In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Susceptibility Testing for Individual Drugs and Combination of Drugs
2.3. Galleria mellonella Mucormycosis Model for the Evaluation of the Effect of Antifungal Drugs Combinations
2.4. Data Analysis
3. Results
3.1. Individual Drug and Combined Susceptibility Testing
3.2. Galleria mellonella Infection and Antifungal Treatment Model
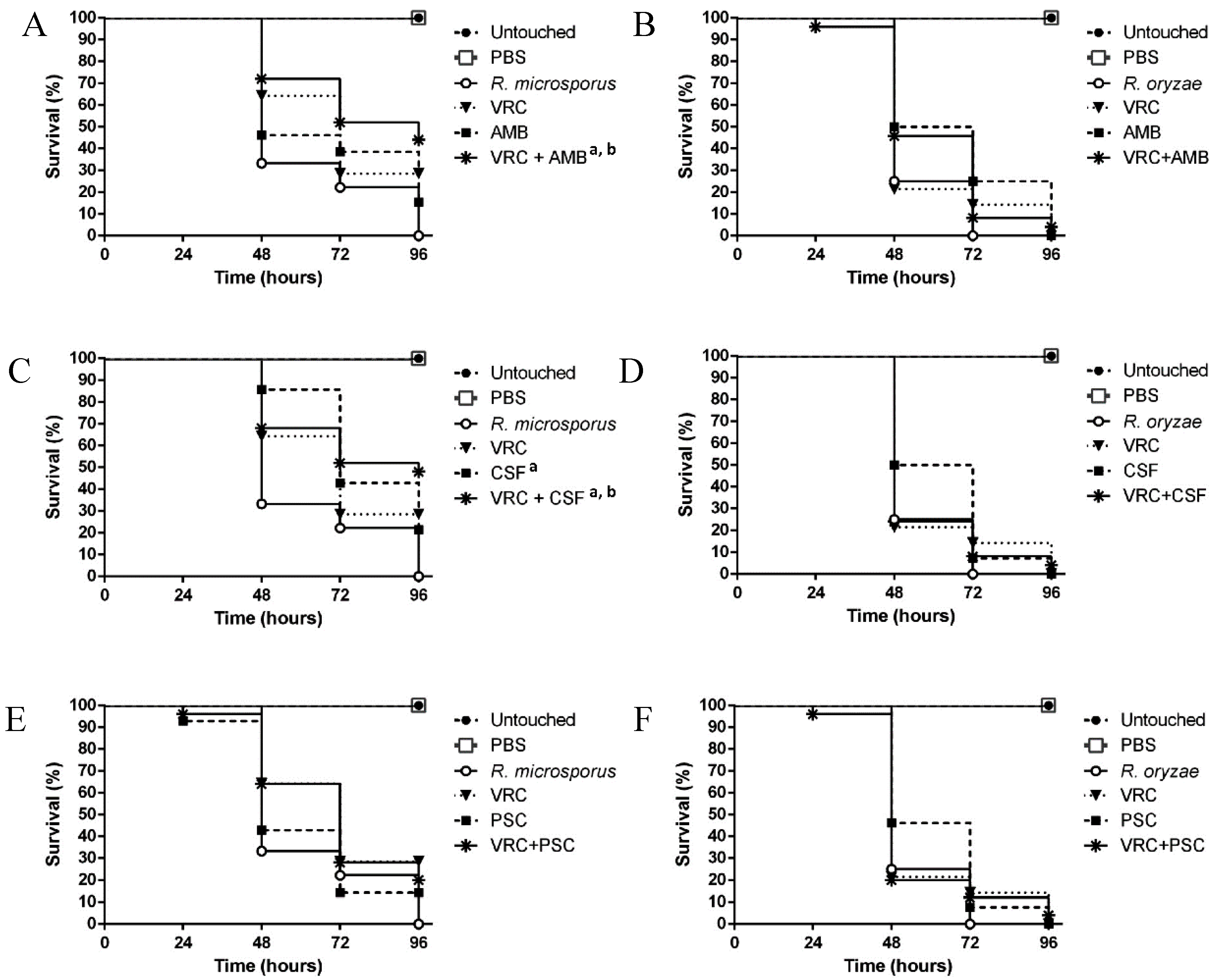
3.3. Evaluation of the Efficacy of the Drug Combinations in the Galleria mellonella Model
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef] [Green Version]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance((R)): Focus on mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.; Roilides, E.; Lewis, R.E.; Lortholary, O.; Petrikkos, G.; Kontoyiannis, D.P.; Walsh, T.J. Combination therapy for mucormycosis: Why, what, and how? Clin. Infect. Dis. 2012, 54 (Suppl. 1), S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Wiederhold, N.P.; Alqarihi, A.; Uppuluri, P.; Azie, N.; Edwards, J.E., Jr.; Ibrahim, A.S. Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. J. Antimicrob. Chemother. 2017, 72, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebremariam, T.; Fu, Y.; Edwards, J.E., Jr.; Spellberg, B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob. Agents Chemother. 2008, 52, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebremariam, T.; Luo, G.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. Combination therapy of murine mucormycosis or aspergillosis with iron chelation, polyenes, and echinocandins. Antimicrob. Agents Chemother. 2011, 55, 1768–1770. [Google Scholar] [CrossRef]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus enhances the potency of posaconazole against Rhizopus oryzae in vitro and in an experimental model of mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef]
- Spellberg, B.; Fu, Y.; Edwards, J.E., Jr.; Ibrahim, A.S. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 2005, 49, 830–832. [Google Scholar] [CrossRef]
- Sugar, A.M.; Liu, X.P. Combination antifungal therapy in treatment of murine pulmonary mucormycosis: Roles of quinolones and azoles. Antimicrob. Agents Chemother. 2000, 44, 2004–2006. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, S.; Walther, G.; van den Ende, A.H.G.G.; de Hoog, G.S. Diversity and delimitation of Rhizopus microsporus. Fungal Divers. 2014, 64, 145–164. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.A.A.; Staplers, J.A. A revision of the genus Rhizopus. II. The Rhizopus microsporus group. Stud. Mycol. 1984, 25, 20–34. [Google Scholar]
- Schipper, M. A revision of the genus Rhizopus. I. The Rhizopus stolonifer group and Rhizopus oryzae. Stud. Mycol. 1984, 25, 1–19. [Google Scholar]
- Machouart, M.; Larche, J.; Burton, K.; Collomb, J.; Maurer, P.; Cintrat, A.; Biava, M.F.; Greciano, S.; Kuijpers, A.F.; Contet-Audonneau, N.; et al. Genetic identification of the main opportunistic Mucorales by PCR-restriction fragment length polymorphism. J. Clin. Microbiol. 2006, 44, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.G.; Afeltra, J.; Meis, J.F.; Verweij, P.E. Activity and post antifungal effect of chlorpromazine and trifluopherazine against Aspergillus, Scedosporium and zygomycetes. Mycoses 2007, 50, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Pawlowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.L.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G.S. DNA barcoding in Mucorales: An inventory of biodiversity. Persoonia 2013, 30, 11–47. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI Standard M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Kloezen, W.; Parel, F.; Bruggemann, R.; Asouit, K.; Helvert-van, P.M.; Fahal, A.; Mouton, J.; van de Sande, W. Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med. Mycol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 2003, 41, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S. Recent advances in the treatment of mucormycosis. Curr. Infect. Dis. Rep. 2010, 12, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, B.; Schilling, A.; Anagnostopolous, I.; Siehl, I.; Thiel, E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 2004, 45, 1351–1360. [Google Scholar] [CrossRef]
- Skiada, A.; Lanternier, F.; Groll, A.H.; Pagano, L.; Zimmerli, S.; Herbrecht, R.; Lortholary, O.; Petrikkos, G.L. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013, 98, 492–504. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Bowman, J.C.; Avanessian, V.; Brown, K.; Spellberg, B.; Edwards, J.E., Jr.; Douglas, C.M. Caspofungin inhibits Rhizopus oryzae 1,3-beta-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 2005, 49, 721–727. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J.; Gamaletsou, M.N.; Rammaert, B.; Sipsas, N.V.; Zeller, V.; Roilides, E.; Kontoyiannis, D.P.; Henry, M.; Petraitis, V.; Moriyama, B.; et al. Bone and joint infections caused by mucormycetes: A challenging osteoarticular mycosis of the twenty-first century. Med. Mycol. 2017. [Google Scholar] [CrossRef]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; van de Sande, W.W.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Kaerger, K.; Schwartze, V.U.; Dolatabadi, S.; Nyilasi, I.; Kovacs, S.A.; Binder, U.; Papp, T.; Hoog, S.; Jacobsen, I.D.; Voigt, K. Adaptation to thermotolerance in Rhizopus coincides with virulence as revealed by avian and invertebrate infection models, phylogeny, physiological and metabolic flexibility. Virulence 2015, 6, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Buitrago, M.; Gomez-Lopez, A.; Mellado, E. An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp strains. Virulence 2015, 6, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Forastiero, A.; Bernal-Martinez, L.; Mellado, E.; Cendejas, E.; Gomez-Lopez, A. In vivo efficacy of voriconazole and posaconazole therapy in a novel invertebrate model of Aspergillus fumigatus infection. Int. J. Antimicrob. Agents 2015, 46, 511–517. [Google Scholar] [CrossRef]
- Gago, S.; Garcia-Rodas, R.; Cuesta, I.; Mellado, E.; Alastruey-Izquierdo, A. Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis virulence in the non-conventional host Galleria mellonella. Virulence 2014, 5, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gago, S.; Serrano, C.; Alastruey-Izquierdo, A.; Cuesta, I.; Martin-Mazuelos, E.; Aller, A.I.; Gomez-Lopez, A.; Mellado, E. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses 2017, 60, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Forastiero, A.; Bernal-Martinez, L.; Cuenca-Estrella, M.; Mellado, E.; Zaragoza, O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med. Mycol. 2013, 51, 461–472. [Google Scholar] [CrossRef]
- Afeltra, J.; Vitale, R.G.; Mouton, J.W.; Verweij, P.E. Potent synergistic in vitro interaction between nonantimicrobial membrane-active compounds and itraconazole against clinical isolates of Aspergillus fumigatus resistant to itraconazole. Antimicrob. Agents Chemother. 2004, 48, 1335–1343. [Google Scholar] [CrossRef]
- Gamarra, S.; Rocha, E.M.; Zhang, Y.Q.; Park, S.; Rao, R.; Perlin, D.S. Mechanism of the synergistic effect of amiodarone and fluconazole in Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 1753–1761. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Mellado, E.; Gomez-Lopez, A.; Alcazar-Fuoli, L.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Differences in interactions between azole drugs related to modifications in the 14-alpha sterol demethylase gene (cyp51A) of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2005, 49, 2119–2121. [Google Scholar] [CrossRef]
- Bellanger, A.P.; Albert, N.D.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Effect of Preexposure to Triazoles on Susceptibility and Virulence of Rhizopus oryzae. Antimicrob. Agents Chemother. 2015, 59, 7830–7832. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case—Control observational study of 27 recent cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Cosimi, L.A.; Baden, L.R. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N. Engl. J. Med. 2004, 350, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Siwek, G.T.; Dodgson, K.J.; de Magalhaes-Silverman, M.; Bartelt, L.A.; Kilborn, S.B.; Hoth, P.L.; Diekema, D.J.; Pfaller, M.A. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin. Infect. Dis. 2004, 39, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Lozano-Chiu, M.; Paetznick, V.; Rex, J.H. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 2002, 46, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Franzot, S.P.; Casadevall, A. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 1997, 41, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Afeltra, J.; Meis, J.F.; Verweij, P.E. In vitro susceptibilities of zygomycetes to combinations of antimicrobial agents. Antimicrob. Agents Chemother. 2002, 46, 2708–2711. [Google Scholar] [CrossRef] [PubMed]
- Maurer, E.; Hortnagl, C.; Lackner, M.; Grassle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Florl, C.; Binder, U. Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. Med. Mycol. 2018. [Google Scholar] [CrossRef]


| Isolate Nº | Organism | Isolation Site | MIC (mg/L) | ∑FIC Index (Interpretation) c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VRC + PSC | VRC + AMB | VRC + CSF | ||||||||||
| VRC | PSC | AMB | CSF | MIC | HA | MIC | HA | MIC | HA | |||
| LMDM-165 | R. microsporusa | Osteomyelitis | 8.00 | 1.00 | 2.00 | 16.00 | 0.75 (NI) | Yes | 0.75 (NI) | Yes | 0.75 (NI) | Yes |
| LMDM-156 | R. microsporusa | Osteomyelitis | 4.00 | 1.00 | 2.00 | 16.00 | 1.00 (NI) | Yes | 2.04 (NI) | No | 1.00 (NI) | Yes |
| LMDM-157 | R. microsporusa | Surgical wound | 4.00 | 0.50 | 1.00 | 16.00 | 0.50 (S) | Yes | 1.03 (NI) | No | 0.75 (NI) | Yes |
| LMDM-158 | R. microsporusa | Surgical wound | 8.00 | 0.50 | 2.00 | 16.00 | 1.00 (NI) | Yes | 1.04 (NI) | No | 2.00 (NI) | Yes |
| LMDM-159 | R. microsporusa | Surgical wound | 4.00 | 1.00 | 2.00 | 16.00 | 1.00 (NI) | No | 1.03 (NI) | No | 1.50 (NI) | No |
| LMDM-164 | R. microsporusa | Osteomyelitis | 4.00 | 1.00 | 2.00 | 16.00 | 1.00 (NI) | No | 1.01 (NI) | Yes | 1.00 (NI) | No |
| LMDM-167 | R. microsporusa | Hospital environment | 4.00 | 1.00 | 2.00 | 16.00 | 1.02 (NI) | Yes | 1.03 (NI) | No | 1.25 (NI) | No |
| LMDM-168 | R. microsporusa | Hospital environment | 4.00 | 1.00 | 2.00 | 16.00 | 1.03 (NI) | No | 2.50 (NI) | No | 2.00 (NI) | No |
| LMDM-176 | R. microsporusa | Osteomyelitis | 8.00 | 1.00 | 2.00 | 16.00 | 0.63 (NI) | Yes | 2.25 (NI) | Yes | 1.25 (NI) | No |
| LMDM-184 | R. microsporusa | Osteomyelitis | 8.00 | 1.00 | 2.00 | 16.00 | 0.75 (NI) | Yes | 1.03 (NI) | Yes | 1.03 (NI) | No |
| LMDM-379 | R. microsporusa | Rhinocerebral | 8.00 | 1.00 | 4.00 | 16.00 | 0.63 (NI) | Yes | 1.00 (NI) | No | 0.75 (NI) | Yes |
| LMDM-596 | R. microsporusa | Rhinocerebral | 2.00 | 1.00 | 1.00 | 16.00 | 1.06 (NI) | No | 2.00 (NI) | No | 1.03 (NI) | No |
| LMDM-1073 | R. microsporusb | Rhinocerebral | 2.00 | 1.00 | 0.50 | 16.00 | 1.06 (NI) | No | 1.06 (NI) | No | 2.00 (NI) | No |
| LMDM-1074 | R. microsporusb | Rhinocerebral | 2.00 | 1.00 | 0.25 | 16.00 | 0.75 (NI) | No | 0.62 (NI) | No | 2.00 (NI) | No |
| LMDM-1127 | R. microsporusb | Hospital environment | 8.00 | 2.00 | 1.00 | 16.00 | 0.50 (S) | No | 0.50 (S) | No | 0.75 (NI) | No |
| n = 15 | R. microsporus | 4.59 | 0.95 | 1.45 | 16.00 | 0.82 | 1.14 | 1.18 | ||||
| LMDM-597 | R. oryzae | Rhinocerebral | 8.00 | 1.00 | 2.00 | 16.00 | 0.75 (NI) | No | 0.75 (NI) | No | 0.75 (NI) | No |
| LMDM-1126 | R. oryzae | Rhinocerebral | 8.00 | 1.00 | 1.00 | 16.00 | 0.75 (NI) | No | 0.50 (S) | No | 1.00 (NI) | No |
| LMDM-1075 | R. oryzae | Rhinocerebral | 4.00 | 1.00 | 0.25 | 16.00 | 1.00 (NI) | No | 0.62 (NI) | No | 2.00 (NI) | No |
| n = 3 | R. oryzae | 6.35 | 1.00 | 0.79 | 16.00 | 0.83 | 0.61 | 1.14 | ||||
| LMDM-1122 | S. racemosum | Rhinocerebral | 4.00 | 1.00 | 0.50 | 16.00 | 1.00 (NI) | Yes | 1.00 (NI) | Yes | 2.00 (NI) | Yes |
| LMDM-1123 | S. racemosum | Hospital environment | 4.00 | 1.00 | 0.25 | 16.00 | 0.75 (NI) | No | 1.00 (NI) | No | 2.00 (NI) | Yes |
| LMDM-1124 | S. racemosum | Hospital environment | 16.00 | 2.00 | 0.50 | 16.00 | 1.00 (NI) | No | 1.00 (NI) | No | 2.00 (NI) | No |
| LMDM-576 | S. racemosum | Rhinocerebral | 16.00 | 2.00 | 2.00 | 16.00 | 0.50 (S) | Yes | 0.50 (S) | Yes | 1.00 (NI) | Yes |
| n = 4 | S. racemosum | 8.00 | 1.40 | 0.59 | 16.00 | 0.78 | 0.88 | 1.75 | ||||
| LMDM-1128 | L. blakesleeana | Cutaneous ulcera | 4.00 | 0.50 | 0.50 | 16.00 | 0.75 (NI) | Yes | 1.00 (NI) | Yes | 2.00 (NI) | No |
| LMDM-1121 | L. corymbifera | Hospital environment | 8.00 | 1.00 | 0.25 | 16.00 | 0.50 (S) | Yes | 1.00 (NI) | No | 2.00 (NI) | Yes |
| LMDM-1125 | L. ramosa | Clinical d | 16.00 | 1.00 | 1.00 | 16.00 | 0.31 (S) | No | 0.62 (NI) | No | 1.00 (NI) | No |
| n = 3 | Lichtheimia spp. | 8.00 | 0.80 | 0.50 | 16.00 | 0.49 | 1.09 | 1.45 | ||||
| n = 25 | Mucormycetes | 5.74 | 1.03 | 1.03 | 16.00 | 0.81 | 1.09 | 1.45 | ||||
| n = 10 | non-R. microsporus | 8.00 | 1.15 | 0.62 | 16.00 | 0.73 | 0.80 | 1.70 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, D.; Leonardelli, F.; Dudiuk, C.; Vitale, R.G.; Del Valle, E.; Giusiano, G.; Gamarra, S.; Garcia-Effron, G. In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. J. Fungi 2019, 5, 5. https://doi.org/10.3390/jof5010005
Macedo D, Leonardelli F, Dudiuk C, Vitale RG, Del Valle E, Giusiano G, Gamarra S, Garcia-Effron G. In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. Journal of Fungi. 2019; 5(1):5. https://doi.org/10.3390/jof5010005
Chicago/Turabian StyleMacedo, Daiana, Florencia Leonardelli, Catiana Dudiuk, Roxana G. Vitale, Eleodoro Del Valle, Gustavo Giusiano, Soledad Gamarra, and Guillermo Garcia-Effron. 2019. "In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis" Journal of Fungi 5, no. 1: 5. https://doi.org/10.3390/jof5010005
APA StyleMacedo, D., Leonardelli, F., Dudiuk, C., Vitale, R. G., Del Valle, E., Giusiano, G., Gamarra, S., & Garcia-Effron, G. (2019). In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. Journal of Fungi, 5(1), 5. https://doi.org/10.3390/jof5010005





