Cortinarius and Tomentella Fungi Become Dominant Taxa in Taiga Soil after Fire Disturbance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Sample Plots
2.2. Soil Sample Collection
2.3. The Extraction and Sequencing of Soil Fungal DNA
2.4. Sequence Data Analysis
3. Results
3.1. The Physicochemical Properties of the Soil Are Affected by Past Fires
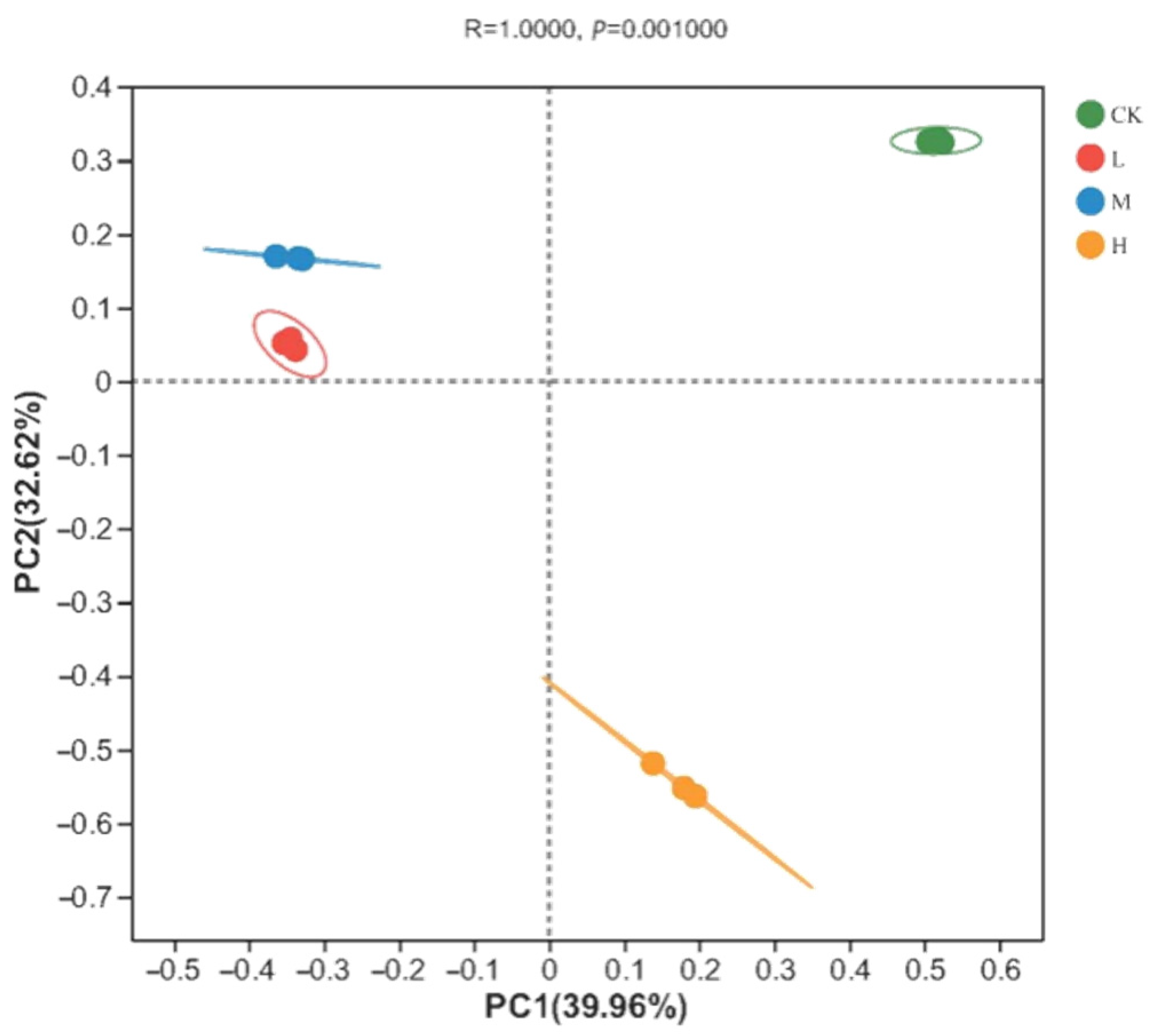
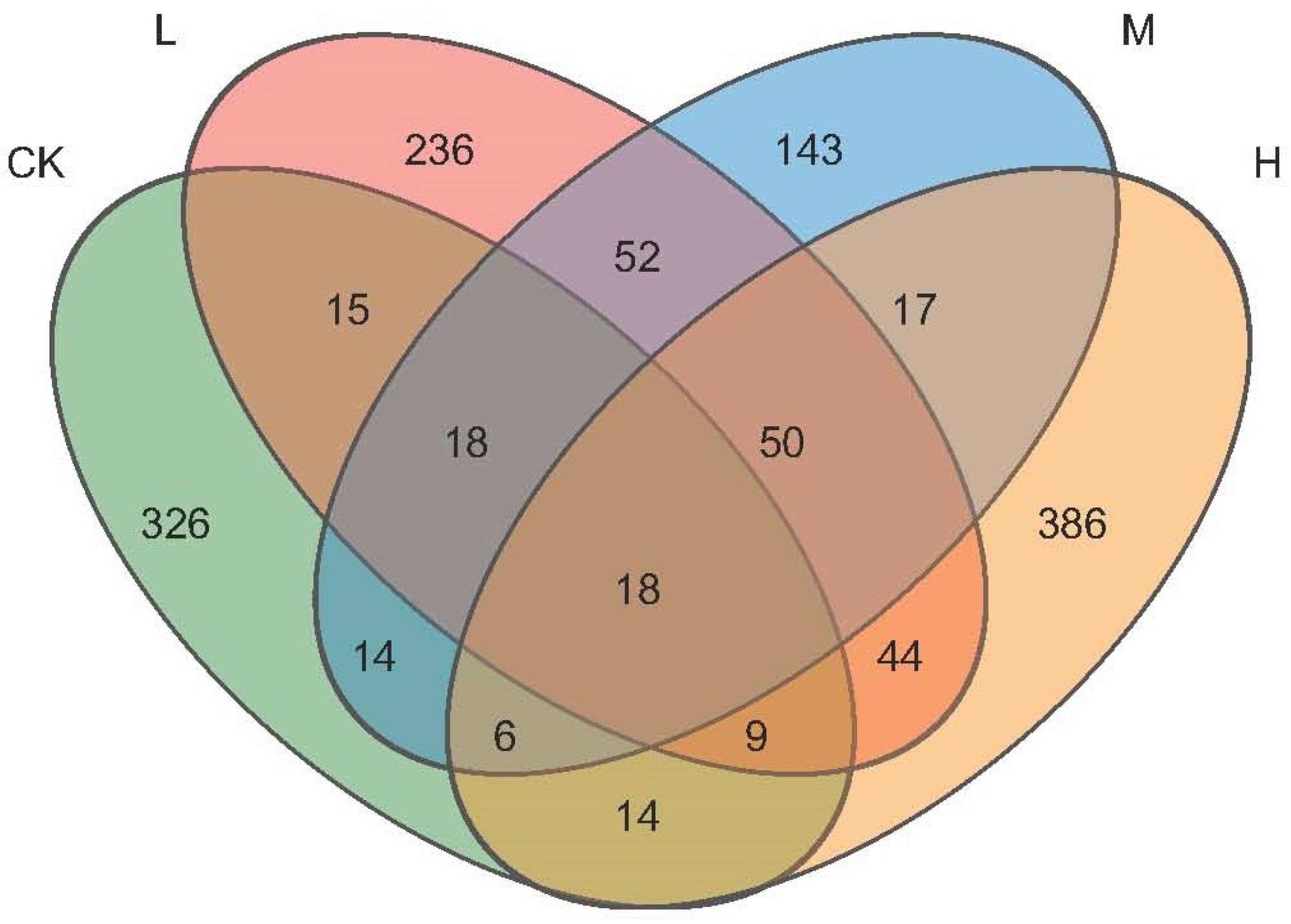
3.2. Soil Fungal Diversity Varies between the Fire Sites
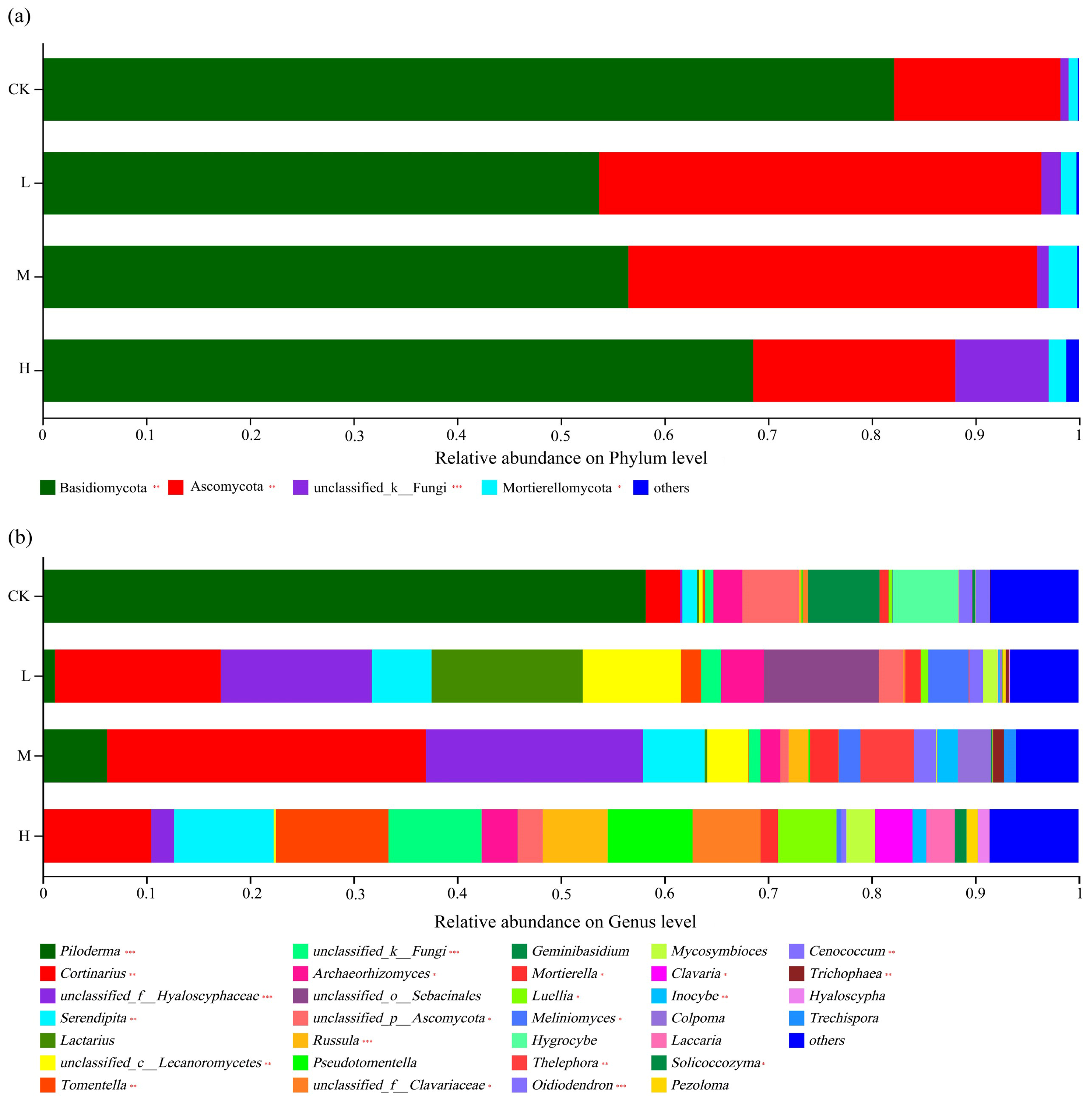
3.3. The Structural Composition of Soil Fungal Communities at the Phylum and Genus Levels
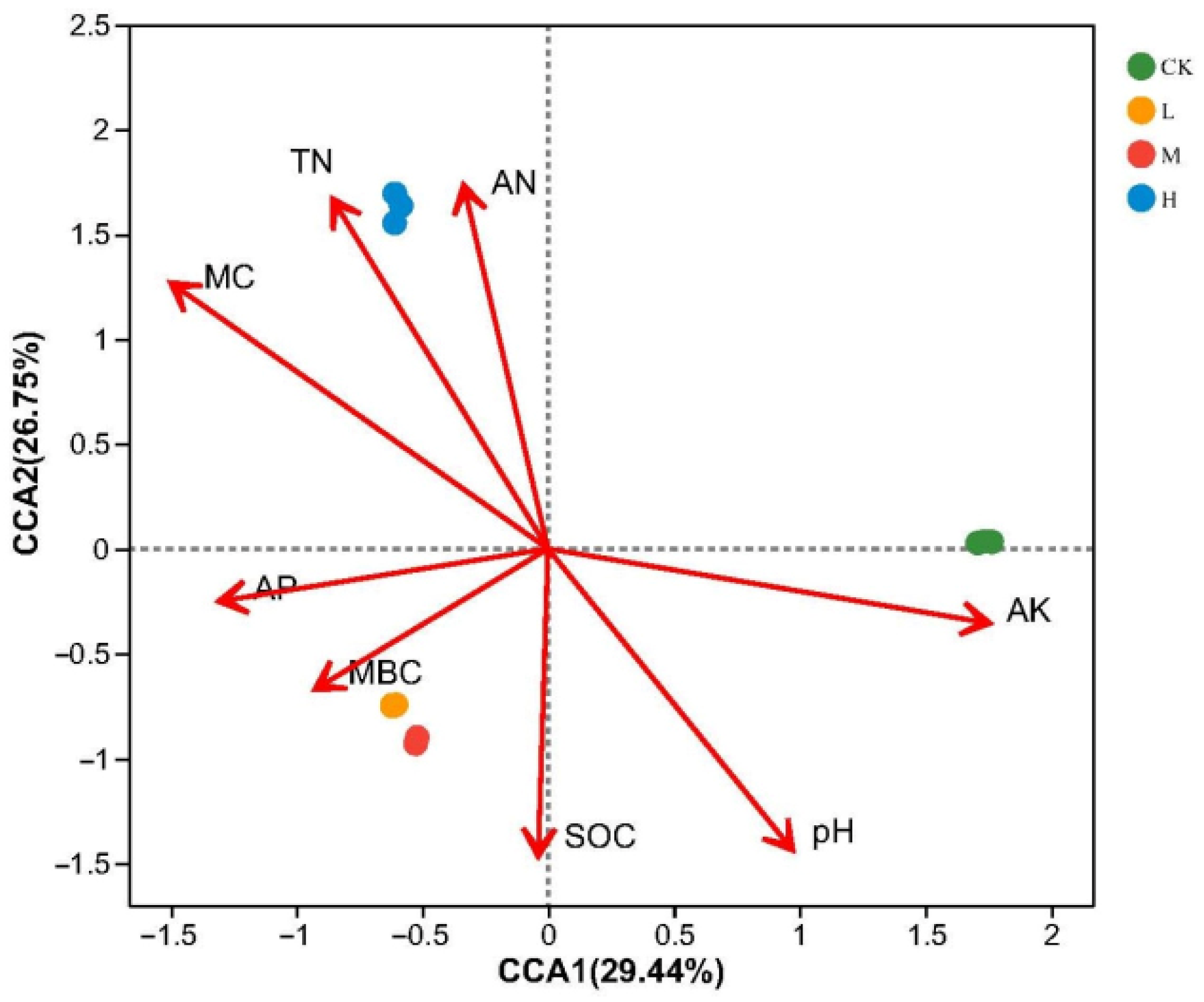
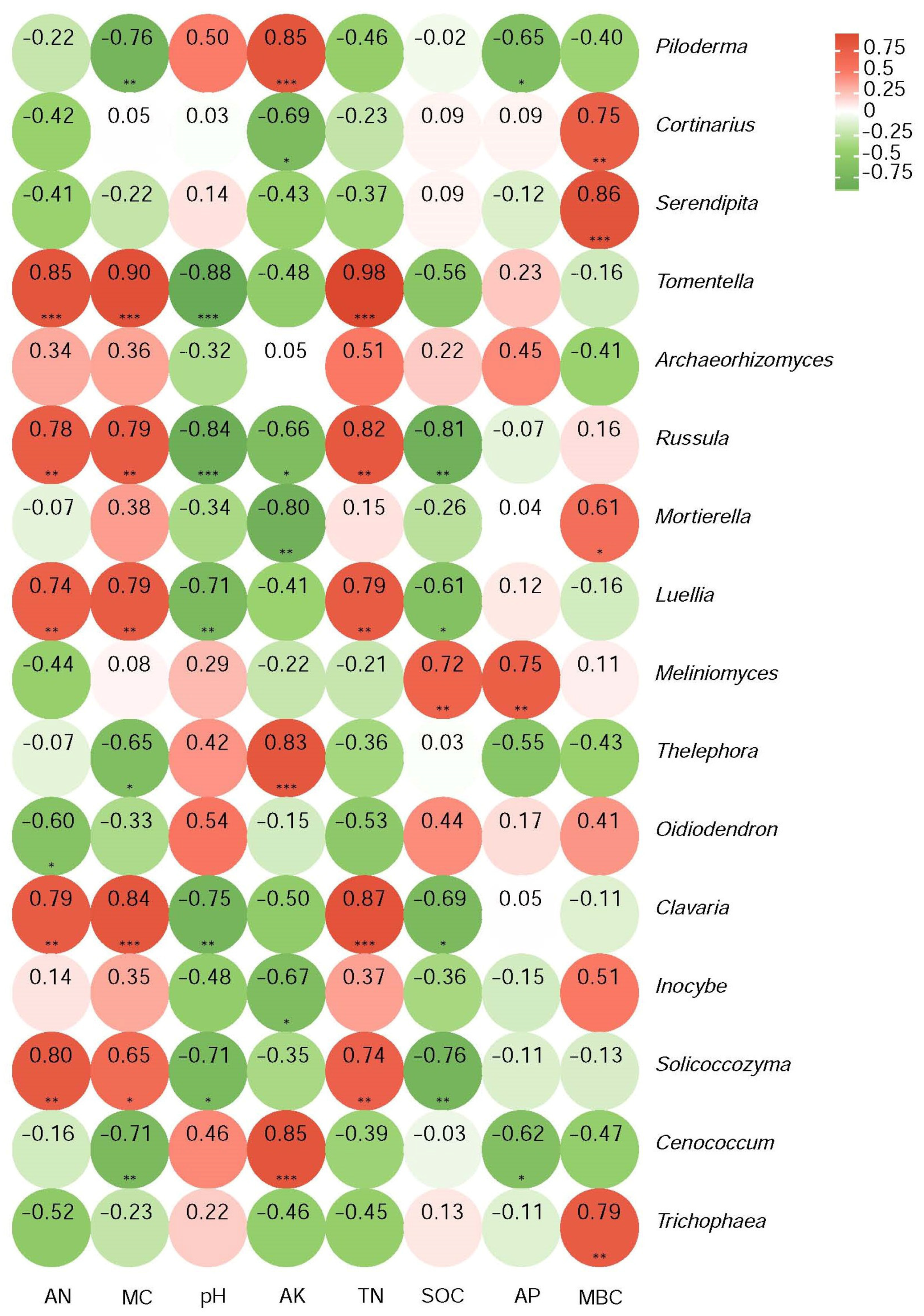
3.4. Correlation Analysis of Factors Influencing the Structure and Diversity of the Fungal Communities in the Soil Types
3.5. Predictive Analyses of Soil Fungal Function in Fire Sites
4. Discussion
4.1. The Effects of Fire Disturbance on Soil Physicochemical Properties
4.2. The Effects of Fire Disturbance on Fungal Alpha Diversity
4.3. The Effects of Fire Disturbance on Soil Fungal Community Composition
4.4. The Effects of Fire Disturbance on Potential Functional Taxa of Soil Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, H.D. Fire: Plant functional types and patch mosaic burning in fire-prone ecosystems. Earth Environ. 2008, 32, 421–437. [Google Scholar] [CrossRef]
- Keeley, J.E.; McGinnis, T.W. Impact of prescribed fire and other factors on cheatgrass persistence in a Sierra Nevada ponderosa pine forest. Int. J. Wildland Fire 2007, 16, 96–106. [Google Scholar] [CrossRef]
- Day, N.J.; Corrière, S.; Baltzer, J.L. Annual dynamics and resilience in post-fire boreal understory vascular plant communities. For. Ecol. Manag. 2017, 401, 264–272. [Google Scholar] [CrossRef]
- Kardol, P.; Yang, T.; Arroyo, D.N.; Teste, F.P. Plant-soil feedback in the’real world’: How does fire fit into all of this? Plant Soil. 2023, 485, 91–102. [Google Scholar] [CrossRef]
- Comer, J.; Perkins, L. Resistance of the soil microbial community to land-surface disturbances of high-intensity winter grazing and wildfire. J. Environ. Manag. 2021, 279, 111596. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Liu, X.J.; Pan, C.D. Effects of recovery time after fire and fire severity on stand structure and soil of larch forest in the Kanas National Nature Reserve, Northwest China. J. Arid. Land. 2019, 11, 811–823. [Google Scholar] [CrossRef]
- Avetisyan, D.; Velizarova, E.; Filchev, L. Post-Fire Forest Vegetation State Monitoring through Satellite Remote Sensing and In Situ Data. Remote Sens. 2022, 14, 6266. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Zaitsev, A.S.; Korobushkin, D.I.; Saifutdinov, R.A.; Butenko, K.O.; de Vries, F.T.; Ekschmitt, K.; Degtyarev, M.I.; Gorbunova, A.Y.; Kostina, N.V.; et al. Forest fire induces short-term shifts in soil food webs with consequences for carbon cycling. Ecol. Lett. 2020, 24, 438–450. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, H.Z.; Moriya, K.; Sakai, T.; Cao, C.X. Monitoring of post-fire forest regeneration under different restoration treatments based on ALOS/PALSAR data. New For. 2018, 49, 105–121. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Rupp, T.S.; Olson, M.; Verbyla, D. Modeling impacts of fire severity on successional trajectories and future fire behavior in Alaskan boreal forests. Landscape Ecol. 2011, 26, 487–500. [Google Scholar] [CrossRef]
- Gustine, R.N.; Hanan, E.J.; Robichaud, P.R.; Elliot, W.J. From burned slopes to streams: How wildfire affects nitrogen cycling and retention in forests and fire-prone watersheds. Biogeochemistry 2022, 157, 51–68. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I. Short-term effects of wildfire on microbial biomass and abundance in black pine plantation soils in Turkey. Ecol. Indic. 2009, 9, 1151–1155. [Google Scholar] [CrossRef]
- Jentsch, P.C.; Bauch, C.T.; Anand, M. Fire mitigates bark beetle outbreaks in serotinous forests. Theor. Ecol. 2021, 14, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Wu, J.; Kumari, D. Fungal community composition analysis of 24 different urban parks in Shanghai, China. Urban. Ecosyst. 2019, 22, 855–863. [Google Scholar] [CrossRef]
- Shi, Y.; Dang, K.K.; Dong, Y.H.; Feng, M.M.; Wang, B.R.; Li, J.G.; Chu, H.Y. Soil fungal community assembly processes under long-term fertilization. Eur. J. Soil. Sci. 2020, 71, 716–726. [Google Scholar] [CrossRef]
- Yin, Y.L.; Wang, Y.Q.; Li, S.X.; Liu, Y.; Zhao, W.; Ma, Y.S.; Bao, G.S. Soil microbial character response to plant community variation after grazing prohibition for 10 years in a Qinghai-Tibetan alpine meadow. Plant Soil. 2021, 458, 175–189. [Google Scholar] [CrossRef]
- Smrž, J.; Čatská, V. Mycophagous mites and their internal associated bacteria cooperate to digest chitin in soil. Symbiosis 2010, 52, 33–40. [Google Scholar] [CrossRef]
- Tapia-Veloz, E.; Gozalbo, M.; Guillén, M.; Dashti, A.; Bailo, B.; Köster, P.; Santín, M.; Carmena, D.; Trelis, M. Prevalence and associated risk factors of intestinal parasites among schoolchildren in Ecuador, with emphasis on the molecular diversity of Giardia duodenalis, Blastocystis sp. and Enterocytozoon bieneusi. PLoS Negl. Trop. Dis. 2023, 17, e0011339. [Google Scholar] [CrossRef]
- Tajik, S.; Ayoubi, S.; Lorenz, N. Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil. Ecol. 2020, 149, 103514. [Google Scholar] [CrossRef]
- Perez-Valera, E.; Goberna, M.; Faust, K.; Raes, J.; Garcia, C.; Verdu, M. Fire modifies the phylogenetic structure of soil bacterial co-occurrence networks. Environ. Microbiol. 2017, 19, 317–327. [Google Scholar] [CrossRef]
- Espinosa, J.; Dejene, T.; Guijarro, M.; Cerdá, X.; Madrigal, J.; Martín-Pinto, P. Fungal diversity and community composition responses to the reintroduction of fire in a non-managed Mediterranean shrubland ecosystem. For. Ecosyst. 2023, 10, 100110. [Google Scholar] [CrossRef]
- Li, J.J. Effect of Different Water and Soil Conservation Measures on the Diversity of Soil Microbes and Their Number; Fujian Agriculture and Forestry University: FuZhou, China, 2012. [Google Scholar]
- Meng, M.; Wang, B.; Zhang, Q.L.; Tian, Y. Driving force of soil microbial community structure in a burned area of Daxing’anling, China. J. For. Res. 2021, 32, 1723–1738. [Google Scholar] [CrossRef]
- Day, N.J.; Dunfield, K.E.; Johnstone, J.F.; Mack, M.C.; Turetsky, M.R.; Walker, X.J.; White, A.L.; Baltzer, J.L. Wildfire severity reduces richness and alters composition of soil fungal communities in boreal forests of western Canada. Glob. Change Biol. 2019, 25, 2310–2324. [Google Scholar] [CrossRef]
- Tian, J.Q.; Wang, H.J.; Vilgalys, R.; Ho, M.C.; Flanagan, N.; Richardson, C.J. Response of fungal communities to fire in a subtropical peatland. Plant Soil 2021, 466, 525–543. [Google Scholar] [CrossRef]
- Kutorga, E.; Adamonyte, G.; Irsenaite, R.; Juzenas, S.; Kasparavicius, J.; Markovskaja, S.; Motiejunaite, J.; Treigiene, A. Wildfire and post-fire management effects on early fungal succession in Pinus mugo plantations, located in Curonian Spit (Lithuania). Geoderma 2012, 191, 70–79. [Google Scholar] [CrossRef]
- Weng, Y.T.; Li, Z.G.; Luo, S.S.; Su, Z.W.; Di, X.Y.; Yang, G.; Yu, H.Z.; Han, D.D. Drivers of changes in soil properties during post-fire succession on Dahurian larch forest. J. Soils Sediments 2021, 21, 3556–3571. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Chang, Y.; Liu, M.; Li, Y.; Ren, B.; Shi, S. Effects of Fire Severity and Topography on Soil Black Carbon Accumulation in Boreal Forest of Northeast China. Forests 2018, 9, 408. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.P.; Li, Y.H.; Han, D.X.; Cong, J.X.; Gao, C.Y. Aerobic and anaerobic burning alter trace metal availability in peat soils: Evidence from laboratory experiments. Eur. J. Soil Sci 2023, 74, e13385. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Q.L.; Hao, S. Effects of fire disturbance on Larix gmelinii growth-climate relationship. Ecol. Indic 2022, 143, 109377. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, S.; Du, J.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Yang, L.B.; Wu, S.; Zhou, T. Warming changes the composition and diversity of fungal communities in permafrost. Ann. Microbiol. 2023, 73, 7. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C.K.; Pan, X.C.; Bai, S.B. Effects of Simulated Acid Rain on Soil Bacterial Community Diversity in Buffer Zone of Broad-Leaved Forest Invaded by Moso Bamboo. Res. Environ. Sci. 2020, 6, 1478–1487. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zhai, X.Z.; Guo, X.; Wang, Y.J.; Wang, J.H.; Zhou, Z.M.; Jiang, T.T. Bacterial community characteristics and key driving factors of surface sediments in Huailai section of Yongding River in winter. J. Environ. Eng. Technol. 2019, 9, 544–551. [Google Scholar] [CrossRef]
- He, J.; Yan, B.; Li, J.S.; Fu, G.; Qi, Y.; Ma, Y.W.; Qiao, M.P. Altitude Distribution Patterns and Regional Differences of Soil Bacterial Community in Northern Slopes in the Middle Qinling Mountains. Res. Environ. Sci. 2019, 32, 1374–1383. [Google Scholar] [CrossRef]
- Wu, B.; Wang, B.Z.; Wang, L.X.; He, Y.D.; Wang, A.B.; Li, C.; Liu, Y.X. Effects of Flower Forcing Fertilizers on Bacterial Community Diversity in Rhizosphere Soil of Wax Apple. Res. Environ. Sci. 2018, 31, 732–741. [Google Scholar] [CrossRef]
- Sui, X.; Yang, L.B.; Cui, F.X.; Zhu, D.G.; Luo, Q.S.; Ni, H.W. Soil of Pinus koraiensis Forest in Tangwanghe National Park: Fungi Diversity. Chin. Agric. Sci. Bull. 2019, 35, 84–90. [Google Scholar] [CrossRef]
- Jin, M.Y.; Hou, L.; Wang, L.Q.; Song, X.Y. Effects of Different Forest Fire Intensities on Soil Physicochemical Properties of Pinus Densata Forest in Southeastern Tibet. J. Plateau Agric. 2022, 6, 424–432. [Google Scholar] [CrossRef]
- Sun, M.X.; Jia, W.W.; Wu, Y. Effect of Forests fire on soil Chemical Properties in Northern Daxing’an Mountains. J. Northeast. For. Univ. 2009, 37, 33–35. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliot, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Cui, X.Y.; Wang, H.Q. Spatiotemporal variations of soil moisture content in the Larix gmelinii forest under interference of experimental forest fire in northern Great Xing’an Mountains of northeastern China. J. Beijing For. Univ. 2020, 42, 94–101. [Google Scholar] [CrossRef]
- Yang, L.B.; Cui, F.X.; Huang, Q.Y.; Zhu, D.G.; Xu, F. Analysis on bacterial diversity and composition of Larixg melinii fallen wood with different decomposition levels. J. Cent. South Univ. For. Technol. 2021, 41, 93–100+182. [Google Scholar] [CrossRef]
- Mcintosh, P.D.; Laffan, M.D.; Hewitt, A.E. The role of fire and nutrient loss in the genesis of the forest soils of Tasmania and southern New Zealand. For. Ecol. Manag 2005, 220, 185–215. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.P.; Wang, X.; Wei, X.R.; Gao, H.L.; Zhang, Y.J.; Cheng, Z.M. Effects of wildfire and topography on soil nutrients in asemiarid restored grassland. Plant Soil 2018, 428, 123–136. [Google Scholar] [CrossRef]
- Gu, H.Y.; Jin, J.B.; Chen, X.W.; Wang, E.H.; Zhou, Y.Y.; Chai, Y.F. The Long-term Impacts on Chemical Properties of Larix gmelini Forest on the Northern Slope of Greater Hinggan Mountains from a Forest Fire of Varying Fire Intensity. J. Nat. Resour. 2010, 25, 1114–1121. [Google Scholar] [CrossRef]
- Chen, J.G. Research on the Absorption and Utilization of Plants to Kalium. Agric. Sci. Technol. Equip. 2015, 1, 5–6. [Google Scholar] [CrossRef]
- Covington, W.W.; Sackett, S.S. Soil mineral nitrogen changes following prescribed burning in ponderosa pine. For. Ecol. Manag. 1992, 54, 175–191. [Google Scholar] [CrossRef]
- Liu, K.; Shu, L.; He, C. Effects of Prescribed Fire on Meadow Soil Chemical Properties in Nanwenghe Nature Reserve. Sustainability 2022, 14, 9984. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183–184, 80–91. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R. Study on Diversity and Community Characteristics of Soil Fungi at Burned Forest Sites for the Great Xing’an. North. Hortic. 2017, 10, 159–166. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Z.; Pang, Q.; Liang, X.; Li, H.; Sui, X.; Li, C.; Song, F. Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus. Microorganisms 2022, 10, 2059. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weng, X.; Zhang, R.; Yang, L.; Liu, Y.; Sui, X. The Diversity and Composition of Soil Microbial Community Differ in Three Typical Wetland Types of the Sanjiang Plain, Northeastern China. Sustainability 2022, 14, 14394. [Google Scholar] [CrossRef]
- Zhao, X.L.; Xie, Y.H.; Ma, X.J.; Wang, S.K. Vegetation structure and its relationship with soil physicochemical properties in restoring sandy grassland in Horqin Sandy Land. J. Desert Res. 2022, 42, 134–141. [Google Scholar] [CrossRef]
- Bai, X.L.; Zhang, E.; Wu, J.M.; Ma, D.H.; Zhang, B.Y.; Zhang, C.H.; Tian, F.; Zhao, H.; Wang, B. Effects of Different Modified Materials on Soil Fungal Community Structure in Saline-Alkali Soil. Environ. Sci. 2023, 1–16. [Google Scholar] [CrossRef]
- Bao, Z.H.; Matsushita, Y.; Morimoto, S.; Hoshino, Y.T.; Suzuki, C.; Nagaoka, K.; Takenaka, M.; Murakami, H.; Kuroyanagi, Y.; Urashima, Y.; et al. Decrease in fungal biodiversity along an available phosphorous gradient in arable Andosol soils in Japan. Can. J. Microbiol. 2013, 59, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Chungu, D.; Ng’andwe, P.; Mubanga, H.; Chileshe, F. Fire alters the availability of soil nutrients and accelerates growth of Eucalyptus grandisin Zambia. J. For. Res. 2020, 31, 1637–1645. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Xu, S.; Ciais, P.; Manzoni, S.; Fang, J.Y.; Yu, G.R.; Tang, X.L.; Zhou, P.; Wang, W.T.; Yan, J.H.; et al. Climate and litter C/N ratio constrain soil organic carbon accumulation. Natl. Sci. Rev. 2019, 6, 746–757. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Y.M.; Wang, Z.B.; Wang, H.X.; Qu, L.Y. The fungal community characteristics of rhizosphere soil of Larix gmelinii in different growth status after severe fire. Acta Ecol. Sin. 2021, 41, 9399–9409. [Google Scholar] [CrossRef]
- Qin, Z.F.; Zhang, H.Y.; Feng, G.; Christie, P.; Zhang, J.L.; Li, X.L.; Gai, J.P. Soil phosphorus availability modifies the relationship between AM fungal diversity and mycorrhizal benefits to maize in an agricultural soil. Soil Biol. Biochem. 2020, 144, 107790. [Google Scholar] [CrossRef]
- Li, P.F.; Liu, M.; Li, G.L.; Liu, K.; Liu, T.S.; Wu, M.; Saleem, M.; Li, Z.P. Phosphorus availability increases pathobiome abundance and invasion of rhizosphere microbial networks by Ralstonia. Environ. Microbiol. 2021, 23, 5992–6003. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhang, J.D.; Wang, J.H.; Liu, D.S. Effects on Soil Microbial Community Structure and Alteration of Potassium by Compost Under Potassium Stress. J. Agro-Environ. Sci. 2006, 25, 121–123. [Google Scholar] [CrossRef]
- Cui, F.X. Effects of Fire Disturbance on Soil Microbial Diversity and Greenhouse Gas Emissions in Xing’an Larch Forest; Northeast Forestry University: Harbin, China, 2022. [Google Scholar]
- Lienhard, P.; Terrat, S.; Mathieu, O.; Levêque, J.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Faivre, C.; Sayphoummie, S.; Panyasiri, K.; et al. Soil microbial diversity and C turnover modified by tillage and cropping in Laos tropical grassland. Environ. Chem. Lett. 2013, 11, 391–398. [Google Scholar] [CrossRef]
- Ma, A.Z.; Zhuang, X.L.; Wu, J.M.; Cui, M.M.; Lv, D.; Liu, C.Z.; Zhuang, G.Q. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Tian, L.M.; Ai, Y.; Chen, S.Y.; MIPAM, T.D. Effects of short-term yak grazing on soil fungal communities in an alpine meadow on the Qinghai-Tibetan Plateau. Acta Prataculturae Sin. 2022, 31, 41–52. [Google Scholar] [CrossRef]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, T.R. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Hansen, P.M.; Semenova-Nelsen, T.A.; Platt, W.J.; Sikes, B.A. Recurrent fires do not affect the abundance of soil fungi in a frequently burned pine savanna. Fungal Ecol. 2019, 42, 1754–5048. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.I.; Levine, C.R.; DiRocco, A.M.; Battles, J.J.; Bruns, T.D. Ectomycorrhizal fungal spore bank recovery after a severe forest fire: Some like it hot. ISME J. 2016, 10, 1228–1239. [Google Scholar] [CrossRef]
- Guo, J.F.; Chen, G.S.; Xie, J.S.; Yang, Z.J.; Yang, Y.S. Effect of heat-disturbance on microbial biomass carbon and microbial respiration in Chinese fir (Cunninghamia lanceolata) forest soils. J. For. Res. 2015, 26, 933–939. [Google Scholar] [CrossRef]
- Docherty, K.M.; Balser, T.C.; Bohannan, B.J.M.; Gutknecht, J.L.M. Soil microbial responses to fire and interacting global change factors in a California annual grassland. Biogeochemistry 2012, 109, 63–83. [Google Scholar] [CrossRef]
- Yang, L.B.; Sui, X.; Zhu, D.G.; Cui, F.X.; Li, J.B.; Song, R.Q.; Ni, H.W. Study on fungal communities characteristics of different Larix gmelini forest typesin cold temperate zone. J. Cent. South Univ. For. Technol. 2017, 37, 76–84. [Google Scholar] [CrossRef]
- Feng, B.; Yang, Z.L. Ectomycorrhizal symbioses: Diversity of mycobionts and molecular mechanisms that entail the development of ectomycorrhizae. Sci. Sin. Vitae 2019, 49, 436–444. [Google Scholar] [CrossRef]
- Holden, S.R.; Gutierrez, A.; Treseder, K.K. Changes in Soil Fungal Communities, Extracellular Enzyme Activities, and Litter Decomposition Across a Fire Chronosequence in Alaskan Boreal Forests. Ecosystems 2013, 16, 34–46. [Google Scholar] [CrossRef]
- Smith, J.E. Mycorrhizal Symbiosis (Third Edition). Soil Sci. Soc. Am. J. 2009, 73, 694. [Google Scholar] [CrossRef]
- Che, R.X.; Wang, S.P.; Wang, Y.F.; Xu, Z.H.; Wang, W.J.; Rui, Y.C.; Wang, F.; Hu, J.M.; Tao, J.; Cui, X.Y. Total and active soil fungal community profiles were significantly altered by six years of warming but not by grazing. Soil Biol. Biochem. 2019, 139, 107611. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.X.; Yang, W.P.; Li, W.G.; Jing, D.D.; Yang, Z.P.; Gao, Z.Q. Effect of forage rape green manure returning to field on soil fungal community in winter wheat field. Acta Microbiol. Sin. 2021, 61, 2869–2882. [Google Scholar] [CrossRef]
- Bowd, E.J.; Egidi, E.; Lindenmayer, D.B.; Wardle, D.A.; Kardol, P.; Cary, G.J.; Foster, C. Direct and indirect effects of fire on microbial communities in a pyrodiverse dry-sclerophyll forest. J. Ecol. 2022, 110, 1687–1703. [Google Scholar] [CrossRef]
- Carson, C.M.; Jumpponen, A.; Blair, J.M.; Zeglin, L.H. Soil fungal community changes in response to long-term fire cessation and N fertilization in tallgrass prairie. Fungal Ecol. 2019, 41, 45–55. [Google Scholar] [CrossRef]

| Fire Intensity | Chao1 | Shannon | Simpson |
|---|---|---|---|
| CK (no fire) | 261 ± 52.37 ab | 2.67 ± 0.69 b | 0.23 ± 0.16 a |
| L (light fire) | 288 ± 7.94 a | 3.74 ± 0.11 a | 0.05 ± 0.01 b |
| M (moderate fire) | 206.67 ± 16.5 b | 3.34 ± 0.1 ab | 0.07 ± 0.01 ab |
| H (heavy fire) | 313 ± 60.56 a | 3.85 ± 0.17 a | 0.04 ± 0.00 b |
| AN | MC | pH | AK | TN | SOC | AP | MBC | |
|---|---|---|---|---|---|---|---|---|
| Chao1 | 0.58 * | 0.45 | −0.55 | 0.04 | 0.64 * | 0.01 | 0.41 | −0.54 |
| Shannon | 0.36 | 0.75 ** | −0.56 | −0.63 * | 0.59 * | 0.04 | 0.65 * | 0.09 |
| Simpson | −0.23 | −0.60 * | 0.42 | 0.64 * | −0.40 | −0.04 | −0.53 | −0.24 |
| R2 | p-Value | |
|---|---|---|
| AN | 0.7631 | 0.008 ** |
| MC | 0.9553 | 0.001 *** |
| pH | 0.7395 | 0.004 ** |
| AK | 0.794 | 0.005 ** |
| TN | 0.8663 | 0.004 ** |
| SOC | 0.5195 | 0.043 * |
| AP | 0.4316 | 0.075 |
| MBC | 0.3122 | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Wu, S.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Cortinarius and Tomentella Fungi Become Dominant Taxa in Taiga Soil after Fire Disturbance. J. Fungi 2023, 9, 1113. https://doi.org/10.3390/jof9111113
Cheng Z, Wu S, Pan H, Lu X, Liu Y, Yang L. Cortinarius and Tomentella Fungi Become Dominant Taxa in Taiga Soil after Fire Disturbance. Journal of Fungi. 2023; 9(11):1113. https://doi.org/10.3390/jof9111113
Chicago/Turabian StyleCheng, Zhichao, Song Wu, Hong Pan, Xinming Lu, Yongzhi Liu, and Libin Yang. 2023. "Cortinarius and Tomentella Fungi Become Dominant Taxa in Taiga Soil after Fire Disturbance" Journal of Fungi 9, no. 11: 1113. https://doi.org/10.3390/jof9111113





