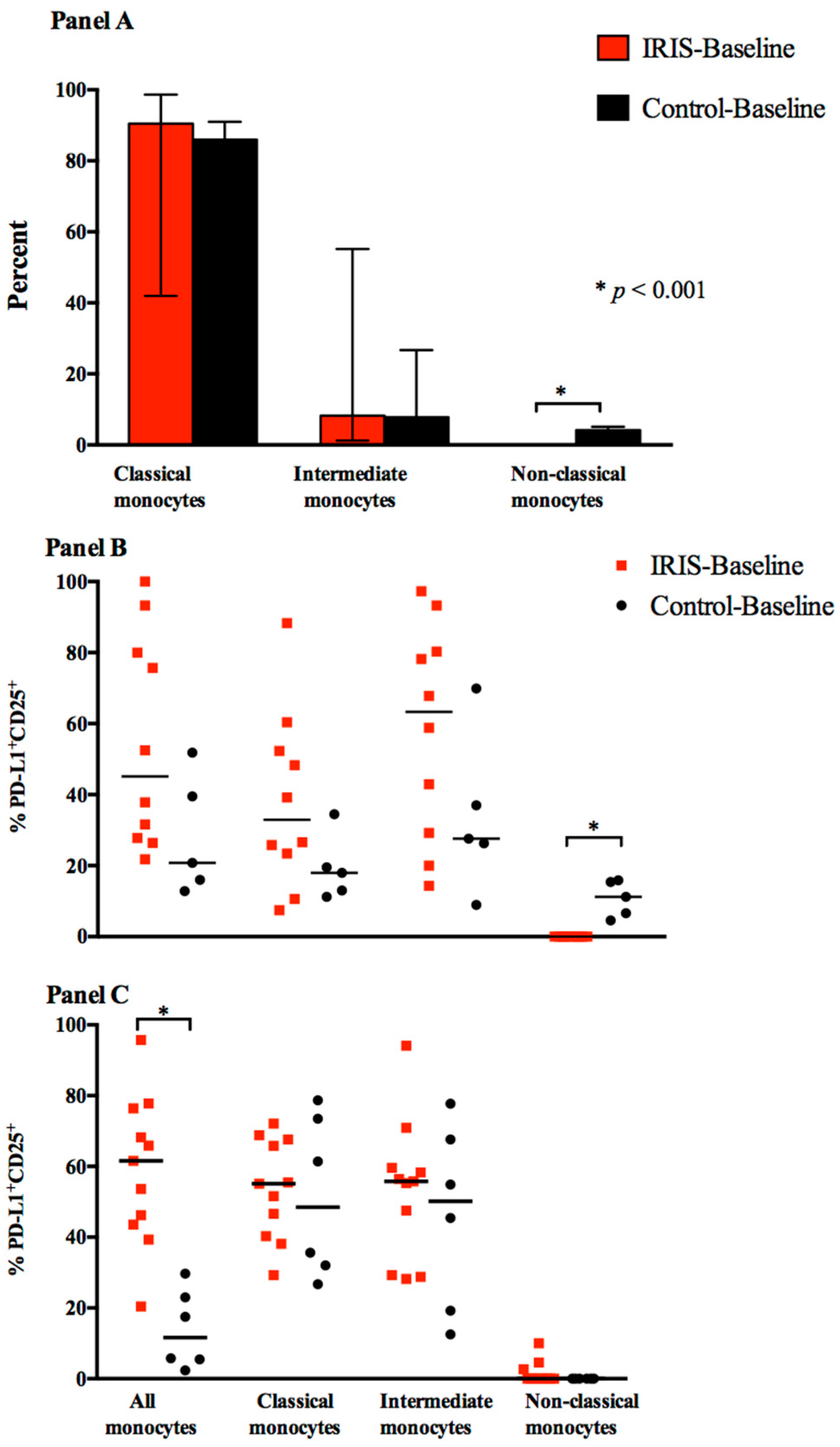
3.1. Baseline Monocyte Subsets
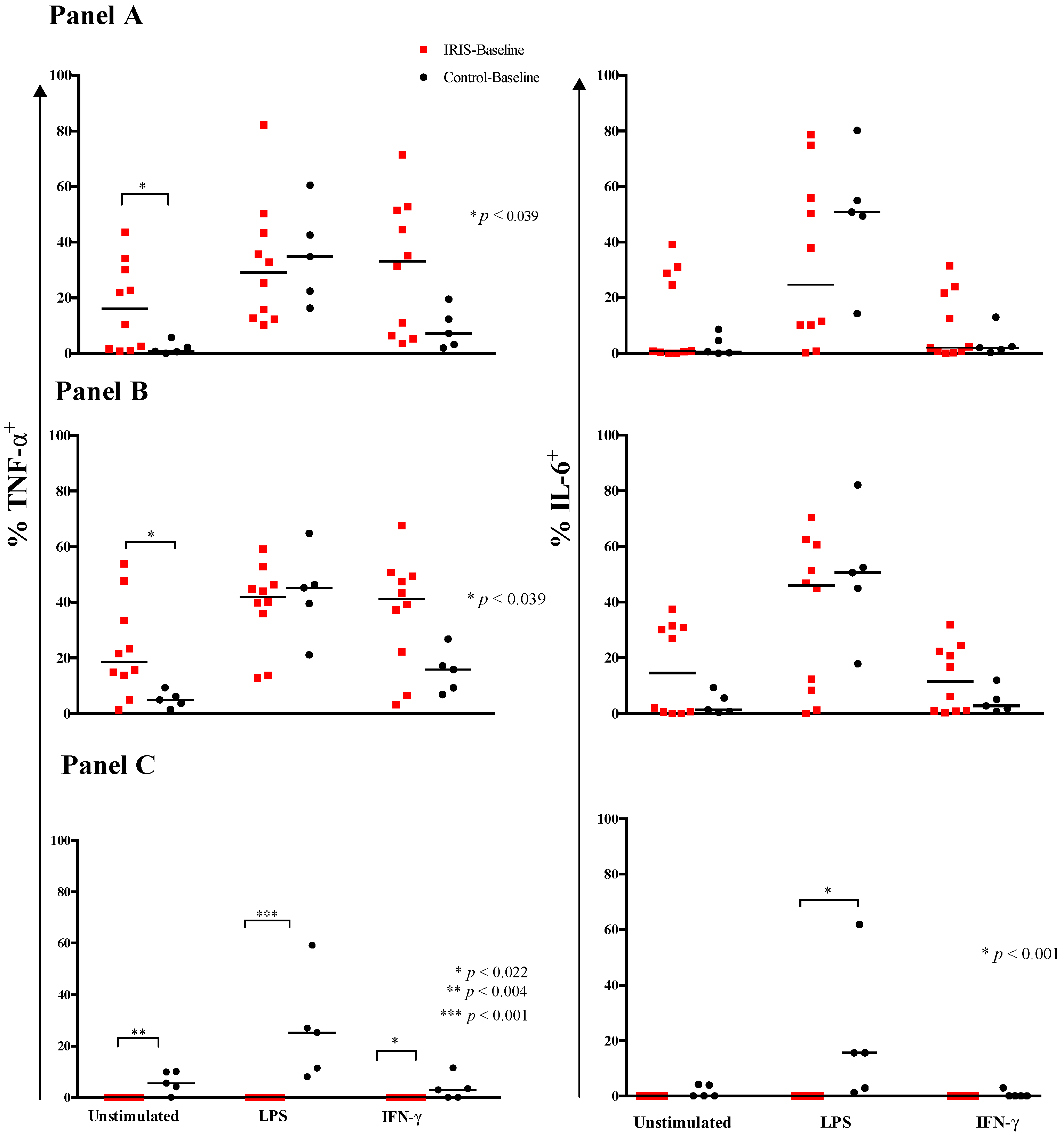
3.2. Cytokine Expression and Responses at Baseline
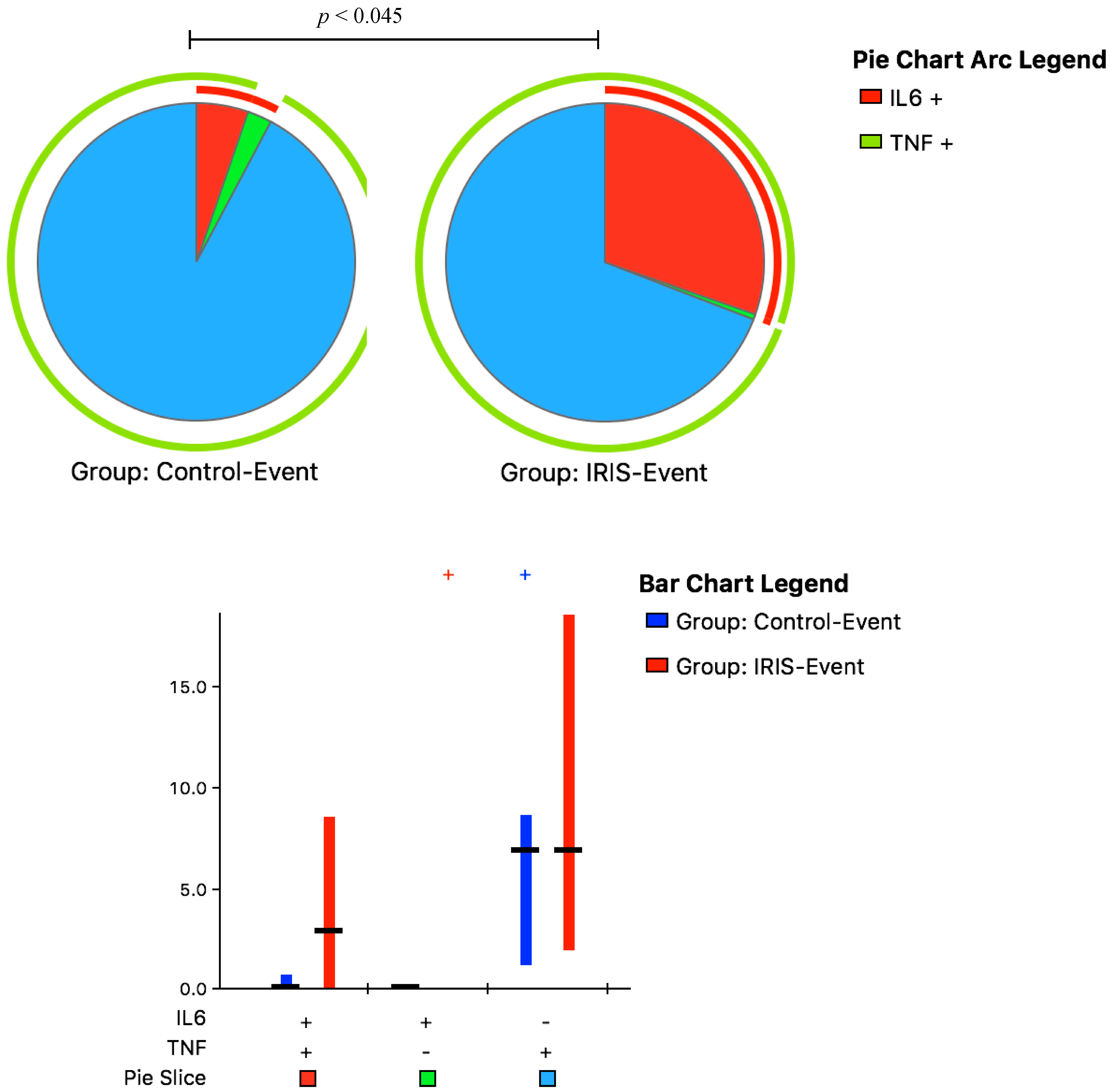
3.3. Cytokine Responses at CM-IRIS
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflict of Interests
References
- French, N.; Gray, K.; Watera, C.; Nakiyingi, J.; Lugada, E.; Moore, M.; Lalloo, D.; Whitworth, J.A.; Gilks, C.F. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002, 16, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.G.; Gangaidzo, I.T.; Heyderman, R.S.; Mielke, J.; Mushangi, E.; Taziwa, A.; Robertson, V.J.; Musvaire, P.; Mason, P.R.; Hakim, J.G.; et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: A prospective study of 406 predominantly adult patients. AIDS 2000, 14, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Lipscomb, M.F. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 1998, 149, 387–396. [Google Scholar] [CrossRef]
- Lortholary, O.; Fontanet, A.; Mémain, N.; Martin, A.; Sitbon, K.; Dromer, F.; French Cryptococcosis Study Group. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS 2005, 19, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Wandel, S.; Colebunders, R.; Attia, S.; Furrer, H.; Egger, M.; for IeDEA Southern and Central Africa. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 251–261. [Google Scholar] [CrossRef]
- French, M.A. HIV/AIDS: Immune reconstitution inflammatory syndrome: A reappraisal. Clin. Infect. Dis. 2009, 48, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, A.M.; Trevenzoli, M.; Sasset, L.; Lanzafame, M.; Marchioro, U.; Meneghetti, F. Multiple cerebral cryptococcomas associated with immune reconstitution in HIV-1 infection. AIDS 2004, 18, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, J.R.; Goddeeris, K.H.; Vanopdenbosch, L.J.; Casselman, J.W. Relapsing meningitis caused by persistent cryptococcal antigens and immune reconstitution after the initiation of highly active antiretroviral therapy. AIDS 2004, 18, 1223–1224. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Meya, D.B.; Bergemann, T.L.; Wiesner, D.L.; Abdu Musubire, J.R.; Lee, S.J.; Kambugu, A.; Janoff, E.N.; Bohjanen, P.R. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: A prospective cohort study. PLoS Med. 2010, 7, e1000384. [Google Scholar] [CrossRef] [PubMed]
- Sungkanuparph, S.; Filler, S.G.; Chetchotisakd, P.; Pappas, P.G.; Nolen, T.L.; Manosuthi, W.; Anekthananon, T.; Morris, M.I.; Supparatpinyo, K.; Kopetskie, H.; et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: A prospective multicenter study. Clin. Infect. Dis. 2009, 49, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, S.A., 3rd; Darcourt, J.; White, A.C., Jr.; Greenberg, S.B.; Hamill, R.J.; Atmar, R.L.; Visnegarwala, F. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2005, 40, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Dorasamy, A.A.; Gosnell, B.I.; Elliott, J.H.; Spelman, T.; Omarjee, S.; Naranbhai, V.; Coovadia, Y.; Ndung’u, T.; Moosa, M.Y.; et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS 2013, 27, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Bonham, S.C.; Meya, D.B.; Wiesner, D.L.; Park, G.S.; Kambugu, A.; Janoff, E.N.; Bohjanen, P.R. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J. Infect. Dis. 2010, 202, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Meintjes, G.; Rebe, K.; Williams, G.N.; Bicanic, T.; Williams, A.; Schutz, C.; Bekker, L.G.; Wood, R.; Harrison, T.S. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: A randomized controlled trial. AIDS 2012, 26, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, L.R.; Mahnke, Y.; Hodge, J.N.; Porter, B.O.; Barber, D.L.; DerSimonian, R.; Greenwald, J.H.; Roby, G.; Mican, J.; Sher, A.; et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 2010, 116, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Greenwald, J.H.; DerSimonian, R.; Roby, G.; Antonelli, L.R.; Sher, A.; Roederer, M.; Sereti, I. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood 2012, 119, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Bourgarit, A.; Carcelain, G.; Martinez, V.; Lascoux, C.; Delcey, V.; Gicquel, B.; Vicaut, E.; Lagrange, P.; Sereni, D.; Autran, B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006, 20, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Andrade, B.B.; Sereti, I.; Sher, A. Immune reconstitution inflammatory syndrome: The trouble with immunity when you had none. Nat. Rev. Microbiol. 2012, 10, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Meya, D.B.; Muzoora, C.; Rolfes, M.A.; Hullsiek, K.H; Musubire, A.; Taseera, K.; Nabeta, H.W.; Schutz, C.; Williams, D.A.; et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014, 370, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Haddow, L.J.; Colebunders, R.; Meintjes, G.; Lawn, S.D.; Elliott, J.H.; Manabe, Y.C.; Bohjanen, P.R.; Sungkanuparph, S.; Easterbrook, P.J.; French, M.A.; et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: Proposed clinical case definitions. Lancet Infect. Dis. 2010, 10, 791–802. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc Biol. 2007, 81, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Nozzi, J.L.; Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytom. A 2011, 79, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chretien, F.; Lortholary, O.; Kansau, I.; Neuville, S.; Gray, F.; Dromer, F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 2002, 186, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Chang, C.C.; Durgiah, R.; Omarjee, S.; Lim, A.; Moosa, M.Y.; Elliot, J.H.; Ndung’u, T.; Lewin, S.R.; French, M.A.; et al. Compartmentalization of innate immune responses in the central nervous system during cryptococcal meningitis/HIV coinfection. AIDS 2014, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.B.; Singh, A.; Narendran, G.; Schechter, M.E.; Nayak, K.; Subramanian, S.; Anbalagan, S.; Jensen, S.M.; Porter, B.O.; Antonelli, L.R.; et al. Mycobacterial Antigen Driven Activation of CD14++CD16− Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. PLoS Pathog. 2014, 10, e1004433. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Van den Bergh, R.; Vu, T.N.; Laukens, K.; Worodria, W.; Loembe, M.M.; Colebunders, R.; Kestens, L.; De Baetselier, P.; Raes, G.; et al. The role of monocytes in the development of Tuberculosis-associated Immune Reconstitution Inflammatory Syndrome. Immunobiology. 2014, 219, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Van den Bergh, R.; Loembe, M.M.; Worodria, W.; Mayanja-Kizza, H.; Colebunders, R.; Mascart, F.; Stordeur, P.; Kestens, L.; De Baetselier, P.; et al. Modulation of the complement system in monocytes contributes to tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2013, 27, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Gauduin, M.C.; Kaur, A.; Ahmad, S.; Yilma, T.; Lifson, J.D.; Johnson, R.P. Optimization of intracellular cytokine staining for the quantitation of antigen-specific CD4+ T cell responses in rhesus macaques. J. Immunol. Methods 2004, 288, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.O.; Ouedraogo, G.L.; Hodge, J.N.; Smith, M.A.; Pau, A.; Roby, G.; Kwan, R.; Bishop, R.J.; Rehm, C.; Mican, J.; et al. d-Dimer and CRP levels are elevated prior to antiretroviral treatment in patients who develop IRIS. Clin. Immunol. 2010, 136, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Fox, Z.; Lundgren, J.D.; Kuller, L.H.; Boesecke, C.; Gey, D.; Skoutelis, A.; Goetz, M.B.; Phillips, A.N.; Group ISfMoATS. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J. Infect. Dis. 2009, 200, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Kalayjian, R.C.; Machekano, R.N.; Rizk, N.; Robbins, G.K.; Gandhi, R.T.; Rodriguez, B.A.; Pollard, R.B.; Lederman, M.M.; Landay, A. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J. Infect. Dis. 2010, 201, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.F.; Price, P.; Brochier, J.; French, M.A. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J. Infect. Dis. 2001, 184, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Price, P.; Keane, N.; Murray, R.; French, M. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002, 3, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincon, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Murdock, B.J.; Huffnagle, G.B.; Olszewski, M.A.; Osterholzer, J.J. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect. Immun. 2014, 82, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, Y.; Kawakami, K. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 2002, 21, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Retini, C.; Pietrella, D.; Monari, C.; Tascini, C.; Beccari, T.; Kozel, T.R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect. Immun. 1995, 63, 2919–2923. [Google Scholar] [PubMed]
- Chaka, W.; Heyderman, R.; Gangaidzo, I.; Robertson, V.; Mason, P.; Verhoef, J.; Verheul, A.; Hoepelman, A.I. Cytokine profiles in cerebrospinal fluid of human immunodeficiency virus-infected patients with cryptococcal meningitis: No leukocytosis despite high interleukin-8 levels. University of Zimbabwe Meningitis Group. J. Infect. Dis. 1997, 176, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Andrade, B.B.; McBerry, C.; Sereti, I.; Sher, A. Role of IL-6 in Mycobacterium avium—Associated immune reconstitution inflammatory syndrome. J. Immunol. 2014, 192, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Sewell, L.D.; Mowad, C.M. Primary cutaneous Cryptococcosis during therapy with methotrexate and adalimumab. J. Drugs Dermatol. 2008, 7, 53–54. [Google Scholar] [PubMed]
- Salmon-Ceron, D.; Tubach, F.; Lortholary, O.; Chosidow, O.; Bretagne, S.; Nicolas, N.; Cuillerier, E.; Fautrel, B.; Michelet, C.; Morel, J.; et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann. Rheum. Dis. 2011, 70, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sitapati, A.M.; Kao, C.L.; Cachay, E.R.; Masoumi, H.; Wallis, R.S.; Mathews, W.C. Treatment of HIV-related inflammatory cerebral cryptococcoma with adalimumab. Clin. Infect. Dis. 2010, 50, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Scemla, A.; Gerber, S.; Duquesne, A.; Parize, P.; Martinez, F.; Anglicheau, D.; Snanoudj, R.; Zuber, M.; Bougnoux, M.E.; Legendre, C.; et al. Dramatic improvement of severe cryptococcosis-induced immune reconstitution syndrome with adalimumab in a renal transplant recipient. Am. J. Transplant. 2015, 15, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Meya, D.B.; Okurut, S.; Zziwa, G.; Rolfes, M.A.; Melander, K.; Cose, S.; Joloba, M.; Naluyima, P.; Palmer, B.E.; Kambugu, A.; et al. Cellular Immune Activation in Cerebrospinal Fluid from Ugandans with Cryptococcal Meningitis and Immune Reconstitution Inflammatory Syndrome. J. Infect. Dis. 2014, 211, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Omarjee, S.; Lim, A.; Spelman, T.; Gosnell, B.I.; Carr, W.H.; Elliott, J.H.; Moosa, M.Y.; Ndung’u, T.; French, M.A.; et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J. Infect. Dis. 2013, 208, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Meintjes, G.; Bicanic, T.; Buffa, V.; Hogan, L.; Mo, S.; Tomlinson, G.; Kropf, P.; Noursadeghi, M.; Harrison, T.S. Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis. PLoS Pathog. 2015, 11, e1004754. [Google Scholar] [CrossRef] [PubMed]
- Scriven, J.E.; Rhein, J.; Hullsiek, K.H.; von Hohenberg, M.; Linder, G.; Rolfes, M.A.; Williams, D.A.; Taseera, K.; Meya, D.B.; Meintjes, G.; et al. Early ART After Cryptococcal Meningitis Is Associated With Cerebrospinal Fluid Pleocytosis and Macrophage Activation in a Multisite Randomized Trial. J. Infect. Dis. 2015, 212, 769–778. [Google Scholar] [CrossRef] [PubMed]

| Variable | Controls (n = 6) | CM-IRIS (n = 11) | p-Value |
|---|---|---|---|
| Male % | 17% | 73% | 0.05 |
| Age years | 35 (±9) | 35 (±8) | 0.937 |
| Baseline CD4 T cells/µL | 8 (5, 166) | 6.5 (4, 28) | 0.828 |
| CD4 T cells/µL > 3 months after ART | 156 (55, 309) | 68 (33, 79) | 0.256 |
| Baseline CD8 T cells/µL | 163 (97, 784) | 256 (140, 591) | 0.515 |
| CD8 T cells/µL > 3 months after ART | 1005 (615, 1086) | 831 (565, 997) | 0.463 |
| Baseline plasma HIV-1 RNA (log10copies/mL) | 4.93 (±0.34) | 5.24 (±0.5) | 0.280 |
| Baseline Cryptococcus log10 colony forming units/mL | 3.97 (2.45, 5.26) | 5.33 (4.96, 5.46) | 0.078 |
| Baseline CSF CrAg titer | 4512 (528, 12,192) | 7,200 (4048, 16,384) | 0.455 |
| Serum CrAg titer at CM-IRIS | 40,960 (8960, 102,400) | - | |
| CSF CrAg titer at CM-IRIS | 12,000 (4672, 16,384) | - | |
| CSF protein (mg/dL) | 60 (47, 68) | 53 (20, 70) | 0.471 |
| Baseline CSF white cells/mL | 19.5 (<5, 45) | <5 (<5, <5) | 0.169 |
| Duration from ART initiation (days) | 67 (48, 92) | 78 (43, 202) | 0.737 |
| Monocyte Population with Intracellular Cytokine Production | Control-Baseline (n = 5) | IRIS-Baseline (n = 10) | p-Value |
|---|---|---|---|
| All Monocytes | |||
| TNF-α+ | 7 (3, 16) | 33 (6, 52) | 0.096 |
| IL-6+ | 2.0 (0.8, 7.7) | 2.0 (0.6, 22) | 0.893 |
| TNF-α+ or IL-6+ | 8 (3, 18) | 35 (6, 53) | 0.050 |
| Activated Monocytes (PD-L1+CD25+) | |||
| TNF-α+ | 11 (4, 21) | 50 (23, 55) | 0.026 |
| IL-6+ | 3 (1, 13) | 27 (6, 28) | 0.018 |
| Activated Classical Monocytes (PD-L1+CD25+) | |||
| TNF-α+ | 13 (7, 26) | 42 (19, 56) | 0.073 |
| IL-6+ | 3 (1, 13) | 14 (1, 29) | 0.196 |
| Intermediate Monocytes | |||
| TNF-α+ | 9 (5, 31) | 29 (9, 52) | 0.291 |
| IL-6+ | 5 (2, 36) | 13 (1, 33) | 0.834 |
| Activated Intermediate Monocytes (PD-L1+CD25+) | |||
| TNF-α+ | 9 (6, 28) | 36 (8, 69) | 0.240 |
| IL-6+ | 5 (1, 41) | 18 (2, 44) | 0.743 |
| Activated Non-Classical monocytes (PD-L1+CD25+) | |||
| TNF-α+ | 3.0 (0, 7) | 0.0 | 0.022 |
| IL-6+ | 0.0 (0, 2) | 0.0 | 0.333 |
| Monocyte Population with Intracellular Cytokine Production | Control-Event (n = 6) | IRIS-Event (n = 11) | p-Value |
|---|---|---|---|
| All Monocytes | |||
| TNF-α+ | 8 (2, 10) | 22 (7, 52) | 0.091 |
| IL-6+ | 0.6 (0.4, 1.3) | 25 (8, 37) | <0.001 |
| TNF-α+ and IL-6+ | 8 (3, 10) | 39 (20, 61) | 0.003 |
| Activated Monocytes (PD-L1+CD25+) | |||
| TNF-α+ | 10 (6, 18) | 36 (24, 64) | 0.007 |
| IL-6+ | 0.4 (0.2, 1.0) | 36 (7, 44) | <0.001 |
| Classical Monocytes | |||
| TNF-α+ | 9 (3, 14) | 29 (23, 47) | 0.005 |
| IL-6+ | 1.5 (0.4, 2.0) | 24 (8, 30) | <0.001 |
| Activated Classical Monocytes | |||
| TNF-α+ | 7 (1, 17) | 26 (17, 45) | 0.010 |
| IL-6+ | 0.6 (0.4, 1.3) | 21 (9, 36) | <0.001 |
| Intermediate Monocytes | |||
| TNF-α+ | 7 (4, 11) | 25 (16, 76) | 0.020 |
| IL-6+ | 0.9 (0.4, 1.5) | 23 (7, 40) | <0.001 |
| Activated Intermediate Monocytes | |||
| TNF-α+ | 6 (5, 13) | 24 (13, 72) | 0.025 |
| IL-6+ | 0.5 (0.1, 0.7) | 25 (8, 46) | 0.003 |
| Non-Classical Monocytes | |||
| TNF-α+ | 0 | 0 (0, 39) | 0.102 |
| L-6+ | 0 | 0 (0, 16) | 0.102 |
| Activated Non-Classical Monocytes | |||
| TNF-α+ | 0 | 0 (0, 80) | 0.353 |
| IL-6+ | 0 | 0 | 0.515 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meya, D.B.; Okurut, S.; Zziwa, G.; Cose, S.; Bohjanen, P.R.; Mayanja-Kizza, H.; Joloba, M.; Boulware, D.R.; Yukari Manabe, C.; Wahl, S.; et al. Monocyte Phenotype and IFN-γ-Inducible Cytokine Responses Are Associated with Cryptococcal Immune Reconstitution Inflammatory Syndrome. J. Fungi 2017, 3, 28. https://doi.org/10.3390/jof3020028
Meya DB, Okurut S, Zziwa G, Cose S, Bohjanen PR, Mayanja-Kizza H, Joloba M, Boulware DR, Yukari Manabe C, Wahl S, et al. Monocyte Phenotype and IFN-γ-Inducible Cytokine Responses Are Associated with Cryptococcal Immune Reconstitution Inflammatory Syndrome. Journal of Fungi. 2017; 3(2):28. https://doi.org/10.3390/jof3020028
Chicago/Turabian StyleMeya, David B., Samuel Okurut, Godfrey Zziwa, Stephen Cose, Paul R. Bohjanen, Harriet Mayanja-Kizza, Moses Joloba, David R. Boulware, Carol Yukari Manabe, Sharon Wahl, and et al. 2017. "Monocyte Phenotype and IFN-γ-Inducible Cytokine Responses Are Associated with Cryptococcal Immune Reconstitution Inflammatory Syndrome" Journal of Fungi 3, no. 2: 28. https://doi.org/10.3390/jof3020028





