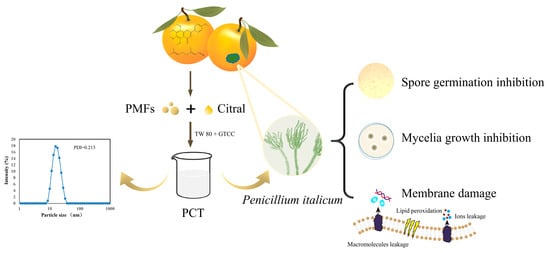
Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungi and Chemicals
2.2. Analysis of the Components of PMFs Dissolved in Citral by UPLC–PDA
2.3. Nanoemulsion Preparation
2.4. Characterization of Nanoemulsions: Particle Size, Polydispersity Index (PDI), and Zeta Potential
2.5. Determination of Minimal Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
2.6. Effect of Nanoemulsion on Spore Germination
2.7. Effect of Nanoemulsion on Mycelial Growth
2.8. Determination of cell Membrane Integrity and Permeability
2.8.1. Morphological Observation
2.8.2. Propidium iodide (PI) Staining
2.8.3. Extracellular Electric Conductivity
2.8.4. Malondialdehyde (MDA)
2.8.5. Total Lipids and Ergosterol
2.9. Statistical Analysis
3. Results
3.1. Analysis of the Components of PMFs Dissolved in Citral by UPLC–PDA
3.2. Characterization of the Nanoemulsions
3.3. MIC and MFC of the CT and PCT Nanoemulsions against P. italicum
3.4. Effect on Spore Germination
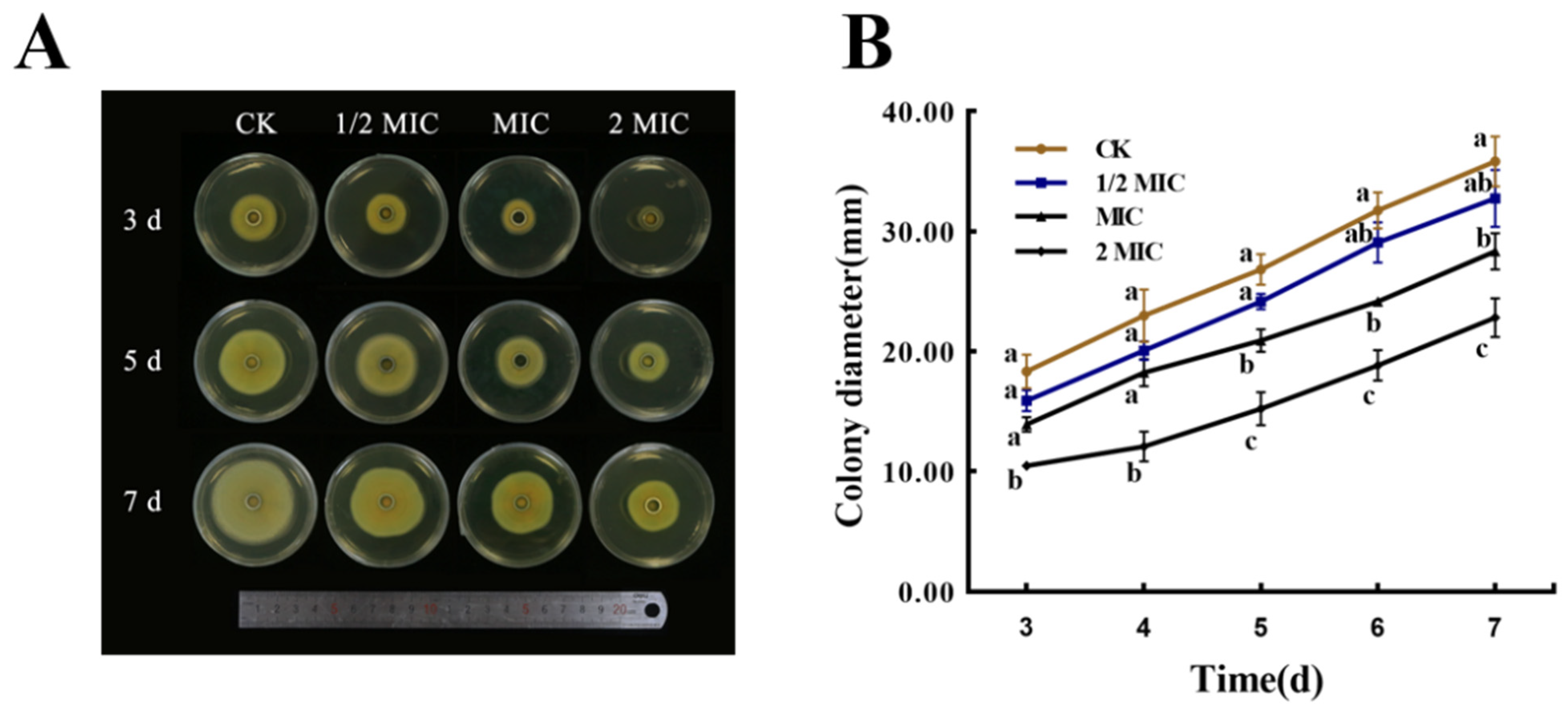
3.5. Effect on Mycelial Growth
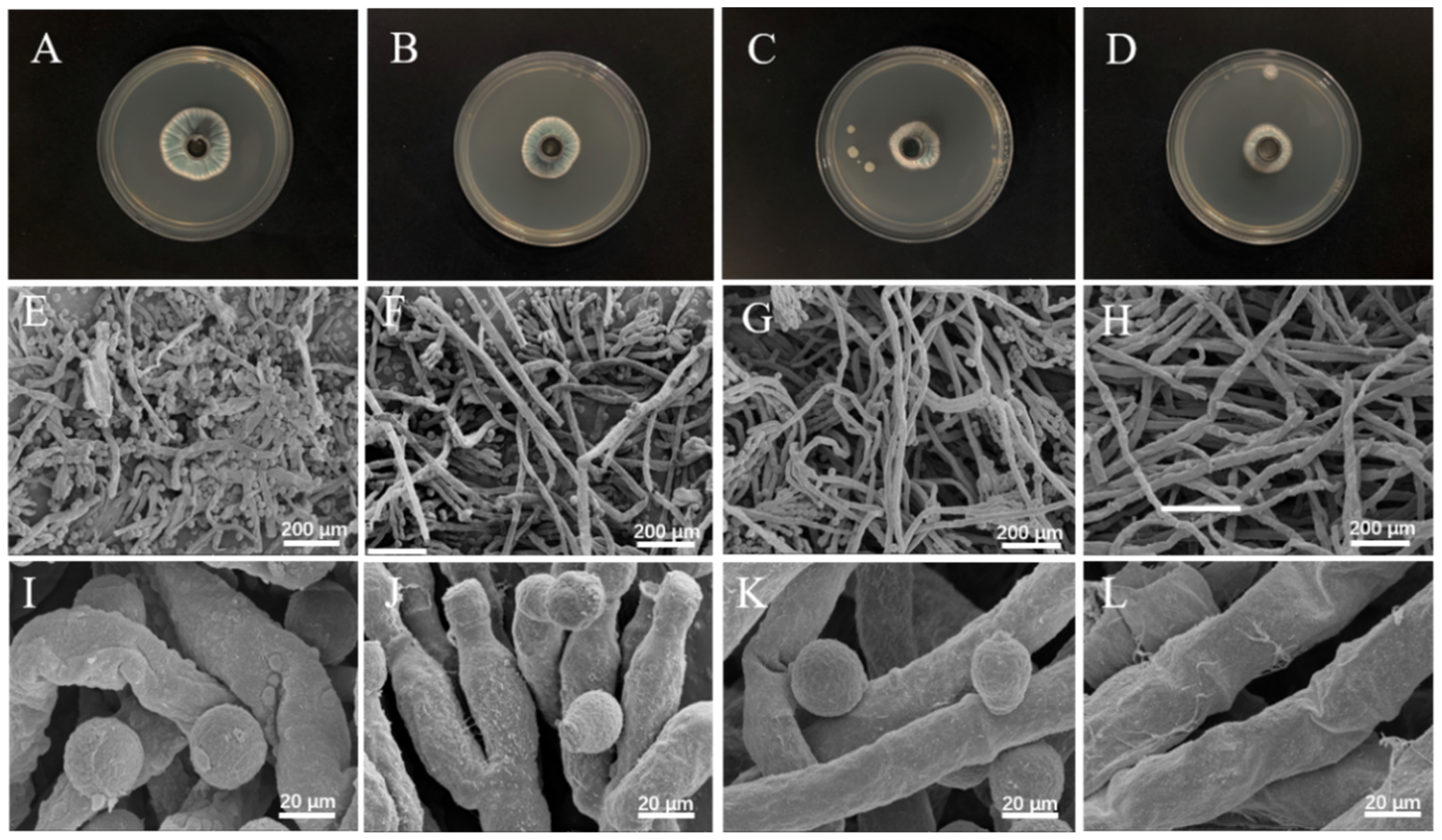
3.6. Micromorphological Analysis by SEM
3.7. Propidium iodide (PI) Staining
3.8. Extracellular Conductivity
3.9. Malondialdehyde (MDA)
3.10. Total Lipid Content
3.11. Ergosterol Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimen, B. Efficient Protoplast Isolation from Ovule-Derived Embryogenic Callus in Citrus Volkameriana. Turk. J. Agric. For. 2020, 44, 567–576. [Google Scholar] [CrossRef]
- Sülü, G.; Kacar, Y.A.; Sulu, G. Identification of Genetic Diversity among Mutant Lemon and Mandarin Varieties Using Different Molecular Markers. Turk. J. Agric. For. 2020, 44, 44. [Google Scholar] [CrossRef]
- Liu, Y.; Benohoud, M.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. Green Extraction of Polyphenols from Citrus Peel By-Products and Their Antifungal Activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Huang, J.; Chen, J.; Nisar, M.F.; Qi, W. Synthesis and Antifungal Activities of Cinnamaldehyde Derivatives against Penicillium digitatum Causing Citrus Green Mold. J. Food Qual. 2020, 2020, e8898692. [Google Scholar] [CrossRef]
- Moussa, H.; El Omari, B.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of Thymol, Carvacrol and Eugenol on Penicillium and Geotrichum Isolates Resistant to Commercial Fungicides and Causing Postharvest Citrus Decay. Can. J. Plant Pathol. 2021, 43, 26–34. [Google Scholar] [CrossRef]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and Its Possible Mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal Activity of Volatile Organic Compounds Produced by Pseudomonas Fluorescens ZX and Potential Biocontrol of Blue Mold Decay on Postharvest Citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Costa, J.H.; Bazioli, J.M.; de Moraes Pontes, J.G.; Fill, T.P. Penicillium digitatum Infection Mechanisms in Citrus: What Do We Know so Far? Fungal Biol. 2019, 123, 584–593. [Google Scholar] [CrossRef]
- Martins, S.J.; Faria, A.F.; Pedroso, M.P.; Cunha, M.G.; Rocha, M.R.; Medeiros, F.H.V. Microbial Volatiles Organic Compounds Control Anthracnose (Colletotrichum lindemuthianum) in Common Bean (Phaseolus Vulgaris L.). Biol. Control 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Manthey, J.A.; Bendele, P. Anti-Inflammatory Activity of an Orange Peel Polymethoxylated Flavone, 3′,4′,3,5,6,7,8-Heptamethoxyflavone, in the Rat Carrageenan/Paw Edema and Mouse Lipopolysaccharide-Challenge Assays. J. Agric. Food Chem. 2008, 56, 9399–9403. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid Composition of Orange Peel and Its Association with Antioxidant and Anti-Inflammatory Activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Díaz, L.; Alvarez, N.; Porras, I.; García-Lidón, A.; Del Río, J.A. Comparative Study of Flavonoid and Scoparone Accumulation in Different Citrus Species and Their Susceptibility to Penicillium digitatum. Food Chem. 2011, 125, 232–239. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S. Chemotherapeutic Effect of Tangeretin, a Polymethoxylated Flavone Studied in 7, 12-Dimethylbenz(a)Anthracene Induced Mammary Carcinoma in Experimental Rats. Biochimie 2014, 99, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Synergistic Inhibition Effect of Citral and Eugenol against Aspergillus Niger and Their Application in Bread Preservation. Food Chem. 2020, 310, 125974. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Zhang, Z.; Yuan, Y.; Yue, T. Effect of Cinnamaldehyde and Citral Combination on Transcriptional Profile, Growth, Oxidative Damage and Patulin Biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Chen, J.; Lin, L.; Wan, C. Possible Fungicidal Effect of Citral on Kiwifruit Pathogens and Their Mechanisms of Actions. Physiol. Mol. Plant Pathol. 2021, 114, 101631. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and Cytotoxicity of Citrus Aurantiifolia Essential Oil and Its Major Constituents: Limonene and Citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Leite, M.C.; Bezerra, A.P.; Sousa, J.P.; Guerra, F.Q.; Lima, E.D. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid. Based Complement. Alternat. Med. 2014, 2014, e378280. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Cheng, D.; He, M.; Pan, S.; Yao, X.; Xu, X. Antifungal Action and Inhibitory Mechanism of Polymethoxylated Flavones from Citrus Reticulata Blanco Peel against Aspergillus niger. Food Control 2014, 35, 354–359. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. Antifungal Activity and Mechanism of Citral, Limonene and Eugenol against Zygosaccharomyces rouxii. LWT 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Chen, Y.; Ji, L.; Yao, W. Synergistic Properties of Citral and Eugenol for the Inactivation of Foodborne Molds in Vitro and on Bread. LWT 2020, 122, 109063. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M. Boosting Antifungal Effect of Essential Oils Using Combination Approach as an Efficient Strategy to Control Postharvest Spoilage and Preserving the Jujube Fruit Quality. Postharvest Biol. Technol. 2020, 164, 111159. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.; Yue, T. Antifungal Mechanism of Cinnamaldehyde and Citral Combination against Penicillium expansum Based on FT-IR Fingerprint, Plasma Membrane, Oxidative Stress and Volatile Profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, R.; Li, Y.; Zhou, Z. Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests. Microorganisms 2021, 9, 969. [Google Scholar] [CrossRef]
- Vuuren, S.F.V.; Viljoen, A.M. Interaction between the Non-Volatile and Volatile Fractions on the Antimicrobial Activity of Tarchonanthus camphoratus. South Afr. J. Bot. 2009, 75, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Zahi, M.R.; Hattab, M.E.; Liang, H.; Yuan, Q. Enhancing the Antimicrobial Activity of D-Limonene Nanoemulsion with the Inclusion of ε-Polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Cheng, D.; Yao, X.; Pan, S. Structure–Activity Relationship of Citrus Polymethoxylated Flavones and Their Inhibitory Effects on Aspergillus niger. J. Agric. Food Chem. 2012, 60, 4336–4341. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral Nanoemulsion Incorporated Edible Coating to Extend the Shelf Life of Fresh Cut Pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Chen, P.; Ference, C.; Sun, X.; Lin, Y.; Tan, L.; Zhong, T. Antimicrobial Efficacy of Liposome-Encapsulated Citral and Its Effect on the Shelf Life of Shatangju Mandarin. J. Food Prot. 2020, 83, 1315–1322. [Google Scholar] [CrossRef]
- Long, Y.; Huang, W.; Wang, Q.; Yang, G. Green Synthesis of Garlic Oil Nanoemulsion Using Ultrasonication Technique and Its Mechanism of Antifungal Action against Penicillium italicum. Ultrason. Sonochem. 2020, 64, 104970. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, L.; Lu, M.; Lu, S.; Li, Z.; Ding, W. Comparative Study on the Fungicidal Activity of Metallic MgO Nanoparticles and Macroscale MgO Against Soilborne Fungal Phytopathogens. Front. Microbiol. 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.; Chen, H.; Xiang, A.; Wang, Y.; Yue, T.; Yuan, Y. Identification and Characterization of Lactobacillus Paracasei Strain MRS-4 Antibacterial Activity against Alicyclobacillus acidoterrestris. LWT 2021, 150, 111991. [Google Scholar] [CrossRef]
- Hu, Z.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.; Liu, F. Mechanism of Antifungal Activity of Perilla Frutescens Essential Oil against Aspergillus flavus by Transcriptomic Analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal Effects of Thymol and Salicylic Acid on Cell Membrane and Mitochondria of Rhizopus stolonifer and Their Application in Postharvest Preservation of Tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zhu, X.; Xie, Y. Antifungal Efficacy of Paeonol on Aspergillus Flavus and Its Mode of Action on Cell Walls and Cell Membranes. LWT 2021, 149, 111985. [Google Scholar] [CrossRef]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal Efficacy of Ursolic Acid in Control of Alternaria Alternata Causing Black Spot Rot on Apple Fruit and Possible Mechanisms Involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Zhou, Z. Exploring the Antifungal Mechanism of Limonin-Loaded Eugenol Emulsion against Penicillium italicum: From the Perspective of Microbial Metabolism. Postharvest Biol. Technol. 2021, 182, 111704. [Google Scholar] [CrossRef]
- da Silva Bomfim, N.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Graton Mikcha, J.M.; de Abreu Filho, B.A.; Machinski, M., Jr. Antifungal and Antiaflatoxigenic Activity of Rosemary Essential Oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A 2020, 37, 153–161. [Google Scholar] [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Pérez Nevado, F.; Aranda, E.; Serradilla, M.J.; de Córdoba, M.G.; Martín, A. Anti-Fungal Activity of Phenolic Sweet Orange Peel Extract for Controlling Fungi Responsible for Post-Harvest Fruit Decay. Fungal Biol. 2021, 125, 143–152. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges. Horticulturae 2020, 6, 102. [Google Scholar] [CrossRef]
- Almada-Ruiz, E.; Martínez-Téllez, M.Á.; Hernández-Álamos, M.M.; Vallejo, S.; Primo-Yúfera, E.; Vargas-Arispuro, I. Fungicidal Potential of Methoxylated Flavones from Citrus for in Vitro Control of Colletotrichum gloeosporioides, Causal Agent of Anthracnose Disease in Tropical Fruits: Fungicidal Potential of Methoxylated Flavones from Citrus. Pest Manag. Sci. 2003, 59, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ye, H.; Shen, Q.; Jiang, X.; Cui, G.; Gu, W.; Zhang, L.; Naqvi, N.I.; Deng, Y.Z. Tangeretin Inhibits Fungal Ferroptosis to Suppress Rice Blast. J. Integr. Plant Biol. 2021, 63, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, J.; Wei, Y.; Han, P.; Dai, K.; Zou, X.; Jiang, S.; Xu, F.; Wang, H.; Sun, J.; et al. Tea Tree Oil Controls Brown Rot in Peaches by Damaging the Cell Membrane of Monilinia fructicola. Postharvest Biol. Technol. 2021, 175, 111474. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral Inhibits Mycelial Growth of Penicillium Italicum by a Membrane Damage Mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Zyl, R.L.; Davids, H.; Van Vuuren, S.F.; Viljoen, A.M. Synergistic and Antagonistic Interactions of Essential Oils on the Biological Activities of the Solvent Extracts from Three Salvia Species. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, J.; Wan, C. Pinocembrin-7-Glucoside (P7G) Reduced Postharvest Blue Mold of Navel Orange by Suppressing Penicillium Italicum Growth. Microorganisms 2020, 8, 536. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Kong, J.; Ju, J.; Zhang, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Xie, Y.; Yao, W. Membrane Damage Mechanism Contributes to Inhibition of Trans-Cinnamaldehyde on Penicillium italicum Using Surface-Enhanced Raman Spectroscopy (SERS). Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Li, Q.; Xu, S.; Zhao, X.; Xue, P.; Feng, X. Antifungal Activity of Zedoary Turmeric Oil against Phytophthora Capsici through Damaging Cell Membrane. Pestic. Biochem. Physiol. 2019, 159, 59–67. [Google Scholar] [CrossRef]
- Li, L.; Xin, Z.; Okwong, R.O.; OuYang, Q.; Che, J.; Zhou, J.; Tao, N. Antofine Inhibits Postharvest Green Mold Due to Imazalil-Resistant Penicillium Digitatum Strain Pdw03 by Triggering Oxidative Burst. J. Food Biochem. 2021, 45, e13751. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal Activity of Lipopeptides from Bacillus Amyloliquefaciens MG3 against Colletotrichum Gloeosporioides in Loquat Fruits. Biol. Control 2020, 146, 104281. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Multifaceted Roles for Lipids in Viral Infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.; Tao, N.; Jing, G. Transcriptional Profiling Analysis of Penicillium digitatum, the Causal Agent of Citrus Green Mold, Unravels an Inhibited Ergosterol Biosynthesis Pathway in Response to Citral. BMC Genom. 2016, 17, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, N.; Jia, L.; Zhou, H. Anti-Fungal Activity of Citrus Reticulata Blanco Essential Oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Effect of Natamycin on Botrytis cinerea and Penicillium expansum—Postharvest Pathogens of Grape Berries and Jujube Fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Ghosoph, J.M.; Schmidt, L.S.; Margosan, D.A.; Smilanick, J.L. Imazalil Resistance Linked to a Unique Insertion Sequence in the PdCYP51 Promoter Region of Penicillium digitatum. Postharvest Biol. Technol. 2007, 44, 9–18. [Google Scholar] [CrossRef]


| Compounds | Retention Time (min) | Concentration (g kg−1) | |
|---|---|---|---|
| 1 | Isosinensetin | 5.46 | 0.265 |
| 2 | Sinensetin | 5.60 | 0.571 |
| 3 | 3′,4′,5,7-Tetrathoxyflavone | 5.78 | 0.067 |
| 4 | Nobiletin | 6.11 | 9.635 |
| 5 | Tangeretin | 6.45 | 8.488 |
| Total | 19.026 |
| Storage Time (Days) | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Fresh | 17.08 ± 0.18 a | 0.215 ± 0.016 a | −22.20 ± 0.80 a |
| 5 | 19.55 ± 0.25 b | 0.240 ± 0.021 a | −15.15 ± 0.45 b |
| 10 | 20.71 ± 0.28 c | 0.219 ± 0.012 a | −11.23 ± 1.27 c |
| 20 | 26.86 ± 0.96 d | 0.309 ± 0.003 b | −11.05 ± 0.75 c |
| Group | Concentration a | Mycelial Growth in PDB (3rd Day) | Mycelial Growth in PDB (6th Day) | MIC | MFC |
|---|---|---|---|---|---|
| CT | 500 | - | - | 125 | 250 |
| 250 | - | - | |||
| 125 | - | + | |||
| 62.5 | + | + | |||
| 31.25 | + | + | |||
| PCT | 500 | - | - | 62.5 | 250 |
| 250 | - | - | |||
| 125 | - | + | |||
| 62.5 | - | + | |||
| 31.25 | + | + | |||
| PDB + Stain | 0 | + | + | ||
| PDB | 0 | - | - |
| Treatment | Spore Germination Rates | ||
|---|---|---|---|
| 5 h | 12 h | 24 h | |
| CK | 4.91 ± 1.25 a | 53.45 ± 1.12 a | 91.69 ± 2.16 a |
| 1/2 MIC | 0.93 ± 0.04 b | 25.56 ± 2.20 b | 48.09 ± 3.64 b |
| MIC | 0 c | 3.19 ± 0.16 c | 8.75 ± 0.40 c |
| 2 MIC | 0 d | 0 d | 0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Li, Y.; Mao, X.; Tao, R.; Tao, B.; Zhou, Z. Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage. J. Fungi 2022, 8, 388. https://doi.org/10.3390/jof8040388
Guo L, Li Y, Mao X, Tao R, Tao B, Zhou Z. Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage. Journal of Fungi. 2022; 8(4):388. https://doi.org/10.3390/jof8040388
Chicago/Turabian StyleGuo, Long, Yi Li, Xiaoxue Mao, Rui Tao, Boyun Tao, and Zhiqin Zhou. 2022. "Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage" Journal of Fungi 8, no. 4: 388. https://doi.org/10.3390/jof8040388