Synergistic Impact of Bioactive Byproduct Extract Leads to Anti-Fusarium and Anti-Mycotoxin Secretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Microorganisms and Evaluated Material
2.2. Byproducts Extraction
2.3. Determination of the Volatile Contents
2.4. Determination of Total Phenolic and Flavonoids
2.5. Determination of Antioxidant Activity
2.6. Determination of Phenolic and Flavonoids Fractions
2.7. Estimation of Fatty Acids in Fixed Byproduct Oil
2.8. Estimation of the Anti-Fusarium Activity for the Crude Extract, Trans-Ferulic, and Hesperidin
2.9. Preparation of the Extract as Liposome
2.10. Determination of the Liposomal Characterization
2.11. Determination of Liposomal Formation Efficiency
2.12. Determination of Antifungal Potency of Liposomal and Non-Liposomal Materials
2.13. Estimation of Extracted Materials on Mycotoxin Secretion
2.14. Determination of Mycotoxin Content
2.15. Statistical Evaluation
3. Results
3.1. Volatile Content Evaluation
3.2. Total Phenolic and Flavonoid Contents
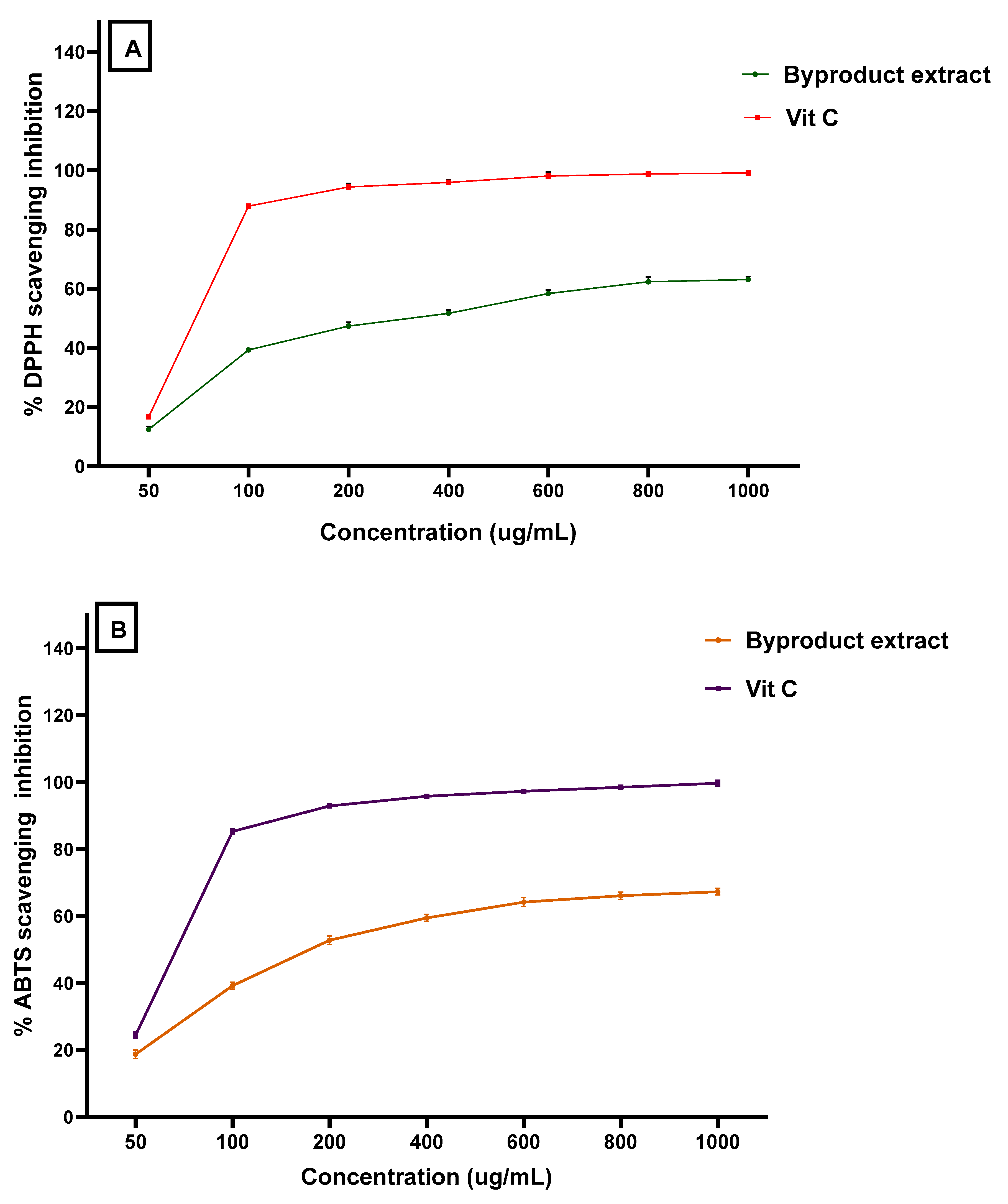
3.3. Antioxidant Activity for the Byproduct Extract
3.4. Phenolic and Flavonoids Fractions Determination
3.5. Fatty Acids in Fixed Oil Byproduct
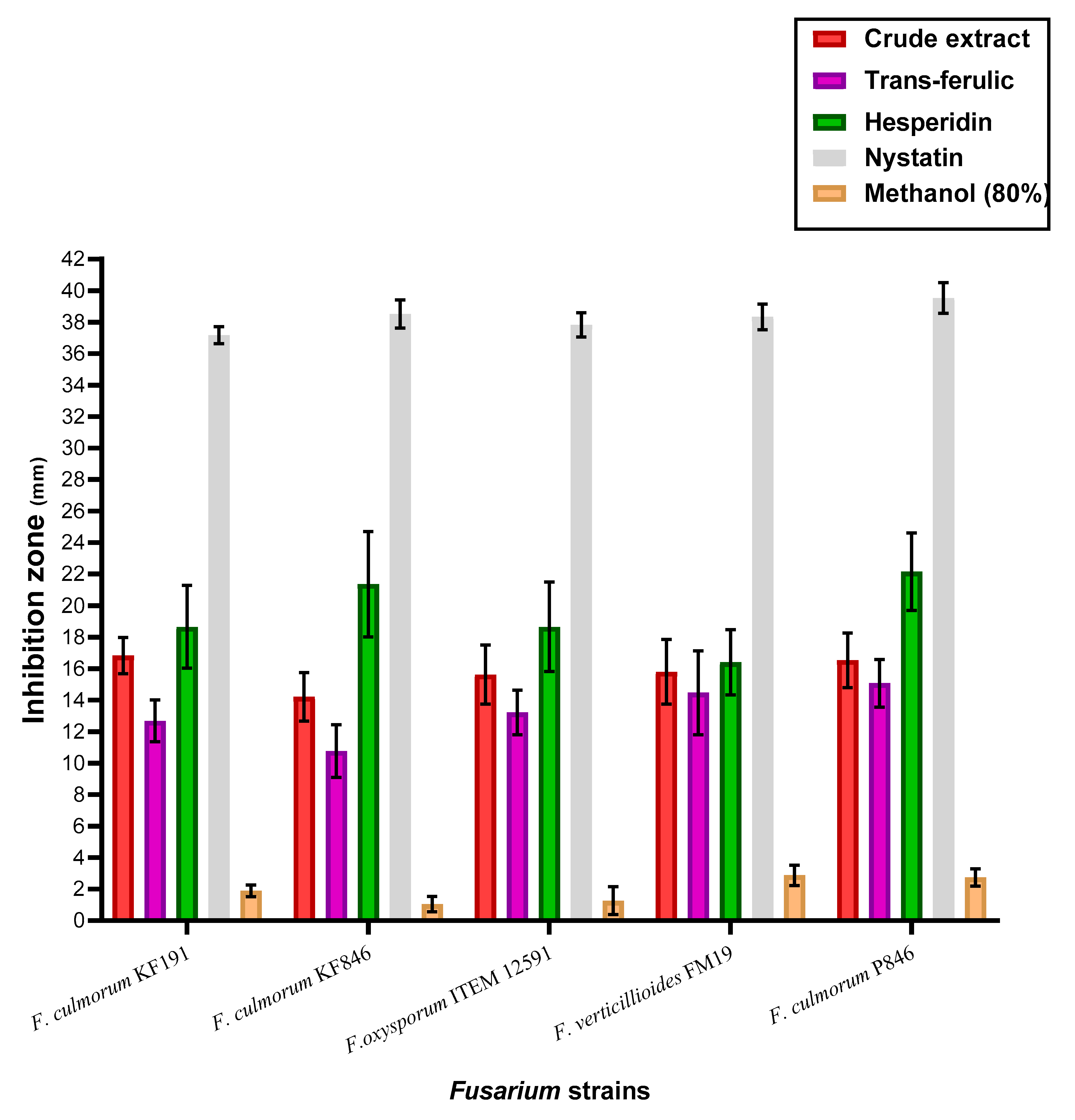
3.6. Anti Fusarium Activity of the Crude Extract, Trans-Ferulic, and Hesperidin
3.7. Liposomal Characterization Evaluation
3.8. Antifungal Potency of Crude and Liposomal Extracts
3.9. Estimation of the Impact on the Mycotoxin Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, H.; De Filippis, A.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Beneficial Effects of Plant Extracts and Bioactive Food Components in Childhood Supplementation. Nutrients 2021, 13, 3157. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentillafruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.-D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef]
- Abu-Sree, Y.H.; Abdel-Fattah, S.M.; Abdel-Razek, A.G.; Badr, A.N. Neoteric approach for peanuts biofilm using the merits of Moringa extracts to control aflatoxin contamination. Toxicol. Rep. 2021, 8, 1685–1692. [Google Scholar] [CrossRef]
- Badr, A.; Shehata, M.; Abdel-Razek, A. Antioxidant Activities and Potential Impacts to Reduce Aflatoxins Utilizing Jojoba and Jatropha Oils and Extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Badr, A.N.; Youssef, M.; Abdel-Razek, A.G.; Shehata, M.G.; Hassanien, M.M.; Amra, H. Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egypt. J. Chem. 2021, 64, 285–298. [Google Scholar] [CrossRef]
- Badr, A.; Ali, H.; Abdel-Razek, A.; Shehata, M.; Albaridi, N. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Said, M.; Elmessery, T.; Abdel-Razek, A.G. Non-traditional Oils Encapsulation as Novel Food Additive Enhanced Yogurt Safety against Aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, S.M.; Badr, A.N.; Abu Seif, F.A.-H.; Al, S.M.; Hass, R.A. Antifungal and Anti-mycotoxigenic Impact of Eco-Friendly Extracts of Wild Stevia. J. Biol. Sci. 2018, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G. Prospective antimycotoxigenic action of wild Opuntia ficus-indica by-products. Czech J. Food Sci. 2020, 38, 308–314. [Google Scholar] [CrossRef]
- Badr, A.N.; Naeem, M.A. Protective efficacy using Cape- golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef]
- Abdel-Razek, A.; Shehata, M.G.; Badr, A.; Gromadzka, K.; Stępień, L. The Effect of Chemical Composition of Wild OpuntiaFicusIndica Byproducts on its Nutritional Quality, Antioxidant and Antifungal Efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Salhi, N.; Saghir, S.A.M.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal Activity of Aqueous Extracts of Some Dominant Algerian Medicinal Plants. BioMed Res. Int. 2017, 2017, 7526291. [Google Scholar] [CrossRef] [Green Version]
- M’hiri, N.; Irina, I.; Cédric, P.; Ghoul, M.; Boudhrioua, N. Antioxidants of Maltease orange peel: Comparative investigation of the efficiency of four extraction methods. J. Appl. Pharm. Sci. 2017, 7, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2016, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; Shehata, M.G. Characterization of Olive Oil By-products: Antioxidant Activity, Its Ability to Reduce Aflatoxigenic Fungi Hazard and Its Aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of Nanoliposomal Vitamin D3 for Potential Application in Beverage Fortification. Adv. Pharm. Bull. 2014, 4 (Suppl. S2), 569–575. [Google Scholar] [CrossRef]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 706–707. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process. Preserv. 2020, 45, e15112. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Muscarella, M.; Iammarino, M.; Nardiello, D.; Palermo, C.; Centonze, D. Determination of deoxynivalenol and nivalenol by liquid chromatography and fluorimetric detection with on-line chemical post-column derivatization. Talanta 2012, 97, 145–149. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical Scavenging Activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrumsativum L.), and Niger (Guizotiaabyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.; Begg, S.N.; Pederick, V.G.; Trappetti, C.; Gregory, M.; Whittall, J.J.; Paton, J.C.; McDevitt, C.A. Arachidonic Acid Stress Impacts Pneumococcal Fatty Acid Homeostasis. Front. Microbiol. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.; Jäger, A.; van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2006, 91, 303–314. [Google Scholar] [CrossRef]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Bajpai, V.; Kim, H.; Kang, S. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT-Food Sci. Technol. 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaemaja cquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Combination of different antifungal agents in oil-in-water emulsions to control strawberry jam spoilage. Food Chem. 2018, 239, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-M.; Kully, M.; Khan, J.K.; Hattori, M.; Daneshtalab, M. Synthesis of chlorogenic acid derivatives with promising antifungal activity. Bioorgan. Med. Chem. 2007, 15, 6830–6833. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Ekiert, H.; Barakat, A.A.; Al-Mana, F.A. Antiproliferative, Antimicrobial, and Antifungal Activities of Polyphenol Extracts from Ferocactus Species. Processes 2020, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Sinapic Acid Affects Phenolic and Trichothecene Profiles of F. culmorum and F. graminearumSensuStricto. Toxins 2017, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Al-Mamary, M.S.; Moussa, Z. Antioxidant Activity: The Presence and Impact of Hydroxyl Groups in Small Molecules of Natural and Synthetic Origin. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; pp. 318–377. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.C.; Chong, Y. Aromatic Hydroxyl Group Plays a Critical Role in Antibacterial Activity of the Curcumin Analogues. Nat. Prod. Commun. 2012, 7, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, G.; Dai, Y.; Wang, Y.; Lee, Y.-W.; Shi, J.; Xu, J. Biodegradation of Deoxynivalenol by a Novel Microbial Consortium. Front. Microbiol. 2020, 10, 2964. [Google Scholar] [CrossRef] [Green Version]


| Compound | RI | Byproduct Content | Identification |
|---|---|---|---|
| Hexanal | 801 | 0.37 ± 0.05 | MS and RI |
| α-Thujene | 928 | 2.67 ± 0.31 | MS and RI |
| α-Pinene | 939 | 3.41 ± 0.54 | MS, RI and ST |
| Sabinene | 972 | 0.88 ± 0.12 | MS and RI |
| β-Pinene | 981 | 4.57 ± 0.24 | MS, RI and ST |
| β-Myrcene | 991 | 7.19 ± 0.81 | MS and RI |
| Octanal | 1006 | 0.39 ± 0.14 | MS and RI |
| α-Terpinene | 1012 | 0.84 ± 0.16 | MS, RI and ST |
| β-phellandrene | 1030 | 3.12 ± 0.06 | MS and RI |
| Limonene | 1033 | 67.54 ± 1.74 | MS and RI |
| γ-Terpinene | 1074 | 4.82 ± 0.98 | MS, RI and ST |
| α-Terpinolene | 1096 | 2.36 ± 0.49 | MS and RI |
| Linalool | 1100 | 0.08 ± 0.03 | MS, RI and ST |
| Nonanal | 1104 | 0.02 ± 0.001 | MS and RI |
| Geranyl | 1149 | ND | MS and RI |
| Citronellal | 1159 | 0.03 ± 0.002 | MS, RI and ST |
| Decanal | 1234 | 0.14 ± 0.01 | MS and RI |
| Ethanone | 1274 | 0.27 ± 0.05 | MS and RI |
| Cadinene | 1275 | 0.34 ± 0.08 | MS, RI and ST |
| α-Cubebene | 1345 | ND | MS and RI |
| Isopiperitone | 1473 | 0.18 ± 0.02 | MS and RI |
| α-Sinensal | 1526 | 0.15 ± 0.03 | MS and RI |
| β-Sinensal | 1675 | 0.54 ± 0.14 | MS and RI |
| Phenolic Acids | Concentrations (mg/100 g) | Flavonoids Compounds | Concentrations (mg/100 g) |
|---|---|---|---|
| Gallic acid | 51.77 ± 2.84 | Catechin | 84.52 ± 1.08 |
| Chlorogenic acid | 125.13 ± 1.05 | Catechol | 125.24 ± 2.74 |
| Protocatechuic acid | 122.31 ± 1.94 | Epicatechins | 27.41 ± 0.88 |
| trans-Ferulic acid | 91.74 ± 1.93 | Rutintrihydrate | 35.17 ± 1.14 |
| trans-Cinnamic acid | 25.22 ± 1.05 | Apigenin 7 glucoside | 44.27 ± 1.67 |
| Vanilic acid | 22.7 ± 0.87 | Quercetin | 63.08 ± 4.51 |
| Caffeic acid | 34.58 ± 1.41 | Luteolin | ND |
| Ferulic acid | 235.54 ± 3.34 | Hesperidin | 492.11 ± 1.15 |
| p-Hydroxybenzoic acid | 2.94 ± 0.67 | Naringenin-7-o-glucoside | 13.97 ± 0.54 |
| p-Coumaric acid | 60.54 ± 1.08 | Kaempferol | 268.56 ± 3.54 |
| Syringic acid | 19.91 ± 1.18 | Isorhamnetin-3-o-rutinoside | 152.81 ± 2.78 |
| Sinapic acid | 121.75 ± 2.86 | Chrysin | ND |
| Total Phenolic acids | 914.36 ± 20.22 | Total Flavonoids | 1307.14 ± 20.03 |
| Carbonnumber | Fatty Acids | Concentration (%) | Notes | Reference for Activity Impact |
|---|---|---|---|---|
| C 12:0 | Lauric | 0.88 ± 0.21 | Short-chain | - |
| C 14:0 | Mayristic | ND | Not detected | - |
| C 16:0 | Palmitic | 0.84 ± 0.11 | Less than 1% | - |
| C 18:0 | Stearic | 4.22 ± 0.41 | Antifungal impact | [34] |
| C 20:0 | Arachidic | 10.09 ± 0.22 | Antifungal impact | [35,36] |
| C 22:0 | Behenic | 0.48 ± 0.08 | Less than 1% | - |
| C 24:0 | Lignoseric | 0.16 ± 0.04 | Less than 1% | - |
| Omega Fatty acid contents | ||||
| C 18:3 n-3 | Linolenic | 8.72 ± 0.37 | Antifungal impact | [37] |
| C 20:5 n-3 | Ecosapentanoic | 0.09 ± 0.005 | Antimicrobial impact | [38] |
| C 22:6 n-3 | Docosahexaenoic | ND | Not detected | - |
| C 14:1 n-5 | Myristoleic | 10.61 ± 0.54 | High content | |
| C 18:1 n-9 | Oleic | 14.71 ± 0.88 | Antifungal impact | [38] |
| C 18:2 n-6 | Linoleic | 18.44 ± 1.05 | Antifungal impact | [38] |
| C 20:4 n-6 | Arachidonic | 0.82 ± 0.04 | Less than 1% | - |
| C 20:2 n-6 | Eicosadienoic | 0.05 ± 0.003 | Antimicrobial impact | [39] |
| C 22:2 n-6 | Docosadienoic | 7.19 ± 0.83 | Antimicrobial impact | [40] |
| C 16:1 n-7 | Palmitoleic | 21.74 ± 0.63 | Major content | |
| C 20:1 n-9 | Gadoleic | 0.26 ± 0.01 | Antimicrobial impact | [36] |
| C 20:3 n-9 | Eicosatrienoic | 0.63 ± 0.08 | Less than 1% | - |
| C 22:1 n-9 | Erucic | 0.02 ± 0.001 | Trace content | - |
| C 24:1 n-9 | Nervonic | 0.05 ± 0.001 | Trace content | - |
| SFA/MUFA/PUFA | 0.25:1.42:1.33 | |||
| Cox value | ||||
| Storage Period (Days) | Particle Size (nm) | Zeta Potential (mv) | PDI | LFE |
|---|---|---|---|---|
| 1 | 89.41 ± 2.11 | −41.24 ± 2.08 | 0.284 ± 0.002 | 99.21% |
| 3 | 96.37 ± 4.27 | −40.7 ± 3.61 | 0.373 ± 0.005 | 98.04% |
| 7 | 107.66 ± 5.44 | −38.14 ± 4.27 | 0.561 ± 0.004 | 91.18% |
| 14 | 145.81. ± 4.81 | −34.81 ± 4.56 | 0.418 ± 0.002 | 87.24% |
| 21 | 177.64 ± 5.74 | −33.63 ± 5.41 | 0.420 ± 0.008 | 82.15% |
| 30 | 237.36 ± 8.63 | −31.05 ± 8.34 | 0.416 ± 0.005 | 77.54% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.N.; Stepien, L.; Drzewiecka, K.; Alharthi, S.S.; Selim, K.; Abdel-Razek, A.G. Synergistic Impact of Bioactive Byproduct Extract Leads to Anti-Fusarium and Anti-Mycotoxin Secretion. J. Fungi 2022, 8, 30. https://doi.org/10.3390/jof8010030
Badr AN, Stepien L, Drzewiecka K, Alharthi SS, Selim K, Abdel-Razek AG. Synergistic Impact of Bioactive Byproduct Extract Leads to Anti-Fusarium and Anti-Mycotoxin Secretion. Journal of Fungi. 2022; 8(1):30. https://doi.org/10.3390/jof8010030
Chicago/Turabian StyleBadr, Ahmed Noah, Lukasz Stepien, Kinga Drzewiecka, Salman S. Alharthi, Khaled Selim, and Adel Gabr Abdel-Razek. 2022. "Synergistic Impact of Bioactive Byproduct Extract Leads to Anti-Fusarium and Anti-Mycotoxin Secretion" Journal of Fungi 8, no. 1: 30. https://doi.org/10.3390/jof8010030









