Production of Microsclerotia by Metarhizium sp., and Factors Affecting Their Survival, Germination, and Conidial Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Species and Strains Used in This Study
2.2. Production of Microsclerotia by M. brunneum and M. robertsii Strains, and Their Germination and Infective Propagule Yield
2.3. Effect of Storage Temperature on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58-Su
2.4. Effect of Incubation Time on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58-Su
2.5. Effect of Soil Texture on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58-Su
2.6. Effects of Soil Temperature and Moisture on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58
2.7. Ultraviolet Radiation (UV-B) Effects on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58-Su
2.8. Statistical Analysis
3. Results
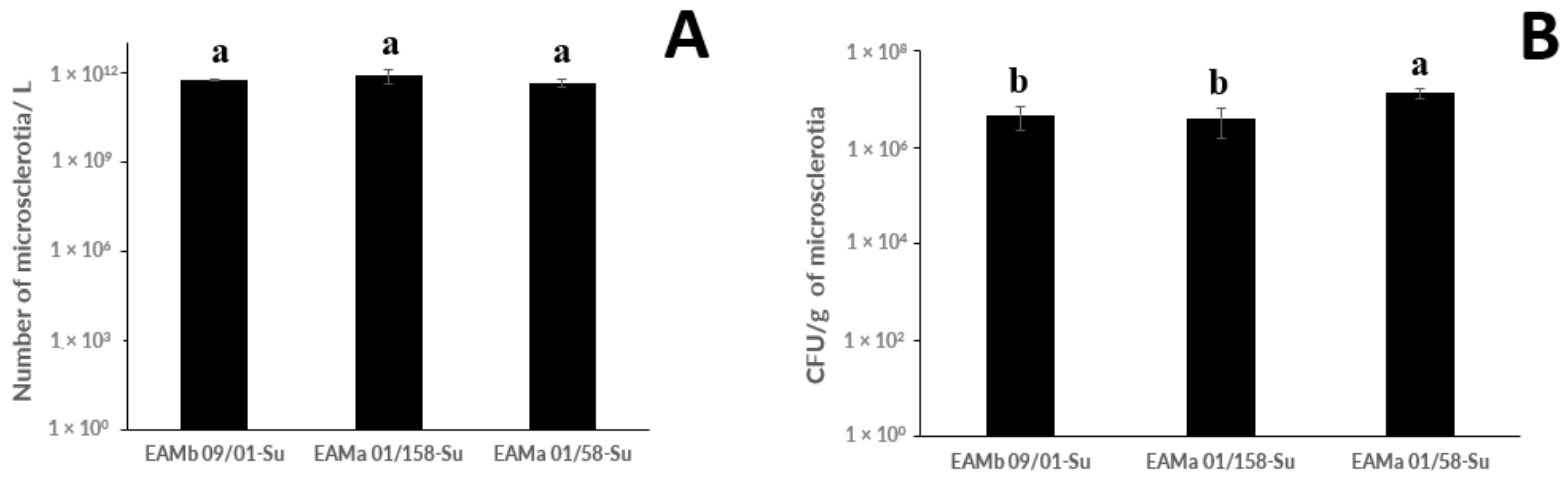
3.1. Production of Microsclerotia, Germination, and Infective Propagule Yield by M. brunneum and M. robertsii Strains
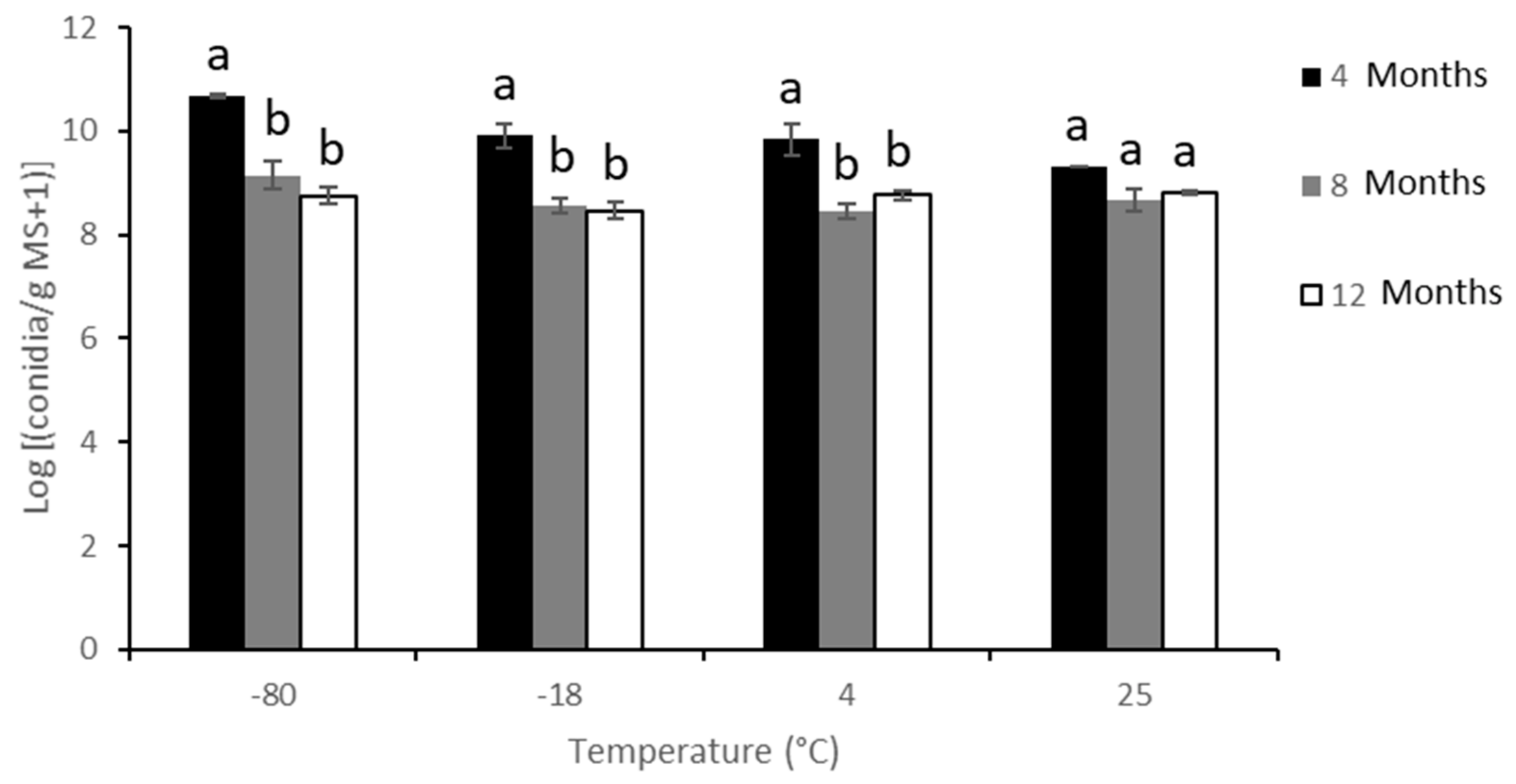
3.2. Effects of Storage Temperature on Microsclerotia Germination and Infective Propagule Yield of M. brunneum Strain EAMa 01/58-Su
3.3. Effects of Incubation Time on Microsclerotia Germination and Colony Yield of M. brunneum Strain EAMa 01/58-Su
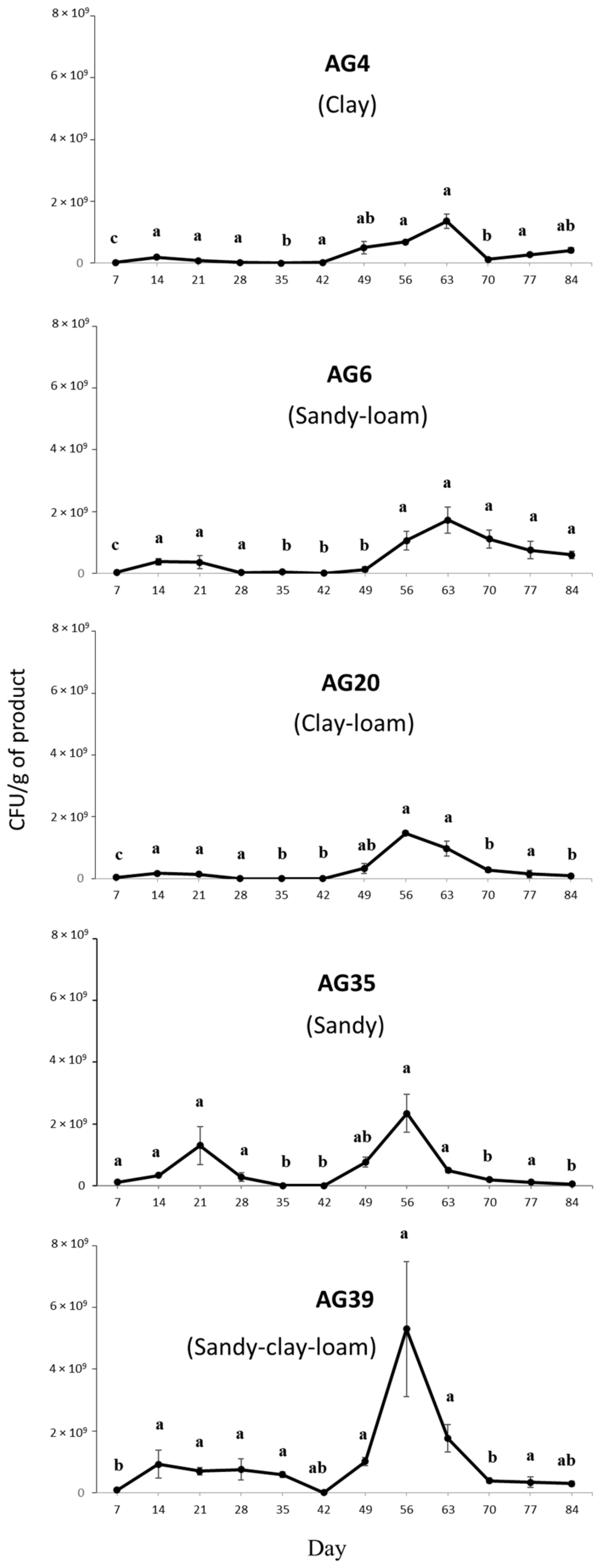
3.4. Effects of Soil Texture on Microsclerotia Germination and Colony Yield of M. brunneum Strain EAMa 01/58-Su
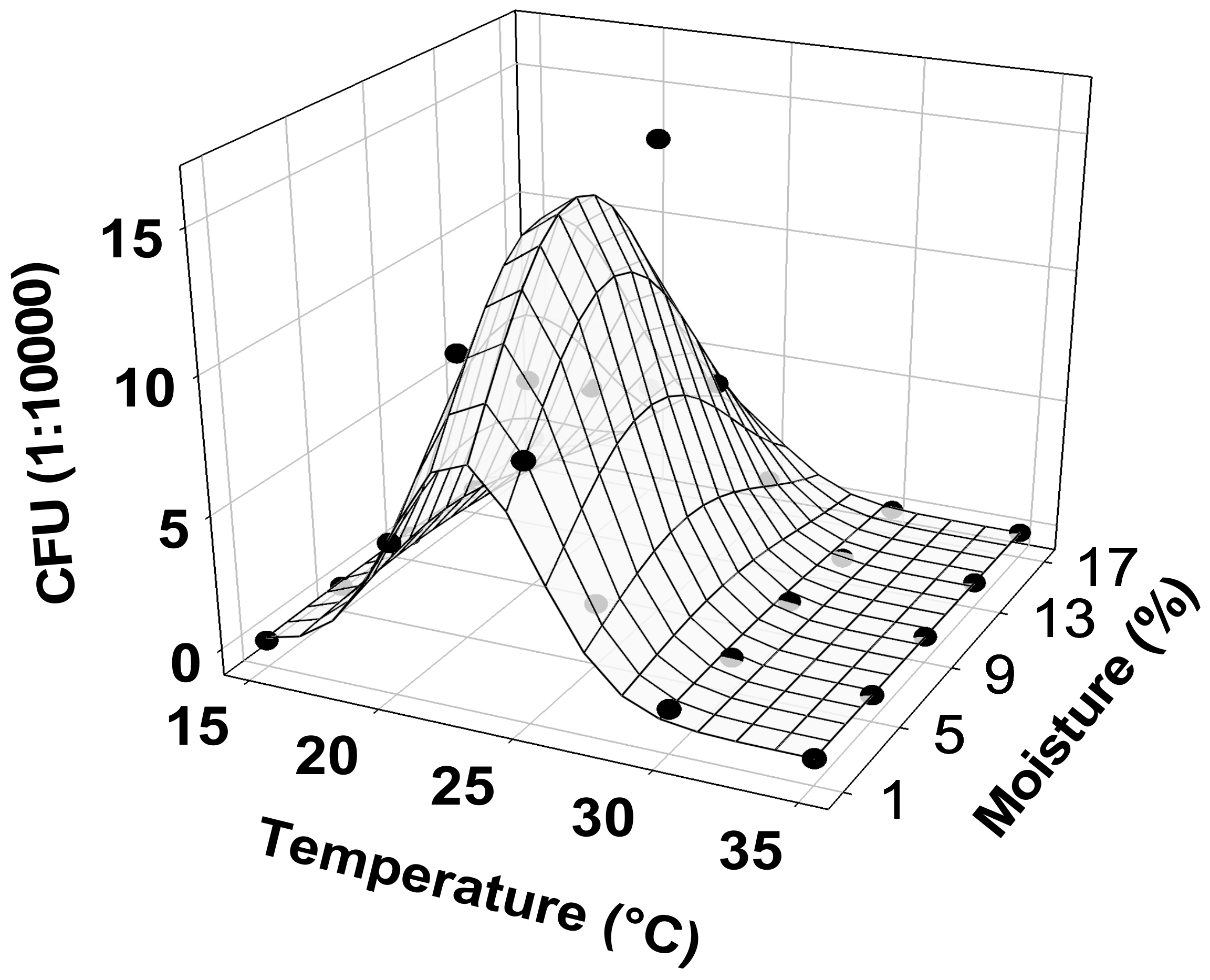
3.5. Effects of Temperature and Moisture on Microsclerotia Germination and Colony Yield of M. brunneum Strain EAMa 01/58-Su
3.6. Effects of Ultraviolet Radiation (UV-B) on Microsclerotia Germination and CFU Yield of M. brunneum Strain EAMa 01/58-Su
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, M.M.; Da Rosa, A.S.; Dal’Boit, S.; Mitchell, D.A.; Krieger, N. Thermal denaturation: Is solid-state fermentation really a good technology for the production of enzymes? Bioresour. Technol. 2004, 93, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T.; Jackson, M.A. Mass production of entomopathogenic Hypocreales. In Manual of Tech-Niques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 255–284. [Google Scholar]
- Jaronski, S.T.; Mascarin, G.M. Mass production of fungal entomopathogens. In Microbial Control of Insect and Mite Pests: From Theory to Practice, 1st ed.; Lacey, L.A., Ed.; Elsevier, Inc.: Sidney, MT, USA, 2016; pp. 141–155. [Google Scholar]
- Quesada-Moraga, E.; Yousef-Naef, M.; Garrido-Jurado, I. Advances in the use of entomopathogenic fungi as biopesticides in suppressing crop insect pests. In Biopesticides for Sustainable Agriculture; Birch, N.N., Glare, T., Eds.; Burleigh Dodds Science Publishing: Cambrige, UK, 2020; pp. 63–98. [Google Scholar]
- Jaronski, S.T.; Jackson, M.A. Efficacy of Metarhizium anisopliae microsclerotial granules. Biocontrol Sci. Technol. 2008, 18, 849–863. [Google Scholar] [CrossRef]
- Willetts, H. The survival of fungal sclerotia under adverse environmental conditions. Biol. Rev. 1971, 46, 387–407. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Development of pilot-scale fermentation and stabilisation processes for the production of microsclerotia of the entomopathogenic fungus Metarhizium brunneum strain F52. Biocontrol Sci. Technol. 2012, 22, 915–930. [Google Scholar] [CrossRef]
- Huarte-Bonnet, C.; Paixao, F.R.S.; Mascarin, G.M.; Santana, M.; Fernandez, E.K.K.; Pedrini, N.E. The entomopathogenic fungus Beauveria bassiana produces microsclerotia-like pellets mediated by ox-idative stress and peroxisome biogenesis. Environ. Microbiol. Rep. 2019, 11, 518–524. [Google Scholar] [CrossRef]
- Rivas-Franco, F.; Hampton, J.G.; Altier, N.A.; Swaminathan, J.; Rostás, M.; Wessman, P.; Saville, D.J.; Jackson, T.A.; Jackson, M.A.; Glare, T.R. Production of microsclerotia from entomopathogenic fungi and use in maize seed coating as delivery for biocontrol against Fusarium graminearum. Front. Sustain. Food Syst. 2020, 4, 606828. [Google Scholar] [CrossRef]
- Villamizar, L.F.; Barrera, G.; Hurst, M.; Destello, Y.R. Characterization of a new strain of Metarhizium novozealandicum with potencial to be developed as a biopesticide. Mycology 2021, 12, 261–278. [Google Scholar] [CrossRef]
- Marciano, A.F.; Mascarin, G.M.; Franco, R.F.F.; Golo, P.S.; Jaronski, S.T.; Fernandes, É.K.K.; Bittencourt, V.R.E.P. Innovative granular formulation of Metarhizium robertsii microsclerotia and blastospores for cattle tick control. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Shearer, J.F.; Jackson, M.A. Liquid Culturing of Microsclerotia of Mycoleptodiscus terrestris a Potential Biological Control Agent for the Management of Hydrilla. Biol. Control. 2006, 38, 298–306. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Kobori, N.N.; de Jesus Vital, R.C.; Jackson, M.A.; Quintela, E.D. Production of mi-crosclerotia by Brazilian strains of Metarhizium spp. Using submerged liquid culture fermentation. World J. Microbiol. Biotechnol. 2014, 30, 1583–1590. [Google Scholar] [CrossRef]
- Jackson, M.A.; Dunlap, C.A.; Jaronski, S.T. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl. 2010, 55, 129–145. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Zárate, C.; Cotes, A.; Villamizar, L. Estudio de la estabilidad en almacenamiento de tres formulaciones oleosas a base de Nomuraea rileyi. In Proceedings of the XXXIII Congreso Nacional de Control Biológico, Ciudad de México, Mexico, 7–12 November 2010. [Google Scholar]
- Kim, J.S.; Je, Y.H.; Woo, E.O.; Park, J.S. Persistence of Isaria fumosorosea (Hypocreales: Cordycipitaceae) SFP-198 conidia in corn oil-based suspension. Mycopathologia 2011, 171, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Behle, R.W.; Jackson, M.A. Effect of fermentation media on the production, efficacy and storage stability of Metarhizium brunneum microsclerotia formulated as a prototype granule. J. Econ. Entomol. 2014, 107, 582–590. [Google Scholar] [CrossRef]
- Lacey, L.; Grzywacz, D.; Shapiro-Ilan, D.; Frutos, R.; Brownbridge, M.; Goettel, M. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar]
- Santos, A.M.; De Brito-Brandão, P.F.; Rivero, L.F.V. Efecto del estrés térmico y la radiación ultravioleta durante la producción masiva de Nomuraea rileyi. Rev. Colomb. Biotecnol. 2017, 19, 82–91. [Google Scholar]
- Garrido-Jurado, I.; Torrent, J.; Barrón, A.; Corpas, A.; Quesada-Moraga, E. Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol. Control. 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Córtes, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef]
- Frac, M.; Hannula, S.E.; Belka, M.; Jedryczka, M. Fungal Biodiversity and their role in soil health. Front. Micobiol. 2018, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- De Crecy, E.; Jaronski, S.; Lyons, B.; Lyons, T.J.; Keyhani, N.O. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotechnol. 2009, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Jurado, I.; Valverde-García, P.; Quesada-Moraga, E. Use of a multiple logistic regression model to determine the effects of soil moisture and temperature on the virulence of entomopathogenic fungi against pre-imaginal Mediterranean fruit fly Ceratitis capitata. Biol. Control. 2011, 59, 366–372. [Google Scholar] [CrossRef]
- Smith, M.E.; Henkel, T.W.; Rollins, J.A. How many fungi make sclerotia? Fungal Ecol. 2015, 13, 211–220. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Production of microsclerotia of the fungal entomopathogen Metarhizium anisopliae and their potential for use as a biocontrol agent for soil-inhabiting insects. Mycol. Res. 2009, 113, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Jurado, I.; Resquín-Romero, G.; Yousef-Naef, M.; Ríos-Moreno, A.; Quesada-Moraga, E. Soil drenching with entomopathogenic fungi for control of the soil-dwelling life stages and adults of the same generation of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2020, 110, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Resquín-Romero, G.; Garrido-Jurado, I.; Quesada-Moraga, E. Combined use of entomopathogenic fungi and their extracts for the control of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Biol. Control. 2016, 92, 101–110. [Google Scholar] [CrossRef]
- Yousef, M.; Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E. Biocontrol of Bactrocera oleae (Diptera: Tephritidae) with Metarhizium brunneum and its extracts. J. Econ. Entomol. 2013, 106, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Yousef, M.; Garrido-Jurado, I.; Quesada-Moraga, E. One Metarhizium brunneum strain, two uses to control Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2014, 107, 1736–1744. [Google Scholar] [CrossRef]
- Yousef, M.; Garrido-Jurado, I.; Ruíz-Torres, M.; Quesada-Moraga, E. Reduction of adult olive fruit fly populations by targeting preimaginals in the soil with the entomopathogenic fungus Metarhizium brunneum. J. Pest Sci. 2017, 90, 345–354. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Composition of Entomopathogenic Fungus and Method for Production and Application for Insect Control. Pat. Appl. 2008, 11, 547–901. [Google Scholar]
- Jackson, M.A.; Payne, A.R. Liquid Culture Production of Fungal Microsclerotia. In Microbial-Based Biopesticides. Methods and Protocols; Glare, T.R., Moran-Diez, M.E., Eds.; Humana Press: New York, NY, USA, 2016; pp. 71–91. [Google Scholar]
- Fernández-Bravo, M.; Flores-León, A.; Calero-López, S.; Gutiérrez-Sánchez, F.; Valverde-García, P.; Quesada-Moraga, E. UV-B radiation-related effects on conidial inactivation and virulence against Ceratitis capitata (Wiedemann) (Diptera; Tephritidae) of phylloplane and soil Metarhizium sp. strains. J. Invertebr. Pathol. 2017, 148, 142–151. [Google Scholar] [CrossRef]
- Stroup, W.W. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications, eBook; CRC Press: New York, NY, USA, 2013; p. 135. [Google Scholar]
- Yousef, M.; Alba-Ramírez, C.; Garrido-Jurado, I.; Mateu, J.; Raya-Díaz, S.; Valverde-García, P.; Quesada Moraga, E. Metarhizium brunneum (Ascomycota; Hypocreales) treatments targeting olive fly in the soil for sustainable crop production. Front. Plant Sci. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzman, D.; Pliester, J.; Leyer, I.; Entling, M.H.; Reineke, A. Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol. 2019, 133, 89–97. [Google Scholar] [CrossRef]
- Song, Z.; Yin, Y.; Jiamg, S.; Liu, J.; Wang, Z. Optimization of culture médium for microsclerotia production by Nomuraea rileyi and análisis of their viability for use as a mycoinsecticide. BioControl 2014, 59, 597–605. [Google Scholar] [CrossRef]
- de Lira, A.C.; Mascarin, G.M.; Júnior, Í.D. Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating. Fungal Biol. 2020, 124, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Lopes Catặo, A.M.; Soares dos Santos, A.; Santos Paixặo, F.R.; Rodrigues Santos, T.; Mercado Martinez, J.; Neves Marreto, R.; Moura Mascarin, G.; Kort Kamp Fernandes, E.; Humber, R.A.; et al. Relative humidity impacts development and activity against Aedes aegypti adults by granular formulations of Metarhizium humberi microsclerotia. Appl. Microbiol. Biotechnol. 2021, 105, 2725–2736. [Google Scholar] [CrossRef]
- Wu, S.; Kostromytska, O.S.; Goble, T.; Hajek, A.E.; Koppenhöfer, A.M. Compatibility of a micro-sclerotial granular formulation of the entomopathogenic fungus Metarhizium brunneum with fungicides. BioControl 2020, 65, 113–123. [Google Scholar] [CrossRef]
- Santos, T.R.; da Paixão, F.R.S.; Catão, A.M.L.; Muniz, E.R.; Ribeiro-Silva, C.S.; Taveira, S.F.; Luz, C.; Mascarin, G.M.; Fernandes, E.K.K.; Marreto, R.N. Inorganic pellets containing microsclerotia of Metarhizium anisopliae: A new technological platform for the biological control of the cattle tick Rhipicephalus microplus. Appl. Microbiol. Biotechnol. 2021, 105, 5001–5012. [Google Scholar] [CrossRef]
- García-Férnandez, P.; Santiago-Álvarez, C.; Quesada-Moraga, E. Pathogenicity and thermal biology of mitosporic fungi as potencial microbial control agents of Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of honey bee, Apis mellifera (Hymenoptera: Apidae). Apidologie 2008, 39, 662–673. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Amprong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Iskandarov, U.S.; Guzalova, A.G.; Davranov, K.D. Effects of nutrient medium composition and temperature on the germination of conidia and the entomopathogenic activity of the fungi. Prikl. Biokhim. Appl. Biochem. Microbiol. 2006, 42, 81–85. [Google Scholar] [CrossRef]
- Thaochan, N.; Benarlee, R.; Prabhakar, C.S.; Hu, Q.B. Impact of temperature and relative humidity on effectiveness of Metarhizium guizhouense PSUM02 against longkong bark eating caterpillar Cossus chloratus Swinhoe under laboratory and field conditions. J.-Asia Pac. Entomol. 2020, 23, 285–290. [Google Scholar] [CrossRef]
- Zaman, S.; ul Hasan, M. Pathogenicity of Entomopathogenic fungi against Sitophilus granarius (L.) (Coleoptera: Curculionidae) under abiotic factors. Pak. J. Agric. Sci. 2020, 57, 79–86. [Google Scholar]
- Tumuhaise, V.; Ekesi, S.; Manainaia, N.K.; Tonnang, H.E.Z.; Tanga, C.M.; Ndegwa, P.N.; Irungu, L.W.; Srinivasan, R.; Mohamed, S.A. Temperature-dependent growth virulence, and mass production po-tential of two candidate isolates of Metarhizium anisopliae (Metschnikoff) Sorokin for managing Maruca vitrata Fabricius (Lepidoptera: Crambidae) on cowpea. Afr. Entomol. 2018, 26, 73–83. [Google Scholar] [CrossRef]
- Lanza, L.M.; Monteiro, A.C.; Malheiros, E.B. Metarhizium anisopliae population in differents soil types and compactness degrees. Ciênc. Rural. 2004, 34, 1757–1762. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, G.S.; Azevedo, J.L. Efeito do solo na germinação de conídios de Metarhizium anisopliae (Metsch.) Sorokin. O Solo 1986, 78, 39–41. [Google Scholar]
- Eilenberg, J.; Hokkanen, H.M.T. An Ecological and Societal Approach to Biological Control, 1st ed.; Springer: New York, NY, USA, 2006; p. 312. [Google Scholar]
- Jaronski, S.T. Soil ecology of the entomopathogenic Ascomycetes: A critical examination of what we (think) we know. In Use of Entomopathogenic Fungi in Biological Pest Management; Maniana, K., Ekesi, S., Eds.; Research SignPosts: Trivandrum, India, 2007; pp. 91–144. [Google Scholar]
- Song, Z.; Shein, L.; Zhong, Q.; Yin, Y.; Wang, Z. Liquid culture production of microsclerotia of Purpureocillium lilacinum for use as bionematicide. Nematology 2016, 18, 719–726. [Google Scholar] [CrossRef]
- Paixão, F.R.S.; Huarte-Bonnet, C.; Ribeiro-Silva, C.S.; Mascarin, G.M.; Fernandes, É.K.K.; Pedrini, N. Tolerance to abiotic factors of microsclerotia and mycelial pellets from Metarhizium robertsii, and molecular and ultrastructural changes during microsclerotial differentiation. Front. Fungal Biol. 2021, 2, 654737. [Google Scholar] [CrossRef]


| Strains * | Fungal Species | Location | Ecosystem of Isolation |
|---|---|---|---|
| EAMa 01/158-Su (CECT 20987) | M. robertsii | Utrera (Seville) | Olive orchard |
| EAMb 09/01-Su (CECT 20784) | M. brunneum | Castilblanco de los Arroyos (Seville) | Holm oak dehesa |
| EAMa 01/58-Su (CECT 20764) | M. brunneum | Hinojosa del Duque (Cordoba) | Wheat |
| Soil Reference | Geographical Location | Soil Type | Soil Composition | ||||
|---|---|---|---|---|---|---|---|
| Locality | Province | Sand (g/Kg) | Silt (g/Kg) | Clay (g/kg) | Textural Class | ||
| AG4 | Córdoba | Córdoba | Inceptisol | 224 | 318 | 458 | Clay |
| AG6 | Obejo | Córdoba | Entisol | 660 | 230 | 110 | Sandy loam |
| AG20 | Luque | Córdoba | Inceptisol | 405 | 245 | 350 | Clay loam |
| AG35 | Pozoblanco | Córdoba | Alfisol | 860 | 90 | 50 | Sandy |
| AG39 | Fuente Obejuna | Córdoba | Alfisol | 630 | 110 | 260 | Sandy clay loam |
| Factor | Parameter | Estimate | Std. Error | Likelihood Ratio χ2 (df = 1) | Probability |
|---|---|---|---|---|---|
| Intercept | β0 | −35.3 | 9.1 | 56.9 | <0.0001 |
| Temperature | β1 | 3.3 | 0.8 | 66.6 | <0.0001 |
| Moisture | β2 | 0.2 | 0.1 | 4.3 | 0.0389 |
| Temperarture2 | β4 | −0.1 | 0 | 70.9 | <0.0001 |
| Moisture2 | β5 | 0 | 0 | 6.5 | 0.011 |
| Treatment (h) | CFU ± SE (×107) |
|---|---|
| UV-B 0 | 9.3 ± 5.4 a |
| UV-B 4 | 91.0 ± 59.0 a |
| UV-B 8 | 6.0 ± 2.0 a |
| UV-B 24 | 17.0 ± 5.4 a |
| UV-B 48 | 28.0 ± 9.6 a |
| UV-B 72 | 41.0 ± 29.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef-Yousef, M.; Romero-Conde, A.; Quesada-Moraga, E.; Garrido-Jurado, I. Production of Microsclerotia by Metarhizium sp., and Factors Affecting Their Survival, Germination, and Conidial Yield. J. Fungi 2022, 8, 402. https://doi.org/10.3390/jof8040402
Yousef-Yousef M, Romero-Conde A, Quesada-Moraga E, Garrido-Jurado I. Production of Microsclerotia by Metarhizium sp., and Factors Affecting Their Survival, Germination, and Conidial Yield. Journal of Fungi. 2022; 8(4):402. https://doi.org/10.3390/jof8040402
Chicago/Turabian StyleYousef-Yousef, Meelad, Antonia Romero-Conde, Enrique Quesada-Moraga, and Inmaculada Garrido-Jurado. 2022. "Production of Microsclerotia by Metarhizium sp., and Factors Affecting Their Survival, Germination, and Conidial Yield" Journal of Fungi 8, no. 4: 402. https://doi.org/10.3390/jof8040402






