Inhibitory Mechanisms of trans-2-Hexenal on the Growth of Geotrichum citri-aurantii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogen
2.2. Fruit
2.3. Chemicals
2.4. Antifungal Activity of trans-2-Hexenal against G. citri-aurantii
2.5. In Vivo Experiments of trans-2-Hexenal against G. citri-aurantii
2.6. Scanning Electron Microscopy (SEM) of trans-2-Hexenal against G. citri-aurantii
2.7. Transmission Electron Microscopy (TEM) of trans-2-Hexenal against G. citri-aurantii
2.8. Fourier Transform Infrared (FT−IR) Spectroscopy of trans-2-Hexenal against G. citri-aurantii
2.9. Effect of trans-2-Hexenal on the Cell Wall of G. citri-aurantii
2.10. Effect of trans-2-Hexenal on the Cell Wall of G. citri-aurantii
2.10.1. Effect of trans-2-Hexenal on the Cell Wall Integrity of G. citri-aurantii
2.10.2. Effect of trans-2-Hexenal on the Chitin Content of G. citri-aurantii
2.10.3. Effect of trans-2-Hexenal on the β-1,3-Glucan Content of G. citri-aurantii
2.10.4. Effect of trans-2-Hexenal on the Extracellular Alkaline Phosphatase (AKP) Activities of G. citri-aurantii
2.11. Effect of trans-2-Hexenal on the Cell Membrane of G. citri-aurantii
2.11.1. Effect of trans-2-Hexenal on the Cell Membrane Integrity of G. citri-aurantii
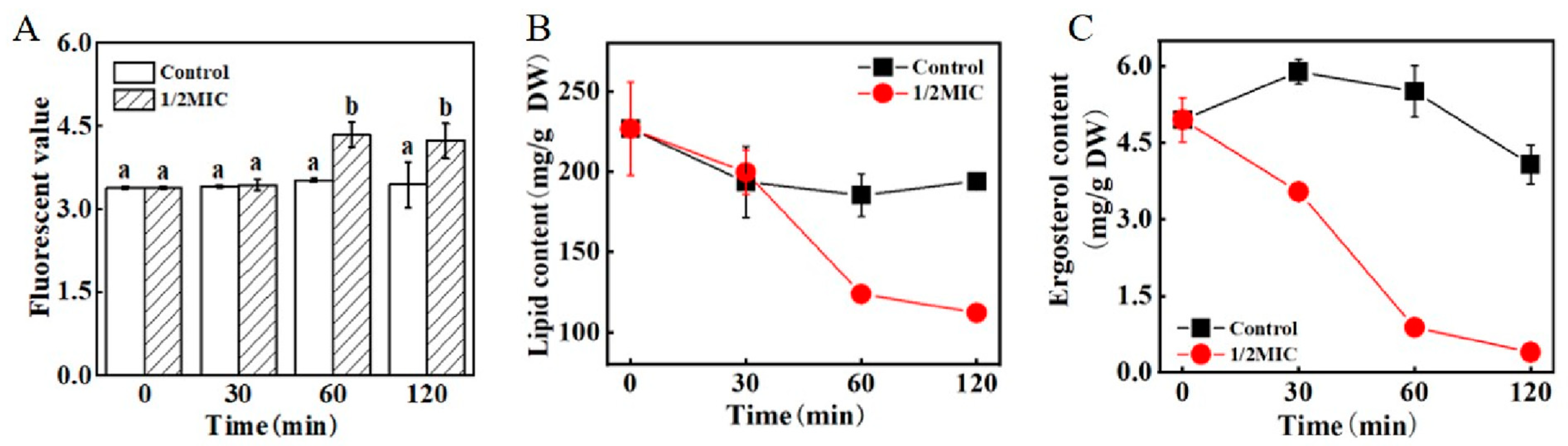
2.11.2. Effect of trans-2-Hexenal on the Total Lipid Content of G. citri-aurantii
2.11.3. Effect of trans-2-Hexenal on the Ergosterol Contents of G. citri-aurantii
2.12. Real-Time Fluorescence Quantitative PCR (RT-qPCR) Analysis
2.13. Molecular Docking
2.14. Statistical Analyses
3. Results
3.1. Antifungal Activity of trans-2-Hexenal against G. citri-aurantii
3.2. In Vivo Experiments of trans-2-Hexenal against G. citri-aurantii
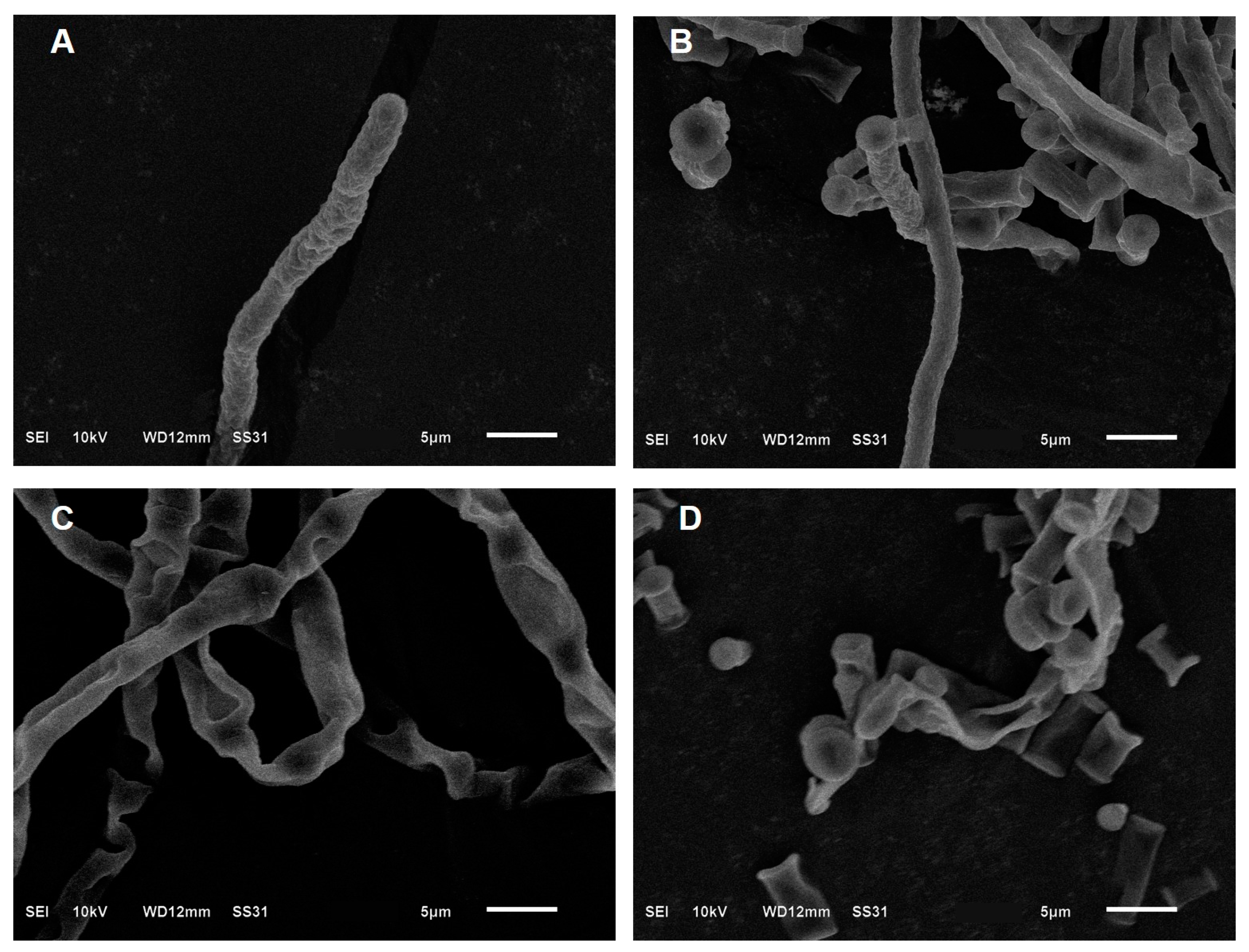
3.3. Scanning Electron Microscopy (SEM) of trans-2-Hexenal against G. citri-aurantii
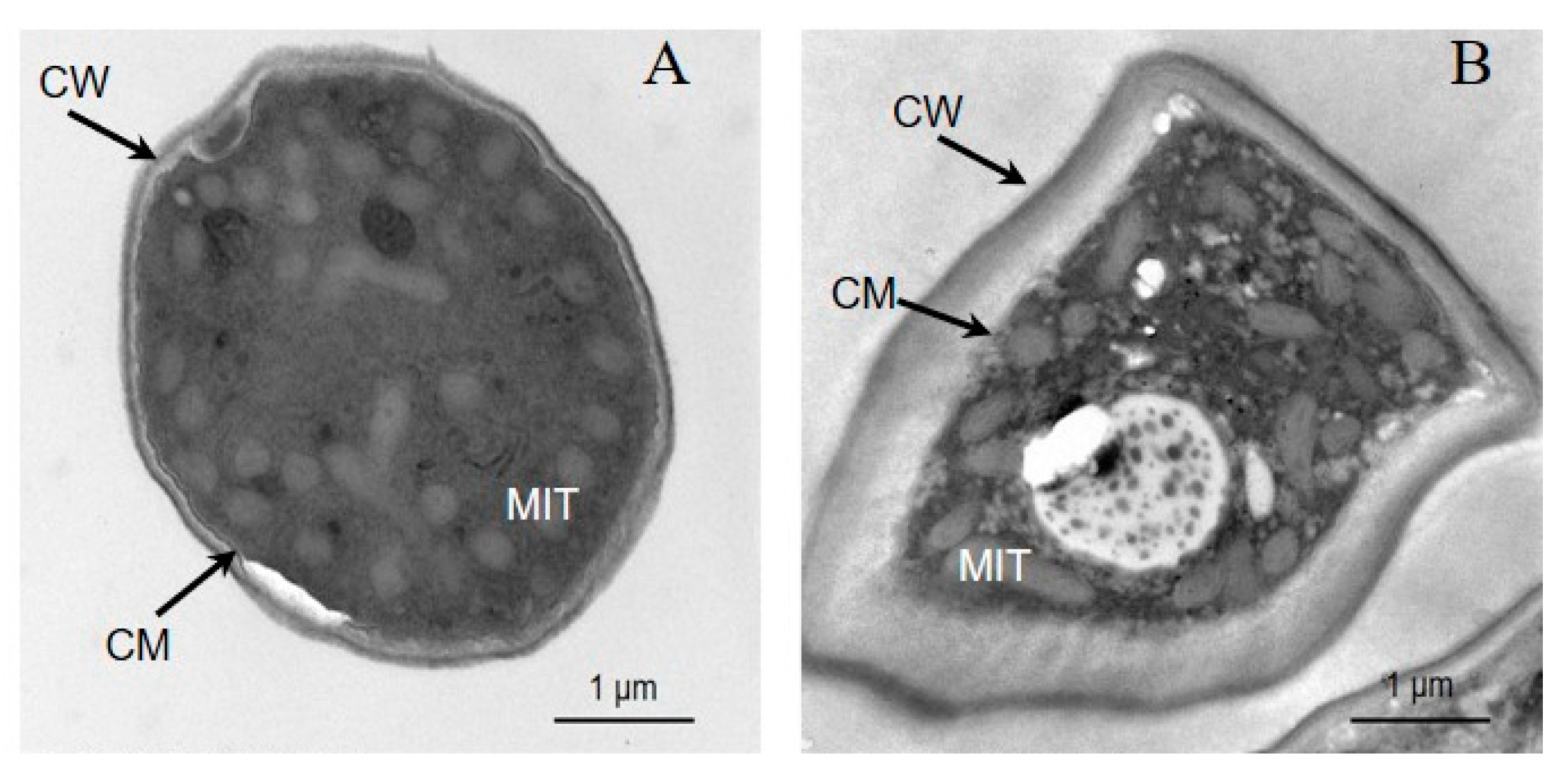
3.4. Transmission Electron Microscopy (TEM) of trans-2-Hexenal against G. citri-aurantii
3.5. FT−IR of trans-2-Hexenal against G. citri-aurantii
3.6. Effect of trans-2-Hexenal on the Cell Wall of G. citri-aurantii
3.7. Effect of trans-2-Hexenal on the Cell Membrane of G. citri-aurantii
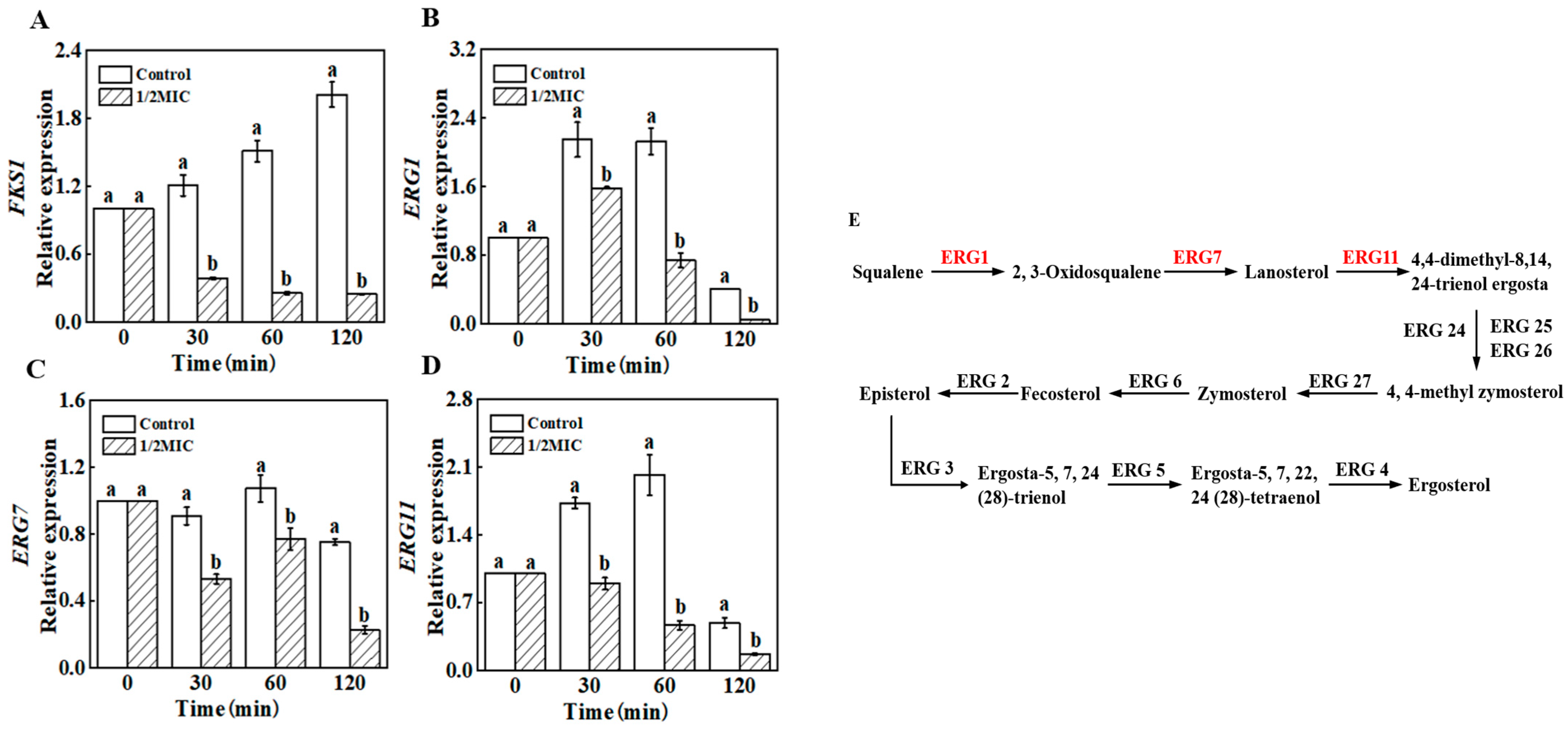
3.8. RT-qPCR
3.9. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferraz, L.P.; Cunha, T.D.; da Silva, A.C.; Kupper, K.C. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 2016, 188–189, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Feng, L.Y.; Sun, J.; Mao, L.T.; Li, X.J.; Jiang, Y.M.; Duan, X.W.; Li, T.T. Antifungal activities of a natural trisaccharide ester against sour rot in mandarin fruit. Postharvest Biol. Technol. 2022, 191, 111981. [Google Scholar] [CrossRef]
- McKay, A.H.; Förster, H.; Adaskaveg, J.E. Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, D.Y.; Wang, Z.; Tian, Z.H.; Fan, Y.; Lu, X.J.; Long, C.A. Genome sequencing and transcriptome analysis of Geotrichum citri-aurantii on citrus reveal the potential pathogenic- and guazatine-resistance related genes. Genomics 2020, 112, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, Y.; Zhu, X.; Yuan, P.; Shan, Y. Inhibitory mechanisms of cinnamic acid on the growth of Geotrichum citri-aurantii. Food Control 2022, 131, 108459. [Google Scholar] [CrossRef]
- Li, Y.H.; Shao, X.F.; Xu, J.Y.; Wei, Y.Y.; Xu, F.; Wang, H.F. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Wuryatmo, E.; Able, A.J.; Ford, C.M.; Scott, E.S. Effect of volatile citral on the development of blue mould, green mould and sour rot on navel orange. Australas. Plant Path. 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ouyang, Q.L.; Duan, B.; Reymick, O.O.; Chen, Y.; Tan, Y.Z.; Zhu, X.R.; Su, D.L.; Li, G.Y.; Tao, N.G. Trans-2-hexenal/beta-cyclodextrin effectively reduces green mold in citrus fruit. Postharvest Biol. Technol. 2022, 187, 111871. [Google Scholar] [CrossRef]
- Arroyo, F.T.; Moreno, J.; Daza, P.; Boianova, L.; Romero, F. Antifungal activity of strawberry fruit volatile compounds against Colletotrichum acutatum. J. Agric. Food Chem. 2007, 55, 5701–5707. [Google Scholar] [CrossRef]
- Myung, K.; Hamilton-Kemp, T.R.; Archbold, D.D. Interaction with and Effects on the profile of proteins of Botrytis cinerea by C6 aldehydes. J. Agric. Food Chem. 2007, 55, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Fu, M.R.; Qu, X.Q.; Liu, J.J.; Bu, J.W.; Feng, S.R.; Zhao, H.D.; Jiao, W.X.; Sun, F. (E)-2-Hexenal-based coating induced acquired resistance in apple and its antifungal effects against Penicillium expansum. LWT-Food Sci. Technol. 2022, 163, 113536. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Sun, H.; Wang, X. Antifungal activity of trans-2-hexenal against Penicillium cyclopium by a membrane damage mechanism. J. Food Biochem. 2017, 41, e12289. [Google Scholar] [CrossRef]
- Dong, Y.P.; Li, C.Y.; Long, H.T.; Liu, Z.T.; Huang, Y.; Zhang, M.; Wang, T.L.; Liu, Y.X.; Bi, Y.; Prusky, D.B. Preparation and use of trans-2-hexenal microcapsules to preserve ‘Zaosu’ pears. Sci. Hortic. 2021, 283, 110091. [Google Scholar] [CrossRef]
- Guo, M.; Feng, J.; Zhang, P.; Jia, L.; Chen, K. Postharvest treatment with trans-2-hexenal induced resistance against Botrytis cinerea in tomato fruit. Australas. Plant Path. 2015, 44, 121–128. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y.L. (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ouyang, Q.L.; Tao, N.G. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef]
- Dou, S.W.; Ouyang, Q.L.; You, K.Y.; Qian, J.J.; Tao, N.G. An inclusion complex of thymol into β-cyclodextrin and its antifungal activity against Geotrichum citri-aurantii. Postharvest Biol. Technol. 2018, 138, 31–36. [Google Scholar] [CrossRef]
- Ouyang, Q.L.; Duan, X.F.; Li, L.; Tao, N.G. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.H.; Yue, T.L. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef]
- Francois, J.M. A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat. Protoc. 2016, 1, 2995–3000. [Google Scholar] [CrossRef]
- Fortwendel, J.R.; Juvvadi, P.R.; Pinchai, N.; Perfect, B.Z.; Alspaugh, J.A.; Perfect, J.R.; Steinbach, W.J. Differential effects of inhibiting chitin and 1,3-β-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2009, 53, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.L.; Okwong, R.O.; Chen, Y.P.; Tao, N.G. Citronellal exerts its antifungal activity by targeting ergosterol biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2021, 25, 402–408. [Google Scholar] [CrossRef]
- Ghfir, B.; Fonvieille, J.L.; Dargent, R. Influence of essential oil of Hyssopus officinalis on the chemical composition of the walls of Aspergillus fumigatus (Fresenius). Mycopathologia 1997, 138, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 11114, 1642–1649. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.M.; Xie, Y.L.; Zhong, Y. o-Vanillin, a promising antifungal agent, inhibits Aspergillus flavus by disrupting the integrity of cell walls and cell membranes. Appl. Microbiol. Biotechnol. 2021, 105, 5147–5158. [Google Scholar] [CrossRef]
- Healey, K.R.; Paderu, P.; Hou, X.; Ortigosa, C.J.; Bagley, N.; Patel, B.; Zhao, Y.N.; Perlin, D.S. Differential regulation of echinocandin targets Fks1 and Fks2 in Candida glabrataby the Post-Transcriptional Regulator Ssd1. J. Fungi 2020, 4, 143. [Google Scholar] [CrossRef]
- García-Rodriguez, L.J.; Trilla, J.A.; Castro, C.; Valdivieso, M.H.; Durán, A.; Roncero, C. Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 2000, 478, 84–88. [Google Scholar] [CrossRef]
- Ma, L.; Salas, O.; Bowler, K.; Bar-Peled, M.; Sharon, A. UDP-4-keto-6-deoxyglucose, a transient antifungal metabolite, weakens the fungal cell wall partly by inhibition of UDP-galactopyranose mutase. MBio 2017, 8, e01559-17. [Google Scholar] [CrossRef]
- Douglas, C.M.; Foor, F.; Marrinan, J.A.; Morin, N.; Nielsen, J.B.; Dahl, A.M.; Mazur, P.; Baginsky, W.; Li, W.; el-Sherbeini, M. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-D-glucan synthase. Proc. Natl. Acad. Sci. USA 1994, 91, 12907–12911. [Google Scholar] [CrossRef] [PubMed]
- Ju, J. Study on the Synergistic Inhibitory Mechanism of Eugenol and Citral against Penicillium Roqueforti and Aspergillus niger; Jiangnan University: Wuxi, China, 2021; pp. 27–35. [Google Scholar]
- Li, L.; Tang, X.; Ouyang, Q.L.; Tao, N.G. Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit. Postharvest Biol. Technol. 2019, 151, 19–25. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, X.; Fu, M. Inhibiting effect of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol. Technol. 2017, 123, 94–101. [Google Scholar] [CrossRef]
- Sant, D.G.; Tupe, S.G.; Ramana, C.V.; Deshpande, M.V. Fungal cell membrane-promising drug target for antifungal therapy. J. Appl. Microbiol. 2016, 121, 1498–1510. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An apoptotic ‘eat me’signal: Phosphatidylserine exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef]
- Vartabedian, V.F.; Savage, P.B.; Teyton, L. The processing and presentation of lipids and glycolipids to the immune system. Immunol. Rev. 2016, 272, 109–119. [Google Scholar] [CrossRef]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef]
- Krumpe, K.; Frumkin, I.; Herzig, Y.; Rimon, N.; Oezbalci, C.; Bruegger, B.; Rapaport, D.; Schuldiner, M. Ergosterol content specifies targeting of tail-anchored proteins to mitochondrial outer membranes. Mol. Biol. Cell 2012, 23, 3927–3935. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Gamarra, S.; Garcia-Effron, G.; Park, S.; Perlin, D.S.; Rao, R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010, 6, e1000939. [Google Scholar] [CrossRef]
- Kumar, M.; Dwivedy, A.K.; Sarma, P.; Dkhar, M.S.; Kayang, H.; Raghuwanshi, R.; Dubey, N.K. Chemically characterised Artemisia nilagirica (Clarke) Pamp. essential oil as a safe plant-based preservative and shelf-life enhancer of millets against fungal and aflatoxin contamination and lipid peroxidation. Plant Biosyst. 2020, 154, 269–276. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or deletion of ergosterol biosynthesis genes alters doubling time, response to stress agents, and drug susceptibility in Saccharomyces cerevisiae. mBio 2018, 9, e01291-18. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Du, S.L.; Sun, H.L.; Liang, Q.W.; Wang, H.H.; Yan, L.M.; Zhang, J.H. Antifungal mechanism of (E)-2-hexenal against Botrytis cinerea growth revealed by transcriptome analysis. Front. Microbiol. 2022, 13, 951751. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | Genes | Primer Sequence (5′-3′) |
|---|---|---|
| CL1729.Contig1_All | FKS1-F | AGGTTGAAGGCAAGCGTACTCT |
| FKS1-R | CAGGAAGTGGCTCAGGAATAGGT | |
| Unigene4828_All | ERG1-F | AAGTCCTACACCTCCAAGGCTAC |
| ERG1-R | GAATATCGGCGTCAGTGAGAACC | |
| Unigene3819_All | ERG7-F | TAACGCATATCCAGGACGACCAA |
| ERG7-R | CGCACAATCTCAATTCGCTCTTC | |
| Unigene3920_All | ERG11-F | CGCCGTAAGGAAGGAAACATTGA |
| ERG11-R | AAGACGAAGTAGCAGCCGAAGT | |
| CL313.Contig2 | Actin1-F | TTACGCCGGTTTCTCCCTCC |
| Actin1-R | GACGATTTCACGCTCGGCAG |
| Concentration (µL/mL) | Inhibitory Rate (%) | ||||
|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | |
| 0.25 | 100.0 ± 0.0 a | 53.7 ± 5.2 b | 24.8 ± 1.7 c | 27.1 ± 3.0 c | 23.2 ± 1.4 c |
| 0.50 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 90.5 ± 1.7 b | 72.9 ± 3.9 b | 64.0 ± 8.7 b |
| 1.00 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| 2.00 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| 4.00 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| Treatments | Incidence Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | |
| Control | 0 ± 0 a | 11 ± 4 a | 22 ± 4 a | 49 ± 4 a | 73 ± 0 a | 89 ± 4 a | 100 ± 0 a |
| 1× MFC trans-2-hexenal | 0 ± 0 a | 0 ± 0 b | 16 ± 4 b | 22 ± 8 b | 60 ± 7 b | 80 ± 0 b | 85 ± 4 b |
| 10× MFC trans-2-hexenal | 0 ± 0 a | 0 ± 0 b | 0 ± 0 c | 9 ± 0 c | 22 ± 8 c | 29 ± 8 b | 33 ± 12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Q.; Shi, S.; Liu, Y.; Yang, Y.; Zhang, Y.; Yuan, X.; Tao, N.; Li, L. Inhibitory Mechanisms of trans-2-Hexenal on the Growth of Geotrichum citri-aurantii. J. Fungi 2023, 9, 930. https://doi.org/10.3390/jof9090930
Ouyang Q, Shi S, Liu Y, Yang Y, Zhang Y, Yuan X, Tao N, Li L. Inhibitory Mechanisms of trans-2-Hexenal on the Growth of Geotrichum citri-aurantii. Journal of Fungi. 2023; 9(9):930. https://doi.org/10.3390/jof9090930
Chicago/Turabian StyleOuyang, Qiuli, Shiwei Shi, Yangmei Liu, Yanqin Yang, Yonghua Zhang, Xingxing Yuan, Nengguo Tao, and Lu Li. 2023. "Inhibitory Mechanisms of trans-2-Hexenal on the Growth of Geotrichum citri-aurantii" Journal of Fungi 9, no. 9: 930. https://doi.org/10.3390/jof9090930




