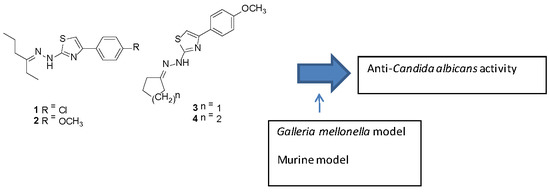
Anti-Candida albicans Activity of Thiazolylhydrazone Derivatives in Invertebrate and Murine Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Microbial Strains and Inoculum Preparation
2.3. Determination of Minimum Inhibitory Concentration (MIC)
2.4. Human Erythrocyte Hemolysis
2.5. Cytotoxicity Assay
2.6. Test of Compound Toxicity in G. mellonella
2.7. G. mellonella Survival
2.8. Murine Models
2.8.1. Animals
2.8.2. Oral Candidiasis
2.8.3. Systemic Candidiasis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antifungal Susceptibility Tests
3.2. Compound Toxicity
3.3. G. mellonella Survival
3.4. In Vivo Assay in Murine Model of Oral and Systemic Candidiasis.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Menezes, E.A.; Cunha, M.D.C.; Dos, S.O.; Ferreira, É.B.; Capelo, L.G.; Braz, B.H.L.; Cunha, F.A. Perfil de suscetibilidade de Candida tropicalis a antifúngicos sistêmicos. Rev. Patol. Trop. 2013, 42, 49–55. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.; Azeredo, J. Silicone colonization by non-Candida albicans Candida species in the presence of urine. J. Med. Microbiol. 2010, 59, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; Powell, A.T.; Saville, S.P.; Mchardy, S.F.; Lopez-Ribot, J.L. A novel small molecule inhibitor of candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Khoury, P.E.; Awad, A.; Wex, B.; Khalaf, R.A. Proteomic analysis of a Candida albicans pir32 null strain reveals proteins involved in adhesion, filamentation and virulence. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Human fungal infections: The Hidden Killers. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef]
- Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A.; Fioravanti, R.; Chimenti, P.; Granese, A.; Carradori, S.; Rivanera, D.; et al. Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg. Med. Chem. Lett. 2007, 17, 4635–4640. [Google Scholar] [CrossRef]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef]
- Lino, C.I.; Gonçalves de Souza, I.; Borelli, B.M.; Silvério Matos, T.T.; Santos Teixeira, I.N.; Ramos, J.P.; Maria de Souza Fagundes, E.; de Oliveira Fernandes, P.; Maltarollo, V.G.; Johann, S.; et al. Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem. 2018, 151, 248–260. [Google Scholar] [CrossRef]
- Fuchs, B.B.; RajaMuthiah, R.; Souza, A.C.R.; Eatemadpour, S.; Rossoni, R.D.; Santos, D.A.; Junqueira, J.C.; Rice, L.B.; Mylonakis, E. Inhibition of bacterial and fungal pathogens by the orphaned drug auronofin. Future Med. Chem. 2016, 8, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.R.; Fuchs, B.B.; Pinhati, H.M.S.; Siqueira, R.A.; Hagen, F.; Meis, J.F.; Mylonakis, E.; Colombo, A.L. Candida parapsilosis resistance to fluconazole: Molecular mechanisms and in vivo impact in infected Galleria mellonella larvae. Antimicrob. Agents Chemother. 2015, 59, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standard Institute. Reference Method for Broth Dilution; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2008; ISBN 1562388649. [Google Scholar]
- Rosch, J.W.; Boyd, A.R.; Hinojosa, E.; Pestina, T.; Hu, Y.; Persons, D.A.; Orihuela, C.J.; Tuomanen, E.I. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J. Clin. Investig. 2010, 120, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, B.B.; O’Brien, E.; El Khoury, J.B.; Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.S.W.; Kao, R.Y.T.; Yuen, K.Y.; Wang, Y.; Yang, D.; Samaranayake, L.P.; Seneviratne, C.J. In vitro and in vivo activity of a novel antifungal small molecule against Candida infections. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010; Volume 28, ISBN 1562386662.
- Prates, R.A.; Fuchs, B.B.; Mizuno, K.; Naqvi, Q.; Kato, I.T.; Ribeiro, M.S.; Mylonakis, E.; Tegos, G.P.; Hamblin, M.R. Effect of Virulence Factors on the Photodynamic Inactivation of Cryptococcus neoformans. PLoS ONE 2013, 8, 10–13. [Google Scholar] [CrossRef]
- Pagano, M.; Faggio, C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef]
- Sá, N.P.; Lima, C.M.; dos Santos, J.R.A.; Costa, M.C.; de Barros, P.P.; Junqueira, J.C.; Vaz, J.A.; Oliveira, R.B.; Fuchs, B.B.; Mylonakis, E.; et al. A phenylthiazole derivative demonstrates efficacy on treatment of the cryptococcosis & candidiasis in animal models. Future Sci. OA 2018, 4, FSO305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá, N.P.; Lima, C.M.; Lino, C.I.C.; Barbeira, P.J.S.; Baltazar, L.M.; Santos, D.A.; Oliveira, R.B.; Mylonakis, E.; Fuchs, B.B.; Johann, S. Heterocycle Thiazole Compounds Exhibit Antifungal Activity through Increase in the Production of Reactive Oxygen Species in the Cryptococcus neoformans—Cryptococcus gattii Species Complex. Antimicrob. Agents Chemother. 2017, 61, 2700–2716. [Google Scholar] [CrossRef]
- Mersch-Sundermann, V.; Knasmüller, S.; Wu, X.J.; Darroudi, F.; Kassie, F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology 2004, 198, 329–340. [Google Scholar] [CrossRef]
- Kamalian, L.; Chadwick, A.E.; Bayliss, M.; French, N.S.; Monshouwer, M.; Snoeys, J.; Park, B.K. The utility of HepG2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol. Vitr. 2015, 29, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Moreno, R.; Idnurm, A.; Heitman, J.; Stephen, B.; Ausubel, F.M.; Diener, A.; Khoury, J.B.; Calderwood, S.B. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef] [PubMed]
- De Sá, N.P.; Lino, C.I.; Fonseca, N.C.; Borelli, B.M.; Ramos, J.P.; Souza-Fagundes, E.M.; Rosa, C.A.; Santos, D.A.; Oliveira, R.B.; Johann, S. Thiazole compounds with activity against Cryptococcus gattii and Cryptococcus neoformans in vitro. Eur. J. Med. Chem. 2015, 102, 233–242. [Google Scholar] [CrossRef]
- Pupulin, Á.R.; Carvalho, P.G.; Nakamura, C.V. Susceptibilidade a antifúngicos e produção de enzimas por leveduras do (levaduras del) gênero Candida isoladas (aisladas) de pacientes com HIV/AIDS. Salud(i)Ciencia 2014, 20, 471–476. [Google Scholar]
- Akpan, A.; Morgan, R. Oral Candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Xu, W.; Solis, N.V.; Ehrlich, R.L.; Woolford, C.A.; Filler, S.G.; Mitchell, A.P. Activation and Alliance of Regulatory Pathways in C. albicans during Mammalian Infection. PLoS Biol. 2015, 13, 1–32. [Google Scholar] [CrossRef]
- Szabo, E.K.; MacCallum, D.M. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol. Lett. 2011, 320, 1–8. [Google Scholar] [CrossRef] [Green Version]








| Thiazolylhydrazones | ||||||
|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 3 | 4 | Fluconazole | Amphotericin B |
| C. albicans (DAY-185) | 0.5 | 0.5 | 4.0 | 8.0 | 0.5 | 1.0 |
| C. albicans (CAN14) | 1.0 | 0.5 | 4.0 | 4.0 | 0.125 | 1.0 |
| C. krusei (ATCC6258) | 1.0 | 2.0 | 2.0 | 4.0 | 64.0 | 8.0 |
| C. glabrata (ATCC90030) | 32 | 16.0 | 32.0 | 32.0 | 4.0 | 2.0 |
| C. parapsilosis (ATCC22019) | 0.5 | 2.0 | 2.0 | 16.0 | 8.0 | 2.0 |
| C. tropicalis (ATCC13803) | 2.0 | 8.0 | 8.0 | 32.0 | >64.0 | 4.0 |
| C. dublinensis (MYA-646) | 1.0 | 2.0 | 2.0 | 8.0 | 1.0 | 1.0 |
| C. neoformans (H99) | 0.5 | 0.5 | 0.25 | 2.0 | 2.0 | 4.0 |
| Thiazolylhydrazones | ||||||
|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 3 | 4 | Fluconazole | Amphotericin B |
| 02A | 1.0 | 1.0 | 0.25 | 8.0 | 4.0 | 0.25 |
| 02B | 0.5 | 1.0 | 0.25 | 8.0 | 2.0 | 0.25 |
| 7 | 2.0 | 2.0 | 2.0 | 16.0 | 1.0 | 0.125 |
| 6 | 1.0 | 1.0 | 0.25 | 16.0 | 1.0 | 0.5 |
| 13 | 0.5 | 1.0 | 0.5 | 8.0 | 1.0 | 0.125 |
| 1 | 1.0 | 1.0 | 0.5 | 8.0 | 1.0 | 0.25 |
| 11 | 1.0 | 1.0 | 0.5 | 16.0 | 1.0 | 0.125 |
| 9 | 1.0 | 2.0 | 0.5 | 16.0 | 0.5 | 0.25 |
| 10 | 0.5 | 1.0 | 0.5 | 16.0 | 0.5 | 0.25 |
| MIC 50 | 1.0 | 1.0 | 0.5 | 16.0 | 1.0 | 0.25 |
| MIC 90 | 2.0 | 2.0 | 2.0 | 16.0 | 2.0 | 0.25 |
| Thiazolylhydrazones | ||||||
|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 3 | 4 | Fluconazole | Amphotericin B |
| 7970A | 2.0 | 4.0 | 4.0 | 2.0 | 1.0 | 0.25 |
| 7652 | 2.0 | 2.0 | 2.0 | 4.0 | 4.0 | 0.25 |
| 7449 | 2.0 | 4.0 | 4.0 | 4.0 | 16.0 | 0.5 |
| 8662 | 2.0 | 4.0 | 4.0 | 4.0 | 16.0 | 0.25 |
| 6901 | 1.0 | 4.0 | 2.0 | 4.0 | 0.25 | 0.5 |
| 6917 | 2.0 | 4.0 | 4.0 | 4.0 | 0.25 | 0.25 |
| 7839 | 2.0 | 4.0 | 4.0 | 4.0 | 0.5 | 0.5 |
| 6933 | 1.0 | 4.0 | 2.0 | 2.0 | 0.25 | 0.5 |
| 7585 | 1.0 | 4.0 | 4.0 | 4.0 | 0.25 | 0.25 |
| 8044 | 1.0 | 2.0 | 2.0 | 2.0 | 0.25 | 0.5 |
| MIC 50 | 2.0 | 4.0 | 4.0 | 4.0 | 0.25 | 0.25 |
| MIC 90 | 2.0 | 4.0 | 4.0 | 4.0 | 16.0 | 0.5 |
| Thiazolylhydrazones | ||||||
|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 3 | 4 | Fluconazole | Amphotericin B |
| 6922 | 4.0 | 16.0 | 8.0 | 8.0 | 2.0 | 0.5 |
| 6927 | 4.0 | 8.0 | 8.0 | 8.0 | 2.0 | 1.0 |
| 6931 | 8.0 | 16.0 | 16.0 | 16.0 | 0.5 | 0.5 |
| 6932 | 4.0 | 8.0 | 8.0 | 16.0 | 0.5 | 0.5 |
| 6943 | 8.0 | 16.0 | 16.0 | >32 | 8.0 | 1.0 |
| 7110 | 4.0 | 4.0 | 4.0 | 8.0 | 1.0 | 0.5 |
| 7221 | 8.0 | 16.0 | 16.0 | 16.0 | 0.5 | 0.5 |
| 7255 | 2.0 | 4.0 | 4.0 | 4.0 | 2.0 | 1.0 |
| 7815 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 0.5 |
| 7871 | 4.0 | 8.0 | 8.0 | 4.0 | 2.0 | 1.0 |
| MIC 50 | 4.0 | 8.0 | 8.0 | 8.0 | 2.0 | 0.5 |
| MIC 90 | 8.0 | 16.0 | 16.0 | 16.0 | 2.0 | 1.0 |
| Thiazolylhydrazones | ||||||
|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 3 | 4 | Fluconazole | Amphotericin B |
| BF113 | 1.0 | 2.0 | 1.0 | 16.0 | 16.0 | 1.0 |
| BF114 | 0.5 | 1.0 | 1.0 | 1.0 | 64.0 | <0.0625 |
| 41292 | 0.5 | 1.0 | 1.0 | 1.0 | 8.0 | 0.125 |
| 41295 | 1.0 | 2.0 | 1.0 | 8.0 | 32.0 | 0.125 |
| 41296 | 2.0 | 4.0 | 2.0 | 32.0 | 16.0 | 1.0 |
| 41297 | 1.0 | 2.0 | 1.0 | 16.0 | 8.0 | 0.125 |
| 41298 | 1.0 | 2.0 | 1.0 | 8.0 | 8.0 | 0.125 |
| 41299 | 0.5 | 0.5 | 0.25 | 4.0 | 4.0 | 0.125 |
| C31 | 0.25 | 0.25 | 0.25 | 8.0 | 4.0 | 0.125 |
| F10 | 1.0 | 1.0 | 1.0 | 4.0 | 4.0 | 0.25 |
| RN01 | 1.0 | 0.5 | 0.25 | 4.0 | 4.0 | 0.125 |
| WP | 0.5 | 1.0 | 0.25 | 8.0 | 8.0 | 1.0 |
| 27JF | 1.0 | 2.0 | 2.0 | 2.0 | 8.0 | 0.25 |
| 28JF | 0.5 | 0.5 | 0.5 | 8.0 | 8.0 | 0.125 |
| 90896 | 1.0 | 1.0 | 0.5 | 8.0 | 32.0 | 0.125 |
| 93 | 1.0 | 1.0 | 1.0 | 1.0 | 4.0 | 0.25 |
| 94 | 0.25 | 0.5 | 1.0 | 0.5 | 8.0 | 0.25 |
| 646B | 1.0 | 2.0 | 2.0 | 2.0 | 1.0 | 0.125 |
| 975 | 1.0 | 1.0 | 0.5 | 16.0 | 2.0 | 0.125 |
| 9220 | 0.5 | 1.0 | 1.0 | 1.0 | 2.0 | 0.125 |
| 9273 | 0.5 | 0.5 | 0.5 | 0.5 | 2.0 | 0.125 |
| 10131 | 0.25 | 0.5 | 0.25 | 0.5 | 8.0 | 0.125 |
| 10211 | 0.5 | 1.0 | 1.0 | 1.0 | 4.0 | 0.125 |
| 10264 | 0.25 | 0.5 | 0.25 | 0.5 | 4.0 | 0.125 |
| 10335 | 0.5 | 0.5 | 2.0 | 0.5 | 8.0 | 0.125 |
| 10379 | 0.5 | 1.0 | 0.25 | 8.0 | 8.0 | 0.125 |
| 92868 | 0.5 | 0.5 | 2.0 | 0.5 | 8.0 | 0.25 |
| MIC 50 | 0.5 | 1.0 | 1.0 | 4.0 | 8.0 | 0.125 |
| MIC 90 | 1.0 | 2.0 | 2.0 | 16.0 | 16.0 | 0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, L.I.B.; Lopes, L.F.F.; De Camargo Ribeiro, F.; De Sá, N.P.; Lino, C.I.; Tharmalingam, N.; De Oliveira, R.B.; Rosa, C.A.; Mylonakis, E.; Fuchs, B.B.; et al. Anti-Candida albicans Activity of Thiazolylhydrazone Derivatives in Invertebrate and Murine Models. J. Fungi 2018, 4, 134. https://doi.org/10.3390/jof4040134
Cruz LIB, Lopes LFF, De Camargo Ribeiro F, De Sá NP, Lino CI, Tharmalingam N, De Oliveira RB, Rosa CA, Mylonakis E, Fuchs BB, et al. Anti-Candida albicans Activity of Thiazolylhydrazone Derivatives in Invertebrate and Murine Models. Journal of Fungi. 2018; 4(4):134. https://doi.org/10.3390/jof4040134
Chicago/Turabian StyleCruz, Lana Ivone Barreto, Larissa Ferreira Finamore Lopes, Felipe De Camargo Ribeiro, Nívea Pereira De Sá, Cleudiomar Inácio Lino, Nagendran Tharmalingam, Renata Barbosa De Oliveira, Carlos Augusto Rosa, Eleftherios Mylonakis, Beth Burgwyn Fuchs, and et al. 2018. "Anti-Candida albicans Activity of Thiazolylhydrazone Derivatives in Invertebrate and Murine Models" Journal of Fungi 4, no. 4: 134. https://doi.org/10.3390/jof4040134