The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review
Abstract
:1. Introduction
2. Composition of DF from Mushrooms
3. Methods of the Preparation and Modification of DFs from Mushrooms
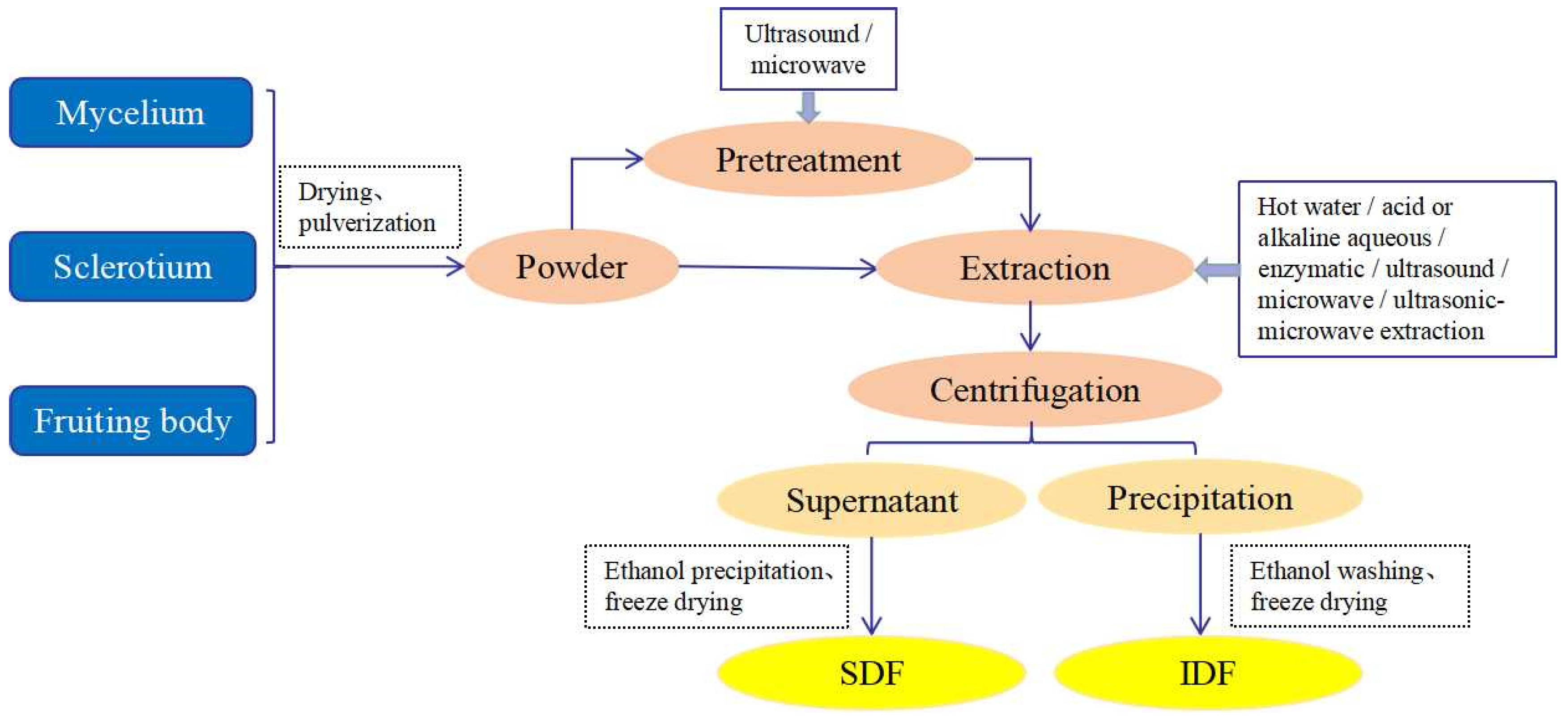
3.1. The Preparation of DFs from Mushrooms
3.2. Methods of Modification of DF from Mushrooms
3.2.1. Physical Modification
3.2.2. Chemical Modification
3.2.3. Biological Modification
3.2.4. Combination Modification
| Modification Methods | Material | DFs | Modification Conditions | Property Changes | Reference | |
|---|---|---|---|---|---|---|
| Physical modification method | High-pressure homogenization | Flammulina velutiper | IDF | 0, 10, 30, and 50 cycles at 700 bar | WHC ↑, interfacial properties ↑, particle size ↓, emulsification Performance ↑ | [61] |
| Extrusion | Lentinula edodes residues | DF | 130 °C, moisture content 40%, 125 r/min | SDF ↑, OHC ↑, GAC ↑, glucose retardation and bile acid retardation index ↑ | [64] | |
| High-temperature cooking | Flammulina velutiper | DF | Liquid-to-material ratio 30:1, 125 °C, 50 min | SDF ↑, improves the physiological indices in obese mice | [74] | |
| High-pressure processing | Agrocybe chaxingu | DF | 400 MPa, 25 °C, 15 min | SDF ↑, polysaccharide solubility ↑, lower viscosity and greater fluidity | [75] | |
| Chemical modification method | Alkaline | Lentinus edodes stem | DF | 13% NaOH, 80% ethanol, alkalization 120 min; 10% C2H2ClNaO2, 50 °C, etherification 3.5 h | WHC ↑, SC ↓, OHC ↑ | [76] |
| Biological modification method | Enzymatic | Lentinus edodes | DF | 1.5% cellulase, solid–liquid ratio 1:25, 50 °C, pH 5.5, 120 min | SC ↑, WHC ↑, OHC ↑, cation exchange capacity ↑, GAC ↑ | [71] |
| Fermentation | Lentinus edodes stem | IDFSDF | Material-liquid 1:10 g/mL, 6% Aspergillus niger, 28 °C, 2 d | IDF: WHC ↑, OHC ↑, SC ↑; SDF: WHC ↑, OHC ↑, SC ↑ | [72] | |
| Combined modification method | Enzymatic-chemical | Auricularia polytricha | DF | 0.4% α-amylase 1.0% protamex, 66 °C, liquid material ratio 41 mL/g | SC ↑, WHC ↑, FAC ↑, GAC ↑, high constipation-relieving activity | [77] |
| Ultrasound-microwave-assisted enzymatic method | Hericium Erinaceus residue | DF | 3% celluloses, ultrasound (1.5 W/mL), 50 °C, 75 min, boiled to stop the enzyme | SDF ↑, particle size ↓, adsorption capacity ↑, better blood lipid-lowering effect in vitro | [78] | |
4. Interaction between DFs and the Gut Microbiota
4.1. The Role of the Gut Microbiota in DF Metabolism
4.2. Effect of DFs on the Composition of the Gut Microbiota
| DF Source | Model | Gut Microbiota Regulation | SCFA Generation | Effect on Host | Reference |
|---|---|---|---|---|---|
| Pleurotus eryngii | HFD-induced obese rat | The relative abundances of Roseburia and Lactobacillus ↓, the relative abundances of Anaerostipes, Clostridium and Lactococcus ↑. | Increased the concentrations of total SCFAs. | Reduced BW gain, adipose tissue weight, FBG level; the expression of FASN and ACC. | [116] |
| Pleurotus eryngii | HFD-fed mice | The relative abundances of Methylobacterium and Lactobacillus ↑, the relative abundances of unidentified_Lachnospiraceae and Helicobacter ↓. | Increased the content of SCFAs, including acetic acid, propionic acid, and butyric acid. | Decreased the weight, promoted the proliferation of beneficial bacteria, reduced the risks of many chronic diseases. | [53] |
| Agaricus blazei Murrill | Hyperlipidemia rats | The ratio of Firmicutes/Bacteroidetes ↓; the abundance of Peptostreptococcaceae, Erysipelaceae, and Clostridium ↑. | Nm | Regulated dyslipidemia in rats with hyperlipidemia possibly by regulating imbalance in the intestinal microflora. | [117] |
| Hericium caput-medusae | One-day-old Arbor Acres male broilers | The count of Lactobacilli and Bifidobacteria ↑, the count of acecum Escherichia coli ↓. | Increased the concentration of propionic acid. | Decreased cholesterol content in broiler chickens. | [118] |
| Flammulina velutipes | Male C57BL/6 J mice | The relative abundance of some beneficial bacteria ↑, such as Akkermansia and Prevotellaceae UCG-001; the relative abundance of some harmful bacteria ↓, such as Lachnospiraceae NK4A136 group and Desulfovibrio. | Nm | Reduced the weight gain, triglycerides and total cholesterol, low-density lipoprotein cholesterol; increased the activity of enzymes related to scavenging ability of oxygen free radicals. | [119] |
| Flammulina velutipes | Mice | The relative abundance of Firmicutes ↓, the relative abundance of Bacteroidetes ↑; the ratio of Firmicutes/Bacteroidetes ↓. | Increased the concentrations of total SCFAs, acetic acid, propionic acid, and n-butyric acid. | Suppressed obesity and immune regulation. | [101] |
| Ganoderma lucidum | C57BL/6NCrlBltw genetic lineage mice | The ratio of Firmicutes/Bacteroidetes, Proteobacteria ↓. | Nm | Reduced body weight gain, chronic inflammation, and insulin resistance in obese individuals. | [120] |
| Poria cocos | C57BL/6J mice | The relative abundance of Lachnospiracea, Clostridium ↑. | Increased butyrate levels. | Activated the intestinal PPAR-γ pathway, modulated gut microbiota to improve hyperglycemia and hyperlipidemia. | [121] |
| Agaricus bisporus | Human | The relative abundance of Firmicutes ↑, the relative abundance of Bacteroidetes ↓. | Increased the concentrations of acetic acid and propionic acid. | Increased the relative abundance of beneficial bacteria, exhibited an effective prebiotic regulation function on human gut microbiota. | [105] |
| Cordyceps militaris | Liver and kidney injury induced by lead acetate in mice | The relative abundance of Clostridium and Bacteroidetes ↑, the relative abundance of Firmicutes ↓. | Nm | Reduced the Pb2+ content and organ index of liver and kidney in mice, had a protective effect on organs against damage in mice. | [122] |
| Pleurotus eryngii | C57BL/6 male mice | The relative abundances of Firmicutes ↓, Bacteroidetes ↑ | Increased the concentrations of Acetate and Propionate. | Regulated the host immune function effectively. | [123] |
| Ganoderma lucidum | Chronic pancreatitis mice | The relative abundance of Bacteroidetes ↓ and that of Firmicutes ↑; at the genus level, the relative abundance of beneficial bacteria such as Lactobacillus, Roseburia, and Lachnospira ↑. | Nm | Indicated beneficial effects on pancreas fibrosis, and impeded an inflammatory response. | [124] |
| Dictyophora indusiata | Antibiotic-induced intestinal microflora disorder in mice | Beneficial bacteria ↑, including Lactobacilli and Ruminococcaceae; harmful bacteria ↓, such as Enterococcus, Bacteroides, and Proteobacteria. | Nm | Enhanced the restoration of gut microbiota and gut barrier integrity, reduce the inflammation and endotoxin levels in mice. | [125] |
| Coprinus comatus | Human | The relative abundances of Bacteroides and Bifidobacterium ↑, the ratio of Firmicutes/Bacteroidetes ↓. | Increased the production of propionic acid and butyric acid. | Demonstrated potential prebiotic effects. | [50] |
| Ganoderma lucidum | C57BL/6J mice | The relative abundances of Actinobacteria at the family level, and Leuconostoc, Lactobacillus spp. ↑. | Nm | Improved low-grade chronic inflammation, ectopic lipid accumulation, and systemic insulin sensitivity. | [126] |
| Hericium erinaceus | Mice | The relative abundance of Lachnospiraceae and Akkermansiaceae ↑, the relative abundance of Rikenellaceae and Bacteroidaceae ↓. | Nm | Promoted the production of NO, IL-6, IL-10, INF-γ, and TNF-α. | [127] |
| Auricularia auricular | ICR mice | The ratio of Firmicutes/Bacteroidetes ↓, the relative abundance of Porphyromoadaceae and Bacteroidaceae ↑. | Increased the concentration of total SCFAs and propanoic acid. | Increased microbial community diversity, and increased the immunoglobulin levels in mouse serum. | [128] |
| Ganoderma lucidum | DSS-induced colitis male Wistar rats | The relative abundance of Firmicutes, Paraprevotella, etc. ↑, the relative abundance of Proteobacteria, Escherichia, etc. ↓. | Increased total SCFAs, acetic acid, propionic acid, and butyric acid. | Enhanced the immunity and reduced inflammatory response and colonic cancer risk. | [129] |
| Ganoderma lucidum | BALB/C mice | The ratio of Firmicutes/Bacteroidetes ↓, the relative abundance of Alistipes ↑. | Nm | Demonstrated tumor-suppressing activity in mice. | [130] |
| Ganoderma lucidum | BALB/c mice | The relative abundance of Oscillospira and unknown genus of Desulfovibrionaceae ↓. | Nm | Prevented colon from shortening and reduced mortality by 30% of mortality in CRC mice. | [131] |
4.3. Effect of DFs on SCFA Production
5. Health Benefits of DFs
5.1. Improving Metabolic Syndromes
5.2. Immunomodulatory Effects
5.3. Antitumor Effects
5.4. Other Beneficial Effects
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Jansma, J.; El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 2021, 9, 16. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.B.; Mayne, J.; Deeke, S.A.; Li, J.; Starr, A.E.; Chen, R.; Singleton, R.; Butcher, J.; Mack, D.R.; et al. In vitro metabolic labeling of intestinal microbiota for quantitative metaproteomics. Anal. Chem. 2016, 88, 6120–6125. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Crow, J.M. Microbiome: That healthy gut feeling. Nature 2011, 480, S88–S89. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Xu, X.F.; Xu, P.P.; Ma, C.W.; Tang, J.; Zhang, X.W. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Petterson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Zhang, H.S.; Sparks, J.B.; Karyala, S.V.; Settlage, R.; Luo, X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015, 9, 770–781. [Google Scholar] [CrossRef]
- Caitriona, M.G.; Paul, D.C. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar]
- Li, D.T.; Wang, P.; Wang, P.P.; Hu, X.S.; Chen, F. Targeting the gut microbiota by dietary nutrients: A new avenue for human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 181–195. [Google Scholar] [CrossRef]
- Collins, S.M.; Denou, E.; Verdu, E.F.; Bercik, P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig. Liver Dis. 2009, 41, 850–853. [Google Scholar] [CrossRef]
- Wang, X.S.; Chen, D.; Li, Y.L.; Zhao, S.L.; Chen, C.Y.; Ning, D.L. Alleviating effects of walnut green husk extract on disorders of lipid levels and gut bacteria flora in high fat diet-induced obesity rats. J. Funct. Foods 2019, 52, 576–586. [Google Scholar] [CrossRef]
- Wang, P.P.; Wang, T.; Zheng, X.J.; Cui, W.; Shang, J.; Zhao, Z.Z. Gut microbiota, key to unlocking the door of diabetic kidney disease. Nephrology 2021, 26, 641–649. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.P.; Yi, X.Y.; Duan, Y.C.; Wang, J.F.; Li, S.S.; Luo, L.L.; Huang, T.Z.; Lnglis, B.; Li, X.; et al. Histopathological features and composition of gut microbiota in rhesus monkey of alcoholic liver disease. Front. Microbiol. 2019, 10, 165. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Issa, M.; Binion, D.G. Clostridium difficile and inflammatory bowel disease. Med. Clin. N. Am. 2010, 94, 135–153. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vetizou, M.; Daillere, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, 271ps1. [Google Scholar] [CrossRef]
- Kotowski, M.A. History of mushroom consumption and its impact on traditional view on mycobiota-an example from Poland. Microb. Biosyst. 2019, 4, 1–13. [Google Scholar]
- Balan, V.; Munafo Jr, J.P.; Pattathil, S.; Merritt, B.B.; Venketachalam, S.; Ng, W. Protocols to evaluate the nutritional and potential health benefits of edible mushrooms. Curr. Biotechnol. 2018, 7, 34–58. [Google Scholar] [CrossRef]
- Wani, B.A.; Bodha, R.H.; Wani, A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Xu, Z.H.; Ding, Z.Y. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO. Diet, Nutrition and the Preparation of Chronic Disease. In Proceedings of the WHO Technical Report Series 916, Geneva, Switzerland, 28 January–1 February 2002. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Dietary fibers from mushroom sclerotia: 1. preparation and physicochemical and functional properties. J. Agric. Food Chem. 2005, 53, 9395–9400. [Google Scholar] [CrossRef]
- Cheung, P.C.K.; Lee, M.Y. Fractionation and characterization of mushroom dietary fiber (Nonstarch polysaccharides) as potential nutraceuticals frorm sclerotia of Pleurotus tuber-regium (Fries) singer. J. Agric. Food Chem. 2000, 48, 3148–3151. [Google Scholar] [CrossRef]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; Della Rocca, G.; Molinari, M.L.; Calabrò, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef]
- Ren, Y.L.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Lu, J.H.; He, R.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Kumari, K. Mushrooms as source of dietary fiber and its medicinal value: A review article. J. Pharmacogn. Phytochem. 2020, 9, 2075–2078. [Google Scholar]
- Cummings, J.H.; Mann, J.I.; Nishida, C.; Vorster, H.H. Dietary fiber: An agreed definition. Lancet 2009, 373, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Shea, N.O.; Arendt, E.K.; Gallagher, E. Dietary fiber and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fiber and fiber-rich by-products of food processing: Characterisation, technological functionality and commerical application: A review. Food Chem. 2010, 124, 411–421. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Yang, W.J.; Pei, F.; Zhao, L.Y.; Hu, Q.H. In vitro fermentation of six kinds of edible mushrooms and its effects on fecal microbiota composition. LWT—Food Sci. Technol. 2018, 96, 627–635. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Mullins, J.T. Regulatory mechanism of β-glucan synthetases in bacteria, fungi and plants. Physiol. Plant. 1990, 78, 309–314. [Google Scholar] [CrossRef]
- Manzi, P.; Pizzoferrato, L. Bata-glucans in edible mushrooms. Food Chem. 2000, 68, 315–318. [Google Scholar] [CrossRef]
- Guo, X.; Meng, H.; Zhu, S.; Tang, Q.; Pan, R.; Yu, S. Stepwise ethanolic precipitation of sugar beet pectins from the acidic extract. Carbohyd. Polym. 2016, 136, 316–321. [Google Scholar] [CrossRef]
- Gong, X.; Wang, S.; Li, Y.; Qu, H. Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep. Purif. Technol. 2013, 107, 273–280. [Google Scholar] [CrossRef]
- Sen, D.; Gosling, A.; Stevens, G.W.; Bhattacharya, P.K.; Barber, A.R.; Kentish, S.E.; Bhattacharjee, C.; Gras, S.L. Galactosyl oligosaccharide purification by ethanol precipitation. Food Chem. 2011, 128, 773–777. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Xu, Y.; Zhu, B.; Piao, J.; Zhu, L.; Yao, L.; Liu, K.; Wang, S.; Zhang, Q.; et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu. Fruits. J. Funct. Foods 2022, 93, 105081. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.N.; Zhou, X.J.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT-Food Sci. Technol. 2021, 145, 111313. [Google Scholar] [CrossRef]
- Sakdasri, W.; Arnutpongchai, P.; Phonsavat, S.; Bumrungthaichaichan, E.; Sawangkeaw, R. Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT-Food Sci. Technol. 2022, 168, 113895. [Google Scholar] [CrossRef]
- Aguilo-Aguayo, I.; Walton, J.; Vinas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT-Food Sci. Technol. 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dong, F.Y.; Liu, X.C.; Lv, Q.; Yang, Y.; Liu, F.; Chen, L.; Wang, T.T.; Wang, Z.; Zhang, Y.M. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohyd. Polym. 2016, 140, 461–471. [Google Scholar]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Qu, H.; Zhou, H.; Yang, H.; Chen, H. Physicochemical characterization, adsorption function and prebiotic effect of chitin-glucan complex from mushroom Coprinus comatus. Int. J. Biol. Macromol. 2022, 206, 255–263. [Google Scholar] [CrossRef]
- Chou, W.T.; Sheih, I.C.; Fang, T.J. The applications of polysaccharides from various mushroom wastes as prebiotics in different systems. J. Food Sci. 2013, 78, M1041–M1048. [Google Scholar] [CrossRef]
- Moreno, R.B.; Ruthes, A.C.; Baggio, C.H.; Vilaplana, F.; Komura, D.L.; Iacomini, M. Structure and antinociceptive effects of β-D-glucans from Cookeina tricholoma. Carbohyd. Polym. 2016, 141, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, D.; Zhang, S.; Liu, X.; Zhao, Y.; Song, C.; Sun, Q. Characterization of insoluble dietary fiber from Pleurotus eryngii and evaluation of its effects on obesity-preventing or relieving effects via modulation of gut microbiota. J. Future Foods 2023, 3, 55–66. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Nutritional value and health benefifits of mushrooms. In Mushrooms as Functional Foods; Cheung, P.C.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 2, pp. 71–109. [Google Scholar]
- Liu, C.; Lin, X.L.; Wan, Z.; Zou, Y.; Cheng, F.F.; Yang, X.Q. The physicochemical properties, in vitro binding capacities and in vivo hypocholesterolemic activity of soluble dietary fiber extracted from soy hulls. Food Funct. 2016, 7, 4830–4840. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, M.A.; Khan, A. Effect of water-soluble gummy fiber, water-insoluble neutral detergent fiber isolated from Syzygium cumini seeds on biliary and fecal bile acids and sterols in rats fed a high cholesterol diet. Int. J. Med. Sci. Public Health 2015, 4, 23–26. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.E.H.; Weickert, M.O. The health benefits of dietary fiber. Nutrients 2020, 12, 3029. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Li, Y.; Li, B.; Liu, S. Water-insoluble dietary-fibers from Flammulina velutiper used as edible stabilizers for oil-in-water Pickering emulsions. Food Hydrocolloid 2020, 101, 105519. [Google Scholar] [CrossRef]
- Wang, H.; Huang, T.; Tu, Z.C.; Ruan, C.Y.; Lin, D. The adsorption of lead(II) ions by dynamic high pressure micro-fluidization treated insoluble soybean dietary fiber. J. Food Sci. Technol. 2016, 53, 2532–2539. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Ma, Q.; Guo, Q.; Santhanam, R.K.; Gao, X.; Chen, Z.; Wang, C.; Chen, H. Physicochemical and functional properties of extruded dietary fiber from mushroom Lentinula edodes residues. Food Biosci. 2019, 32, 100452. [Google Scholar] [CrossRef]
- Huang, L.; Ding, X.; Zhao, Y.; Li, Y.; Ma, H. Modification of insoluble dietary fiber from garlic straw with ultrasonic treatment. J. Food Process. Preserv. 2019, 42, e13399. [Google Scholar] [CrossRef]
- Wei, E.; Yang, R.; Zhao, H.; Wang, P.; Zhao, S.; Zhai, W.; Zhang, Y.; Zhou, H. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019, 123, 280–290. [Google Scholar] [CrossRef]
- Wu, C.; Teng, F.; McClements, D.J.; Zhang, S.; Li, Y.; Wang, Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020, 134, 109251. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, B.Y.; Prudencio, S.H. Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem. 2020, 323, 126776. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liao, A.M.; Thakur, K.; Huang, J.H.; Zhang, J.G.; Wei, Z.J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Tian, H. Effects of carboxymethylation, acidic treatment, hydroxypropylation and heating combined with enzymatic hydrolysis on structural and physicochemical properties of palm kernel expeller dietary fiber. LWT-Food Sci. Technol. 2020, 133, 109909. [Google Scholar] [CrossRef]
- Wang, Z.D. Study on Extraction and Enzymatic Modification of Lentinus edodes Dietary Fiber. Master’s Thesis, Liaoning University, Shenyang, China, 2018. [Google Scholar]
- Pei, Z.W. Study on Properties and Application of Dietary Fiber from Lentinus edodes Stem Modified by Aspergillus niger. Master’s Thesis, Liaoning University, Shenyang, China, 2021. [Google Scholar]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Gong, Z.; Wang, Y.; Cui, W.; Song, S.; Zhang, J.; Jia, F. Modification, physicochemical properties and lipid-lowering and antioxidant activity of dietary fiber from Flammulina velutipes. Food Sci. 2021, 42, 60–98, (In Chinese with English abstract). [Google Scholar]
- Lv, G.; Zhang, Z.; Pan, H.; Fan, L. Effect of physical modifification of mushroom (A. chaxingu) powders on their physical and chemical properties. Food Sci. Technol. Res. 2014, 20, 731–738. [Google Scholar] [CrossRef]
- Wang, C.C. Study on Technologies of Chemical Modification and Properties of Dietary Fiber from Lentinus edodes Stem. Master’s Thesis, Liaoning University, Shenyang, China, 2020. [Google Scholar]
- Jia, F.; Yang, S.; Ma, Y.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction optimization and constipation-relieving activity of dietary fiber from Auricularia polytricha. Food Biosci. 2020, 33, 100506. [Google Scholar] [CrossRef]
- Liu, T.; Wang, N.; Xu, X.; Wang, D. Effect of high quality dietary fiber of Hericium erinaceus on lowering blood lipid in hyperlipidemia mice. J. Future Foods 2022, 2, 61–68. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.M.; Tamura, K.; Dejean, G.; Abbott, D.W.; Brumer, H. Polysaccharide utilization loci: Fueling microbial communities. J. Bacteriol. 2017, 199, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Martens, E.C. Interrogating gut bacterial genomes for discovery of novel carbohydrate degrading enzymes. Curr. Opin. Chem. Biol. 2018, 47, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda, R.H.; Drula, E.; Coutinbo, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- White, B.A.; Lamed, R.; Bayer, E.A.; Flint, H.J. Biomass utilization by gut microbiomes. Annu. Rev. Microbiol. 2014, 68, 279–296. [Google Scholar] [CrossRef]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinbo, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef]
- Biely, P. Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 2012, 30, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, H.; Wu, H. Glycosyltransferase-mediated sweet modification in oral Streptococci. J. Dent. Res. 2015, 94, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Lois, A.S.; Basle, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Gordon, J.I. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef]
- Rivière, A.; Gagnon, M.; Weckx, S.; Roy, D.; De Vuyst, L. Mutual cross-feeding interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides. Appl. Environ. Microb. 2015, 81, 7767–7781. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mraz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between microbes and host intestinal health: Modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Mitsou, E.; Savami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.; Mountzouris, K.; Pletsa, V.; et al. Effects of rich in β-glucans edible mushrooms on aging gut microbiota characteristics: An in vitro study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Yang, J.; Martinez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef]
- Comino, P.; Williams, B.A.; Gidley, M.J. In vitro fermentation gas kinetics and end-products of soluble and insoluble cereal flour dietary fibers are similar. Food Funct. 2018, 9, 898–905. [Google Scholar] [CrossRef]
- Xiang, Q.R.; Li, W.Y.; Feng, T. Regulating effects of dietary fiber and powder of Agaricus bisporus based on in vitro fermentation on human gut microbiota. Sci. Technol. Food Ind. 2022, 44, 130–137. (In Chinese) [Google Scholar]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef]
- Ding, Q.; Nie, S.; Hu, J.; Zong, X.; Li, Q.; Xie, M. In vitro and in vivo gastrointestinal digestion and fermentation of the polysaccharide from Ganoderma atrum. Food Hydrocoll. 2017, 63, 646–655. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microb. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Payling, L.; Fraser, K.; Loveday, S.M.; Sims, I.; Roy, N.; McNabb, W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends Food Sci. Technol. 2020, 97, 233–248. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.; Roy, N.C.; McNabb, W.C. The classification and evolution of bacterial cross-feeding. Front. Ecol. Evol. 2019, 7, 153. [Google Scholar] [CrossRef]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microb. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Falony, G.; Viachou, A.; Verbrugghe, K.; Vuyst, L.D. Cross-feeding between Bifidobacterium longum BB536 and acetate converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microb. 2006, 72, 7835–7841. [Google Scholar] [CrossRef]
- Nakahara, D.; Nan, C.; Mori, K.; Hanayama, M.; Kikuchi, H.; Hirai, S.; Egashira, Y. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020, 59, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Li, X.; Guo, X.; Sheng, Y.; Li, Y.; Xu, G.; Han, X.; An, L.; Du, P. Effects of Agaricus blazei Murrill polysaccharides on hyperlipidemic rats by regulation of intestinal microflora. Food Sci. Nutr. 2020, 8, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.M.; Song, H.; Wang, L.N.; Wu, B.; Ding, G.D.; Jiang, Y.Y.; Yao, X.; Shen, S.J. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. On performance, gut microflora, and cholesterol metabolism in broiler chickens. Livest. Sci. 2014, 167, 276–285. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Song, S.; Zhang, J.; Jia, F. Flammulina velutipes mycorrhizae dietary fiber improves lipid metabolism disorders in obese mice through activating AMPK signaling pathway mediated by gut microbiota. Food Biosci. 2021, 43, 101246. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Yong, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, Z. Using Cordyceps militaris extracellular polysaccharides to prevent Pb2+-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020, 11, 9226–9239. [Google Scholar] [CrossRef]
- Ma, G.; Kimatu, B.M.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef]
- Li, K.; Zhuo, C.; Teng, C.; Yu, S.; Wang, X.; Hu, Y.; Ren, G.; Yu, M.; Qu, J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Ren, X.; Li, M.; Xin, Y. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef]
- Xu, S.; Dou, Y.; Ye, B.; Wu, Q.; Wang, Y.; Hu, M.; Ma, F.; Rong, X.; Guo, J. Ganoderma lucidum polysaccharide insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J. Funct. Foods 2017, 38, 545–552. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohyd. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cheng, N.; Nakata, P.A.; Zhao, L.; Hu, Q. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019, 123, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, Y.; Chen, B.; Zhang, G.; Ou, S.; Luo, J.; Peng, X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019, 63, 1559. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, H.B.; Zhang, Q.W.; Li, Z.P.; Wong, T.L.; Fung, H.Y.; Zhang, J.X.; Bai, S.P.; Lu, A.P.; Han, Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 6172. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Liu, R.; Gao, L.; Ou, S.; Liu, L.; Peng, X. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods 2018, 47, 127–135. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. BBA-Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Hetzel, M.; Brock, M.; Selmer, T.; Pierik, A.J.; Golding, B.T.; Buckel, W. Acryloyl-CoA reductase from Clostridium propionicum: An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur. J. Biochem. 2003, 270, 902–910. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.D.; Flint, H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4449. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microb. 2002, 68, 5186–5190. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Beglinger, C.; Degen, L. Gastrointestinal satiety signals in humans—Physiologic roles for GLP-1 and PYY? Physiol. Behav. 2006, 89, 460–464. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Gunther, C.; Bischoff, S.C. Prebiotic lnulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiotaderived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; AI-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxmia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Nat. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.M.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, Y.; Saito, Y. Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem. Biophys. Res. Commun. 2002, 293, 827–831. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL- 10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yasumura, S.; Atarashi, Y.; Minemura, M.; Miyazaki, T.; Lwamoto, M.; Higuchi, K.; Watanabe, A. Sodium butyrate enhances Fas-mediated apoptosis of human hepatoma cells. J. Hepatol. 2004, 40, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, B.; Gan, Z.; Song, D.; Lu, Z.; Yi, H.; Wu, Y.; Wang, Y.; Du, H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016, 6, 27070. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Serizawa, I.; Araki, Y.; Suzuki, A.; Andoh, A.; Fujitama, Y.; Mitsuyama, K.; Takaki, K.; Toyonaga, A.; Sata, M.; et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J. Gastroenterol. 2003, 38, 134–141. [Google Scholar] [CrossRef]
- Vlassopoulou, M.; Yannakoulia, M.; Pletsa, V.; Zervakis, G.I.; Kyriacou, A. Effects of fungal beta-glucans on health—A systematic review of randomized controlled trials. Food Funct. 2021, 12, 3366. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Stauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immun. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermudez-Humaran, L.G.; Smirnova, N.; Berge, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Z.; Xue, Y.; Zhang, J.; Zhu, J.; Gao, R. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes 2020, 11, 1030–1042. [Google Scholar] [CrossRef]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmstrom, M.; Koskensalo, S.; Bohling, T.; Rautelin, H.; et al. Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Digest. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Fu, Y.X.; Chen, Y.; Guo, X. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021, 33, 988–1000. [Google Scholar] [CrossRef]
- Si, W.; Liang, H.; Bugno, J.; Xu, Q.; Ding, X.; Yang, K.; Fu, Y.; Weichselbaum, R.R.; Zhao, X.; Wang, L. Lactobacillus rhamnosus GG induces cGAS/STING-dependent type I interferon and improves response to immune checkpoint blockade. Gut 2022, 71, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xiao, N.; He, P.; Sun, P. Chemical analysis and antioxidant activity in vitro of dietary fibers extracted from Boletus edulis. Int. J. Biol. Macromol. 2011, 49, 1092–1095. [Google Scholar] [CrossRef] [PubMed]


| Extraction Methods | Materials | Extraction Conditions | Extraction Features | Reference | |
|---|---|---|---|---|---|
| Physical method | Pressurized hot water | Pleurotus sajor-caju | 140 °C, 0.92 MPa, and 40 min | Water as a solvent, low cost, but poor impurity removal | [46] |
| Ultrasound-assisted | Agaricus bisporus | 15 min, 100 mm amplitude, and 1 h of precipitation in 80% ethanol | Less time-consuming and highly efficient, but high cost and little capacity | [47] | |
| Microwave | Cordyceps gunnii mycelia | 1:20 (w/v), 70 °C, 280 W, 5 min | High extraction efficiency, short time and low energy input, but the microwave power and microwave time should be strictly controlled | [48] | |
| Biological method | Enzymatic | Schizophyllum commune | α-amylase, 100 °C, 30 min; protease 60 °C, 30 min | High specificity of enzyme is needed, and the extraction conditions must be strictly controlled | [49] |
| Chemical method | Alkaline | Coprinus comatus | 2% NaOH in a ratio of 1:15, 85 °C, 2 h | High yield, but may degrade some compounds | [50] |
| Acid | Lentinula edodes stipe | 100 °C, 2 h; 0.8 M trichloroacetic acid, 4 °C, 3 h | High yield, but may produce some byproducts | [51] | |
| Combined method | Hot water and alkaline | Cookeina tricholoma | 98 °C, 4 h; 2% KOH (w/v 1:4), 98 °C, 4 h | High yield and purity, but time-consuming | [52] |
| Acid–alkaline combined | Pleurotus eryngii | 0.1 M H2SO4 (1:10 w/v), 60 °C, 2 h; 0.25 M NaOH (1:8 w/v), 60 °C, 2 h | Higher purity, low cost, but may cause excessive degradation | [53] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Dong, Q.; Chen, M.; Zhao, R.; Zha, L.; Zhao, Y.; Zhang, M.; Zhang, B.; Ma, A. The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review. J. Fungi 2023, 9, 1028. https://doi.org/10.3390/jof9101028
Yu C, Dong Q, Chen M, Zhao R, Zha L, Zhao Y, Zhang M, Zhang B, Ma A. The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review. Journal of Fungi. 2023; 9(10):1028. https://doi.org/10.3390/jof9101028
Chicago/Turabian StyleYu, Changxia, Qin Dong, Mingjie Chen, Ruihua Zhao, Lei Zha, Yan Zhao, Mengke Zhang, Baosheng Zhang, and Aimin Ma. 2023. "The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review" Journal of Fungi 9, no. 10: 1028. https://doi.org/10.3390/jof9101028





