Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism
2.3. Pretreatment
2.4. Effect of Substrate Loading on Lipid Yield
2.5. Fed-Batch SSF at Low Enzyme Loading
2.6. Fed-Batch SSF at High Enzyme Loading in the Presence of Tween 80
2.7. Effect of Tween 80 on Enzyme Hydrolysis and Lipid Production
2.8. Separate Hydrolysis and Fermentation (SHF) with Cellulase Recycling
2.9. Chemical Analysis of Lignocellulosic Biomass
2.10. High-Pressure Liquid Chromatography (HPLC) Analysis
2.11. Determination of Lipid Content
3. Results and Discussion
3.1. Chemical Composition of Pretreated Biomass
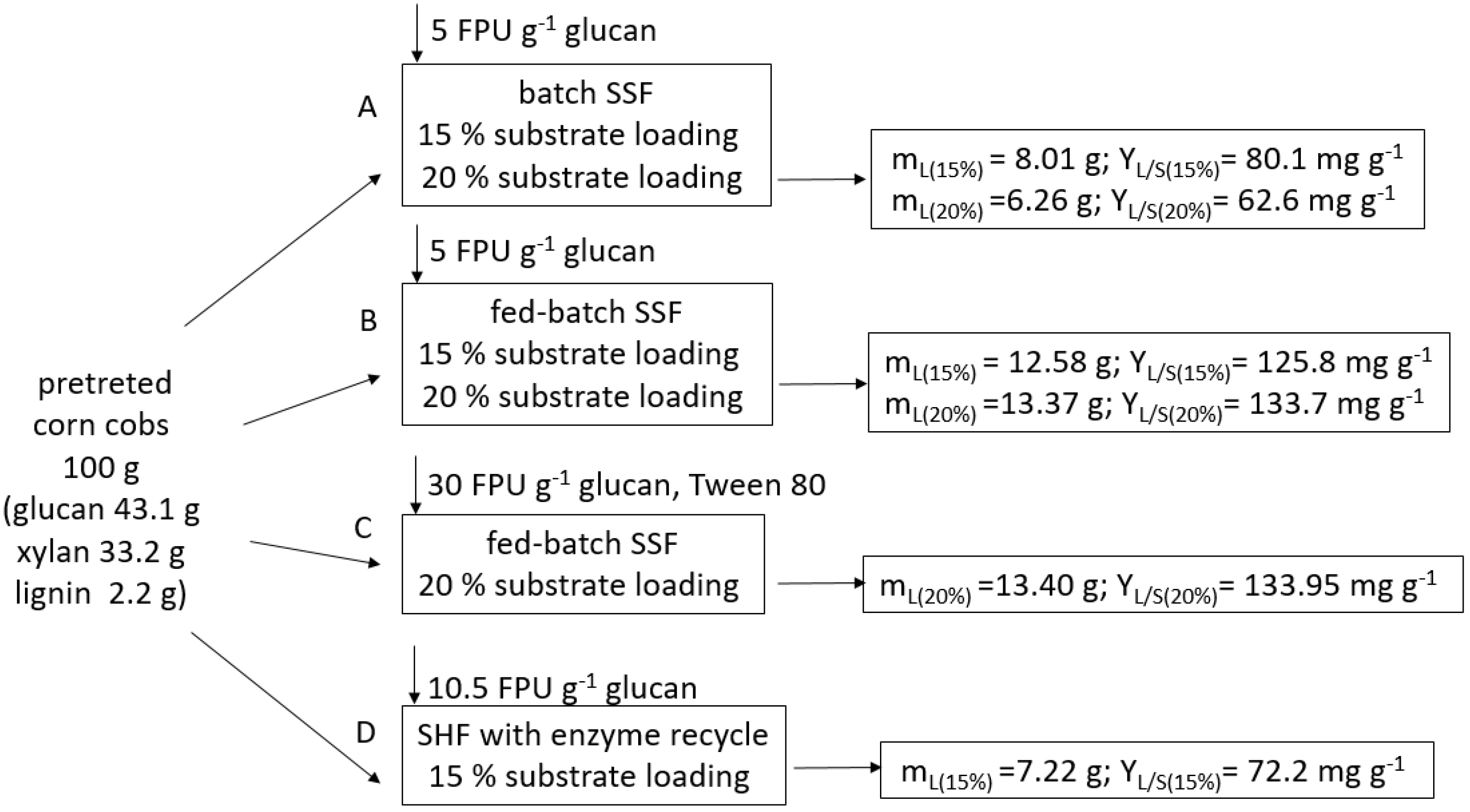
3.2. Effect of Substrate Loading on Lipid Yield
3.3. Fed-Batch SSF at a Low Enzyme Loading
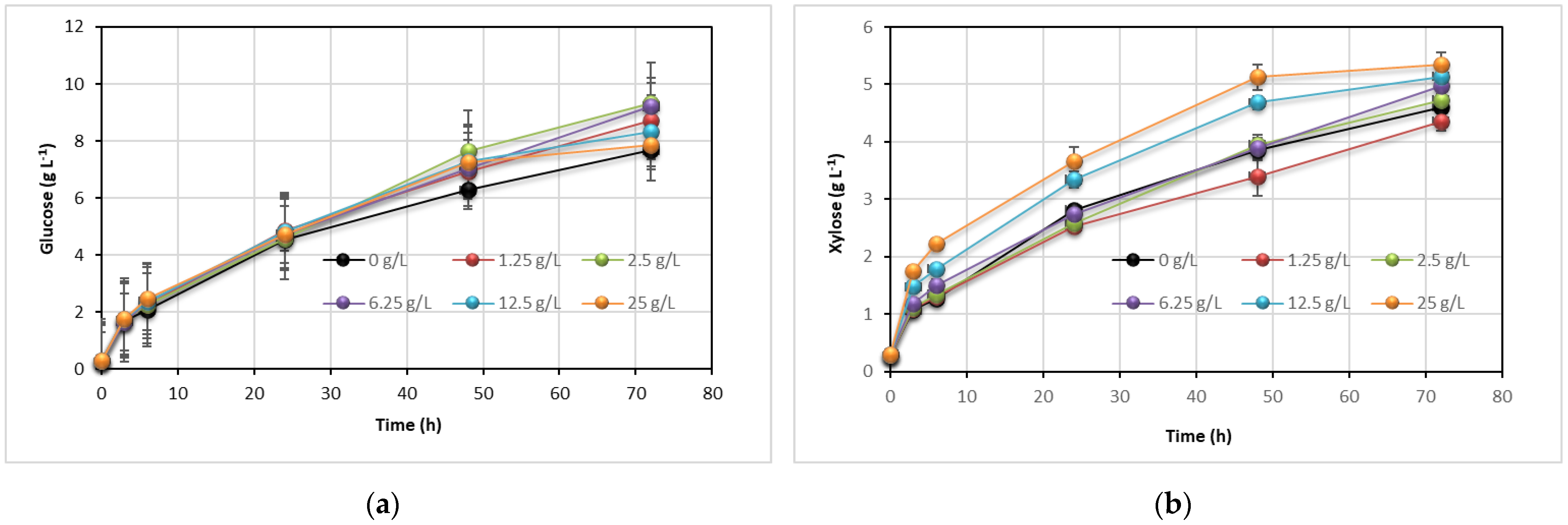
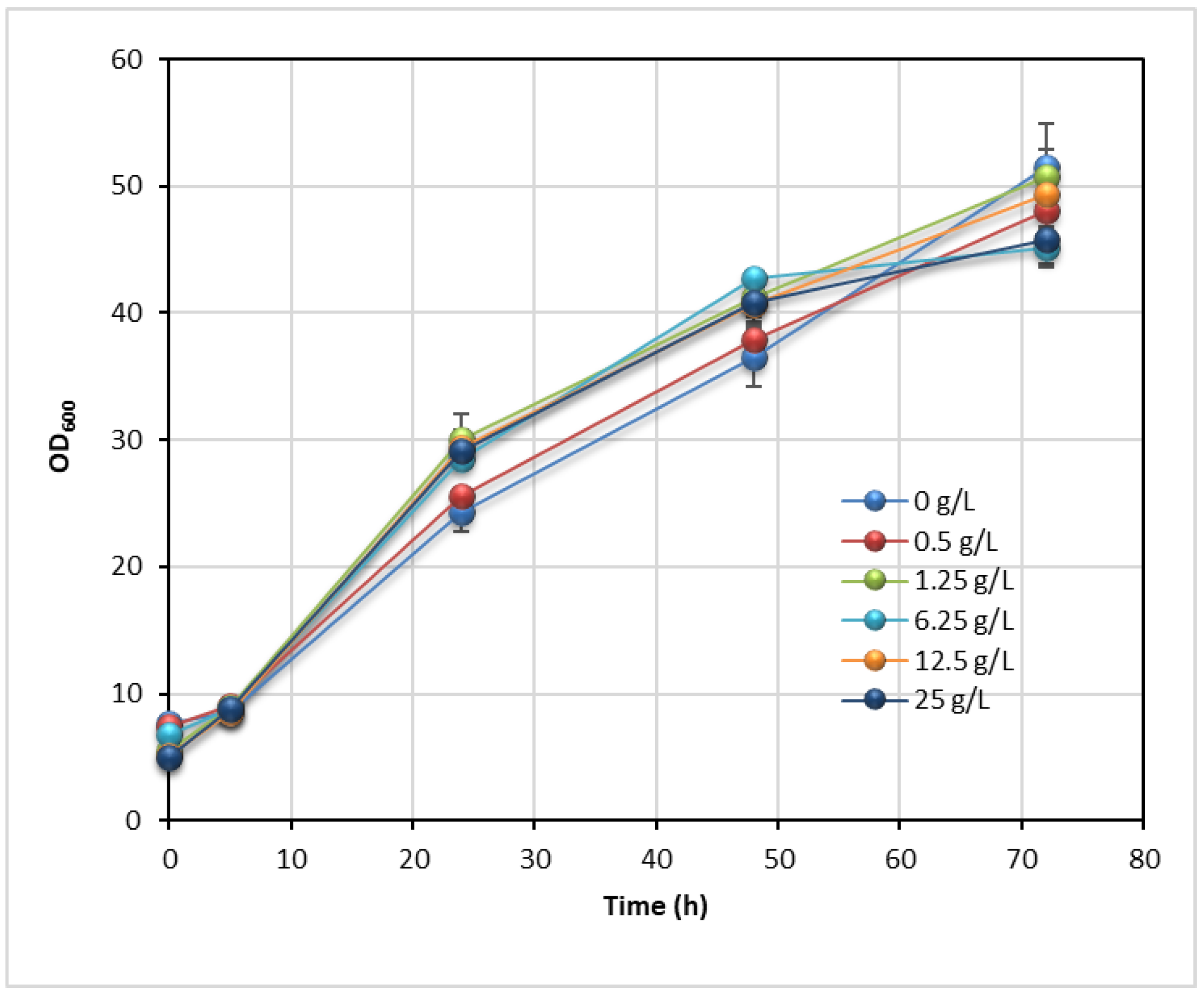
3.4. Effect of Tween 80 on Enzyme Hydrolysis and Lipid Production
3.5. Fed-Batch Cultivation at High Enzyme Loading in the Presence of Tween 80
3.6. Separate Hydrolysis and Fermentation (SHF) with Cellulase Recycle
3.7. Mass Balances of Lipid Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flach, B.; Lieberz, S.; Bolla, S. EU Biofuels Annual 2019. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Biofuels%20Annual_The%20Hague_EU-28_7-15-2019.pdf (accessed on 24 September 2021).
- Renewable Energy Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics&oldid=515129#of_renewable_energy_used_in_transport_activities_in_2019 December 2020 (accessed on 24 September 2021).
- Sönnichsen, N. BP Statistical Review of World Energy 2021. 2021. Available online: https://www.statista.com/statistics/274163/global-biofuel-production-in-oil-equivalent/ (accessed on 24 September 2021).
- Renewables 2020 Analysis and Forecast to 2025. Paris: International Energy Agency. Available online: https://www.iea.org/reports/renewables-2020 (accessed on 7 September 2021).
- Transport and Envionment. 10 Years of EU’s Failed Biofuels Policy Has Wiped out Forests the Size of the Netherlands—Study. Available online: https://www.transportenvironment.org/discover/10-years-of-eus-failed-biofuels-policy-has-wiped-out-forests-the-size-of-the-netherl (accessed on 24 September 2021).
- Parsons, S.; Abeln, F.; McManus, M.C.; Chuck, C.J. Techno-economic analysis (TEA) of microbial oil production from waste resources as part of a biorefinery concept: Assessment at multiple scales under uncertainty. J. Chem. Technol. Biotechnol. 2019, 94, 701–711. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Ivančić Šantek, M.; Miškulin, E.; Petrović, M.; Beluhan, S.; Šantek, B. Effect of carbon and nitrogen source concentrations on the growth and lipid accumulation of yeast Trichosporon oleaginosus in continuous and batch culture. J. Chem. Technol. Biotechnol. 2017, 92, 1620–1629. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Soccol, C.R.; Dalmas Neto, C.J.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; de Souza Vandenberghe, L.P. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Karamerou, E.E.; Parsons, S.; Mcmanus, M.C.; Chuck, C.J. Using techno-economic modelling to determine the minimum cost possible for a microbial palm oil substitute. Biotechnol. Biofuels 2021, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Júnnior, H.; Lopes, S.; Bonturi, N.; Lahtvee, P.; Kerkhoven, E.J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wu, D.; Li, J.; Tyagi, R.D. Economical lipid production from Trichosporon oleaginosus via dissolved oxygen adjustment and crude glycerol addition. Bioresour. Technol. 2019, 273, 288–296. [Google Scholar] [CrossRef]
- Šantek, M.I.; Grubišić, M.; Perečinec, M.G.; Beluhan, S.; Šantek, B. Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Šantek, M.I.; Lisičar, J.; Mušak, L.; Špoljarić, I.V.; Beluhan, S.; Šantek, B. Lipid production by yeast Trichosporon oleaginosus on the enzymatic hydrolysate of alkaline pretreated corn cobs for biodiesel production. Energy Fuels 2018, 32, 12501–12513. [Google Scholar] [CrossRef]
- Taskin, M.; Ortucu, S.; Aydogan, M.N.; Arslan, N.P. Lipid production from sugar beet molasses under non-aseptic culture conditions using the oleaginous yeast Rhodotorula glutinis TR29. Renew. Energy 2016, 99, 198–204. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Tyagi, R.D. Impact of nitrogen on the industrial feasibility of biodiesel production from lipid accumulated in oleaginous yeast with wastewater sludge and crude glycerol. Energy 2021, 217, 119343. [Google Scholar] [CrossRef]
- Patel, A.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Volatile fatty acids (VFAs) generated by anaerobic digestion serve as feedstock for freshwater and marine oleaginous microorganisms to produce biodiesel and added-value compounds. Front. Microbiol. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Carota, E.; Silvia, C.; D’Annibale, A.; Gallo, A.M.; Stazi, S.R.; Petruccioli, M.A. Sustainable use of ricotta cheese whey for microbial biodiesel production. Sci. Total Environ. 2017, 584–585, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [Green Version]
- Casey, G.P.; Magnus, C.A.; Ingledew, W.M. High-gravity brewing: Effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl. Environ. Microbiol. 1984, 48, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Shen, H.; Yang, X.; Wang, Q.; Xie, H.; Zhao, Z.K. Lipid production from corn stover by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghose, T.K. Measurement of Cellulase Activities. Available online: https://doi.org/10.1515/iupac.59.0006 (accessed on 24 September 2021).
- Schneiter, R.; Daum, G. Extraction of yeast lipids. Methods Mol. Biol. 2006, 313, 41–45. [Google Scholar] [PubMed]
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid recovery from oleaginous yeasts: Perspectives and challenges for industrial applications. Fuel 2020, 259, 116292. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2006. Available online: https://www.nrel.gov/docs/gen/fy08/42623.pdf (accessed on 24 September 2021).
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, Y.; Zhao, Z.; Li, H.; Zhao, K.; Zhang, F. Selectively biorefining levoglucosan from NaOH pretreated corncobs via fast pyrolysis. Cellulose 2019, 26, 7877–7887. [Google Scholar] [CrossRef]
- Goldberg, I.; Richard, A.W.; Williams, R. Biotechnology and Food Ingredients; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Da Silva, A.S.A.; Espinheira, R.P.; Teixeira, R.S.S.; De Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P. Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: A critical review. Biotechnol. Biofuels 2020, 13, 1–28. [Google Scholar] [CrossRef] [Green Version]
- López-Linares, J.C.; Romero, I.; Cara, C.; Ruiz, E.; Moya, M.; Castro, E. Bioethanol production from rapeseed straw at high solids loading with different process configurations. Fuel 2014, 122, 112–118. [Google Scholar] [CrossRef]
- Dai, X.; Shen, H.; Li, Q.; Rasool, K.; Wang, Q.; Yu, X.; Zhao, Z.K. Microbial lipid production from corn stover by the oleaginous yeast Rhodosporidium toruloides using the presslp process. Energies 2019, 12, 1053. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, Y.; Yu, Z.; Bao, J. Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon cutaneum. Bioresour. Technol. 2012, 118, 13–18. [Google Scholar] [CrossRef]
- Fei, Q.; Brien, M.O.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced lipid production by Rhodosporidium toruloides using different fed—batch feeding strategies with lignocellulosic hydrolysate as the sole carbon source. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliston, A.; Collins, S.R.A.; Wilson, D.R.; Roberts, I.N.; Waldron, K.W. High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste-paper. Bioresour. Technol. 2013, 134, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Chen, L.; Hu, M.; Sun, D.; Li, A.; Li, Y.; Hu, Z.; Zou, S.; Tu, Y.; Xia, T.; et al. Tween-80 is effective for enhancing steam-exploded biomass enzymatic saccharification and ethanol production by specifically lessening cellulase absorption with lignin in common reed. Appl. Energy 2016, 175, 82–90. [Google Scholar] [CrossRef]
- Kadhum, H.J.; Mahapatra, D.M.; Murthy, G.S. A comparative account of glucose yields and bioethanol production from separate and simultaneous saccharification and fermentation processes at high solids loading with variable PEG concentration. Bioresour. Technol. 2019, 283, 67–75. [Google Scholar] [CrossRef]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. J. Biosci. Bioeng. 2011, 111, 420–424. [Google Scholar] [CrossRef]
- Reitermayer, D.; Kafka, T.A.; Lenz, C.A.; Vogel, R.F. Interrelation between Tween and the membrane properties and high pressure tolerance of Lactobacillus plantarum. BMC Microbiol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Boekema, B.K.H.L.; Beselin, A.; Breuer, M.; Hauer, B.; Koster, M.; Rosenau, F.; Tommassen, J. Hexadecane and Tween 80 stimulate lipase production in Burkholderia glumae by different mechanisms. Appl. Environ. Microbiol. 2007, 73, 3838–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol. Bioprocess Eng. 2011, 16, 23–33. [Google Scholar] [CrossRef]
- Arnesen, S.; Eriksen, S.H.; Olsen, J.; Jensen, B. Increased production of α-amylase from Thermomyces lanuginosus by the addition of Tween 80. Enzyme Microb. Technol. 1998, 23, 249–252. [Google Scholar] [CrossRef]
- Tsuji, M.; Goshima, T.; Matsushika, A.; Kudoh, S.; Hoshino, T. Direct ethanol fermentation from lignocellulosic biomass by Antarctic basidiomycetous yeast Mrakia blollopis under a low temperature condition. Cryobiology 2013, 67, 241–243. [Google Scholar] [CrossRef]
- Ohta, K.; Hayashida, S. Role of Tween 80 and monoolein in a lipid-sterol-protein complex which enhances ethanol tolerance of sake yeasts. Appl. Environ. Microbiol. 1983, 46, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Cheng, C.; Chen, T. Production of Acinetobacter radioresistens lipase using Tween 80 as the carbon source. Enzyme Microb. Technol. 2001, 29, 258–263. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.T.; Santos, M.; Ferreira, C.; Marques, S.; Ferreira, F.C. Conversion of cellulosic materials into glycolipid biosurfactants, mannosylerythritol lipids by Pseudozyma spp. under SHF and SSF processes. Microb. Cell Factories 2014, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negro, M.J.; Oliva, J.M.; Ballesteros, M. Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresour. Technol. 2009, 100, 890–895. [Google Scholar]




| Glucan (%, g g−1) | Xylan (%, g g−1) | Lignin Insoluble in Acid (%, g g−1) | Lignin Soluble in Acid (%, g g−1) | |
|---|---|---|---|---|
| untreated | 38.6 ± 4.44 | 36.8 ± 0.65 | 13.9 ± 0.37 | 0.4 ± 0.30 |
| alkali-pretreated | 43.1 ± 5.08 | 33.2 ± 3.66 | 2.1 ± 1.08 | 0.1 ± 0.08 |
| Cultivation | Substrate (%, g g−1) | Time (d) | wL (%, g g−1) | L (g L−1) | YL/S (mg g−1) | PrL (g L−1 d−1) | ηL (%) |
|---|---|---|---|---|---|---|---|
| B_1 | 5.0 | 3 | 9.10 ± 0.76 | 2.99 ± 0.61 | 59.72 | 0.99 | 21.23 |
| B_2 | 7.5 | 6 | 13.36 ± 0.60 | 5.56 ± 0.61 | 74.07 | 0.93 | 26.33 |
| B_3 | 10.0 | 10 | 20.98 ± 0.66 | 7.47 ± 0.58 | 74.7 | 0.75 | 26.55 |
| B_4 | 12.5 | 10 | 14.37 ± 0.24 | 12.00 ± 0.48 | 92.1 | 0.88 | 32.72 |
| B_5 | 15.0 | 10 | 14.50 ± 0.43 | 12.02 ± 0.87 | 80.1 | 1.20 | 28.47 |
| B_6 | 20.0 | 10 | 9.27 ± 0.17 | 12.52 ± 0.52 | 62.6 | 1.25 | 22.25 |
| Time (d) | Substrate | Solid Residue (g L−1) | wL (%, g g−1) | L (g L−1) | YL/S (mg g−1) | Pr (g L−1 d−1) | ηL (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| No. Batch Additions | Cumulative (%, g g−1) | ||||||||
| FB_1 | 0 | 2 × 5 | 5 | 4.94 ± 0.42 | - | - | - | - | - |
| 7 | % (g g−1) | 10 | 4.98 ± 0.77 | 20.25 ± 1.43 | 10.08 ± 2.20 | 100.8 | 1.44 | 35.83 | |
| 8 | 15 | 8.24 ± 0.70 | 14.15 ± 1.00 | 11.66 ± 1.76 | 77.7 | 1.46 | 27.63 | ||
| 10 | 15 | 8.12 ± 2.15 | 23.23 ± 2.63 | 18.87 ± 2.46 | 125.8 | 1.89 | 44.71 | ||
| FB_2 | 0 | 6 × 2.5 | 5 | 4.96 ± 0.28 | - | - | - | - | - |
| 6 | % (g g−1) | 10 | 5.76 ± 0.41 | 14.83 ± 0.80 | 8.54 ± 0.12 | 85.40 | 1.42 | 30.36 | |
| 10 | 15 | 7.99 ± 0.68 | 24.47 ± 1.73 | 19.54 ± 0.34 | 130.3 | 1.95 | 46.30 | ||
| 13 | 20 | 9.84 ± 0.56 | 27.18 ± 0.77 | 26.74 ± 2.31 | 133.7 | 2.06 | 47.52 | ||
| Tween 80 (g L−1) | X* (g L−1) | L (g L−1) | YL/S (g g−1) | YX/S (g g−1) | ηL (%) | PrL (g L−1 d−1) | PrX (g L−1 d−1) |
|---|---|---|---|---|---|---|---|
| 0.00 | 10.22 ± 0.78 | 4.36 ± 0.21 | 0.109 | 0.256 | 34.2 | 1.45 | 3.41 |
| 0.50 | 10.48 ±0.54 | 4.70 ± 0.28 | 0.117 | 0.260 | 36.5 | 1.57 | 3.49 |
| 1.25 | 10.42 ± 0.98 | 4.50 ± 0.16 | 0.110 | 0.255 | 34.5 | 1.50 | 3.47 |
| 2.50 | 10.53 ± 0.62 | 5.24 ± 0.27 | 0.126 | 0.252 | 39.3 | 1.75 | 3.51 |
| 6.25 | 10.95 ± 0.53 | 5.07 ± 0.23 | 0.122 | 0.264 | 38.2 | 1.69 | 3.65 |
| 12.50 | 11.15 ± 0.31 | 5.43 ± 0.41 | 0.129 | 0.266 | 40.4 | 1.81 | 3.72 |
| 25.00 | 10.70 ± 0.61 | 6.86 ± 0.31 | 0.161 | 0.251 | 50.4 | 2.29 | 3.57 |
| Time (d) | Cumulative Substrate Loading (%, g g−1) | Solid Residue (%, g g−1) | L (g L−1) | YL/S (mg g−1) | PrL (g L−1 d−1) | ηL (%) | |
|---|---|---|---|---|---|---|---|
| FB_3 | 6 | 10 | 4.61 ± 0.33 | 17.65 ± 1.06 | 176.5 | 2.71 | 62.81 |
| 21 | 20 | 6.05 ± 0.63 | 26.8 ± 1.03 | 133.95 | 1.28 | 47.61 | |
| FB_4 | 6 | 10 | 4.52 ± 0.31 | 7.02 ± 0.69 | 70.2 | 1.02 | 25.62 |
| 21 | 20 | 6.92 ± 0.62 | 22.8 ± 1.03 | 114.2 | 1.09 | 40.6 |
| Cycle (no.) | Enzyme Loading (%) * | Glucose (g L−1) | Xylose (g L−1) | XT (g L−1) | wL (%, g g−1) | L (g L−1) | YL/S (mg g−1) | PrL (g L−1 d−1) | ηL (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| after Enzyme Hydrolysis | after Cultivation | after Enzyme Hydrolysis | after Cultivation | |||||||||
| B_7 | 1 | 100 | 67.80 ± 4.28 | 4.33 ± 3.19 | 35.12 ± 1.51 | 21.89 ± 1.17 | 25.21 ± 1.38 | 48.27 ± 5.45 | 12.17 | 0.081 | 1.134 | 28.8 |
| 2 | 90 | 61.67 ± 0.92 | 1.58 ± 0.29 | 37.90 ± 0.71 | 24.98 ± 0.05 | 23.65 ± 2.44 | 44.96 ± 0.80 | 10.63 | 0.071 | 0.994 | 25.2 | |
| 3 | 90 | 62.26 ± 0.69 | 1.83 ± 2.59 | 39.54 ± 0.77 | 28.35 ± 5.16 | 28.20 ± 4.26 | 45.07 ± 0.88 | 12.71 | 0.085 | 1.19 | 30.1 | |
| 4 | 90 | 62.91 ± 0.25 | 4.27 ± 1.22 | 39.89 ± 0.41 | 29.15 ± 0.16 | 24.58 ± 0.98 | 47.69 ± 2.48 | 11.72 | 0.078 | 1.092 | 27.8 | |
| B_8 | 1 | 100 | 68.63 ± 4.73 | 0.37 ± 0.52 | 36.37 ± 1.92 | 17.63 ± 4.83 | 26.01 ± 3.61 | 49.48 ± 1.65 | 12.87 | 0.086 | 1.204 | 30.5 |
| 2 | 80 | 62.07 ± 0.48 | 3.94 ± 0.79 | 37.76 ± 0.10 | 27.80 ± 0.34 | 23.41 ± 0.98 | 44.12 ± 2.10 | 10.33 | 0.069 | 0.966 | 24.5 | |
| 3 | 80 | 61.96 ± 0.84 | 0.83 ± 1.17 | 39.29 ± 0.06 | 24.61 ± 1.20 | 26.95 ± 0.25 | 45.07 ± 0.88 | 12.15 | 0.081 | 1.134 | 28.8 | |
| 4 | 80 | 61.81 ± 0.14 | 3.97 ± 1.70 | 39.61 ± 0.70 | 26.42 ± 3.39 | 25.23 ± 3.16 | 47.16 ± 6.69 | 11.90 | 0.079 | 1.106 | 28.2 | |
| B_9 | 1 | 100 | 65.99 ± 3.90 | 0.00 ± 0.00 | 34.56 ± 1.95 | 18.40 ± 0.60 | 23.88 ± 3.34 | 48.24 ± 1.60 | 11.52 | 0.077 | 1.078 | 27.3 |
| 2 | 70 | 59.93 ± 1.44 | 4.41 ± 1.66 | 35.72 ± 1.17 | 23.35 ± 0.31 | 24.48 ± 1.43 | 44.10 ± 1.15 | 10.80 | 0.072 | 1.008 | 25.6 | |
| 3 | 70 | 61.43 ± 2.15 | 1.82 ± 0.46 | 38.55 ± 1.26 | 25.96 ± 0.12 | 23.30 ± 1.55 | 46.01 ± 3.04 | 10.72 | 0.071 | 0.994 | 25.4 | |
| 4 | 70 | 61.64 ± 0.26 | 2.02 ± 6.65 | 39.29 ± 0.10 | 25.86 ± 1.59 | 23.87 ± 0.91 | 46.64 ± 3.21 | 11.13 | 0.074 | 1.036 | 26.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubišić, M.; Mihajlovski, K.; Gruičić, A.M.; Beluhan, S.; Šantek, B.; Ivančić Šantek, M. Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass. J. Fungi 2021, 7, 934. https://doi.org/10.3390/jof7110934
Grubišić M, Mihajlovski K, Gruičić AM, Beluhan S, Šantek B, Ivančić Šantek M. Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass. Journal of Fungi. 2021; 7(11):934. https://doi.org/10.3390/jof7110934
Chicago/Turabian StyleGrubišić, Marina, Katarina Mihajlovski, Ana Marija Gruičić, Sunčica Beluhan, Božidar Šantek, and Mirela Ivančić Šantek. 2021. "Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass" Journal of Fungi 7, no. 11: 934. https://doi.org/10.3390/jof7110934






