Identification and Functional Characterization of Alternative Transcripts of LncRNA HNF1A-AS1 and Their Impacts on Cell Growth, Differentiation, Liver Diseases, and in Response to Drug Induction
Abstract
:1. Introduction
2. Results
2.1. Identification of HNF1A-AS1 Alternative Transcripts in Human Livers and Cultured Cells
2.2. Design of Real-Time Quantitative PCR (RT-qPCR) Primers for Quantification of the Identified Transcripts
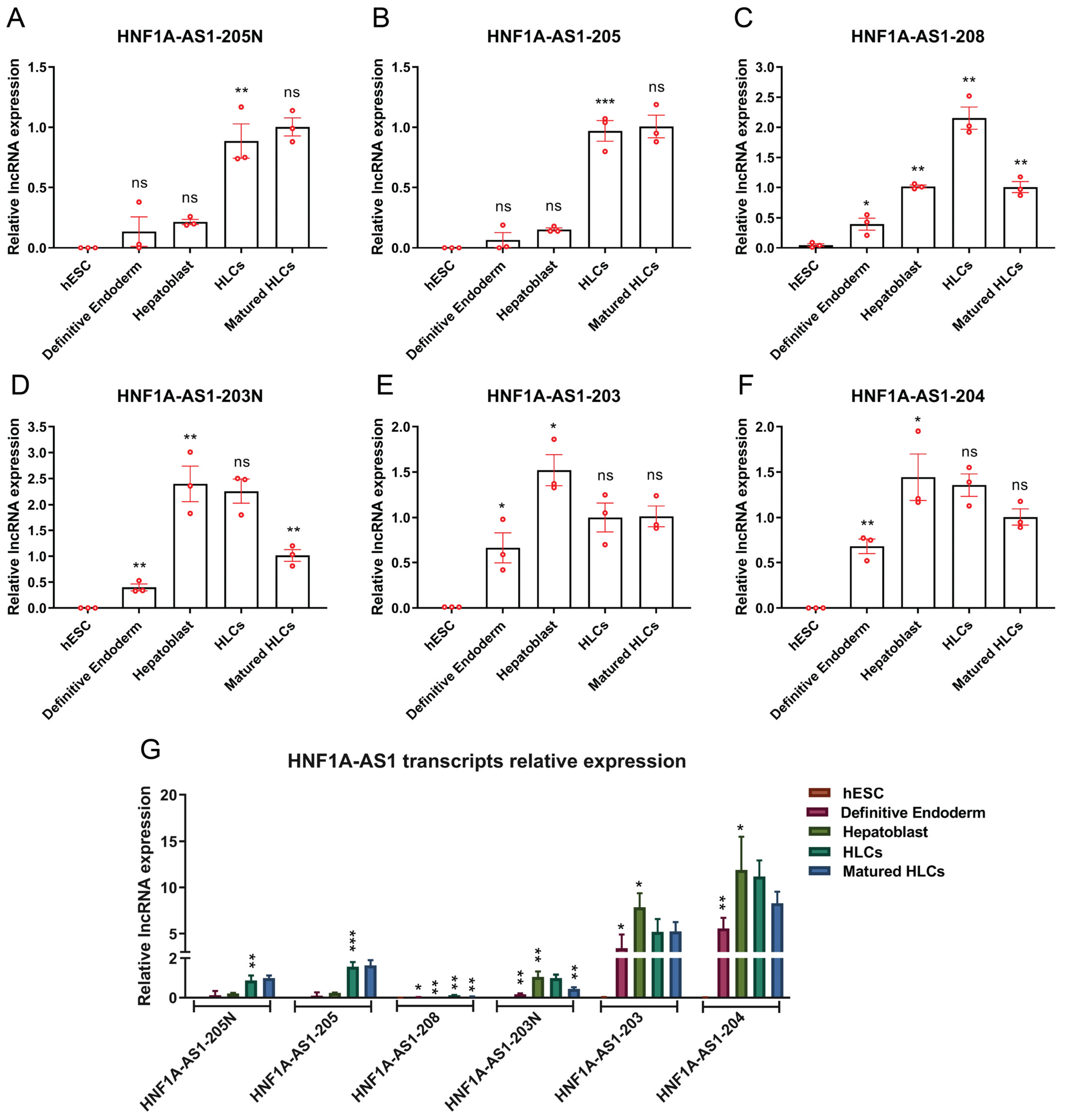
2.3. Expression Patterns of HNF1A-AS1 Transcripts during Stages of Hepatocyte Differentiation from Human Embryonic Stem Cells (hESC)
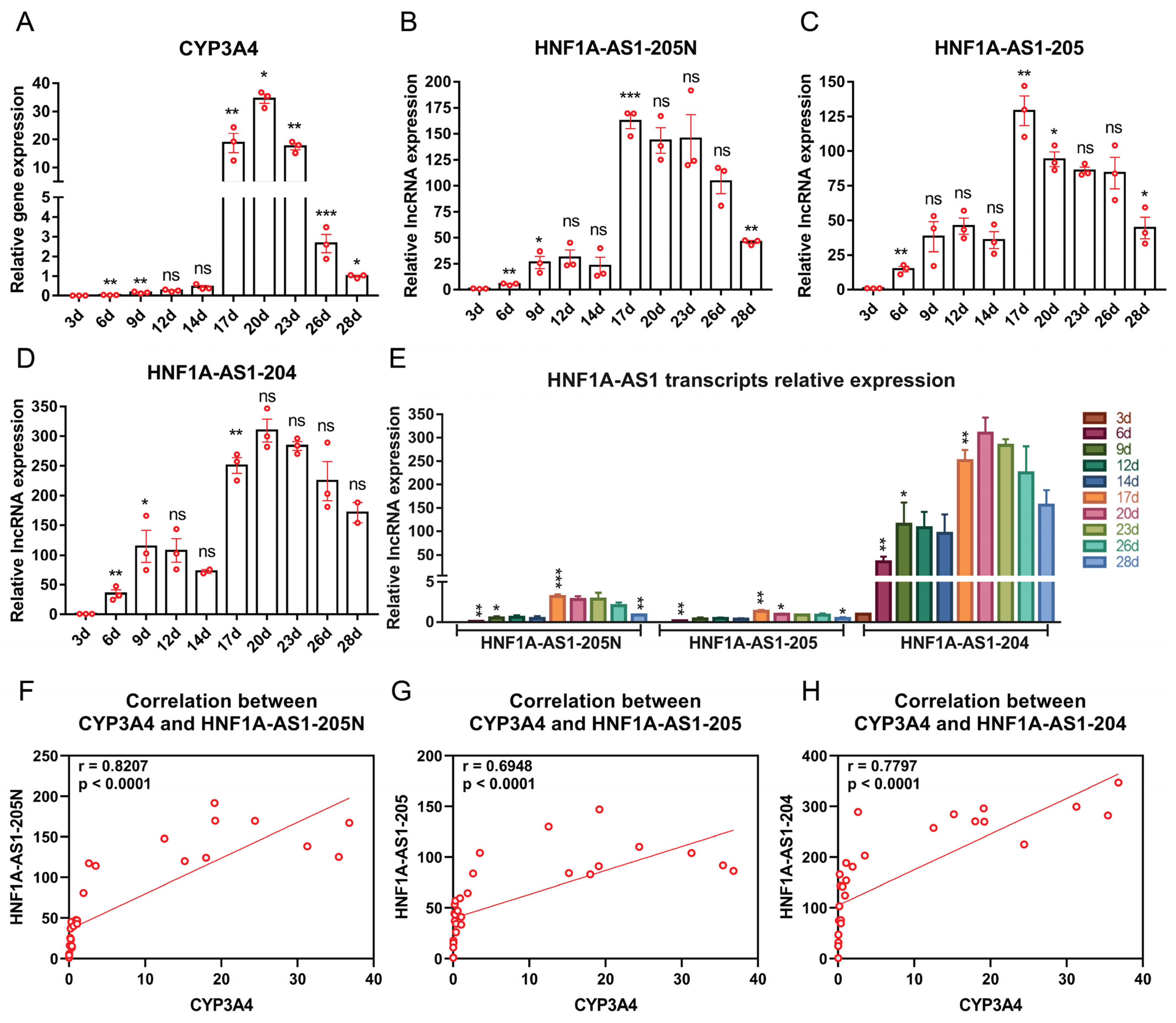
2.4. Expression Patterns of the Identified HNF1A-AS1 Transcripts during HepaRG Cell Growth and Differentiation
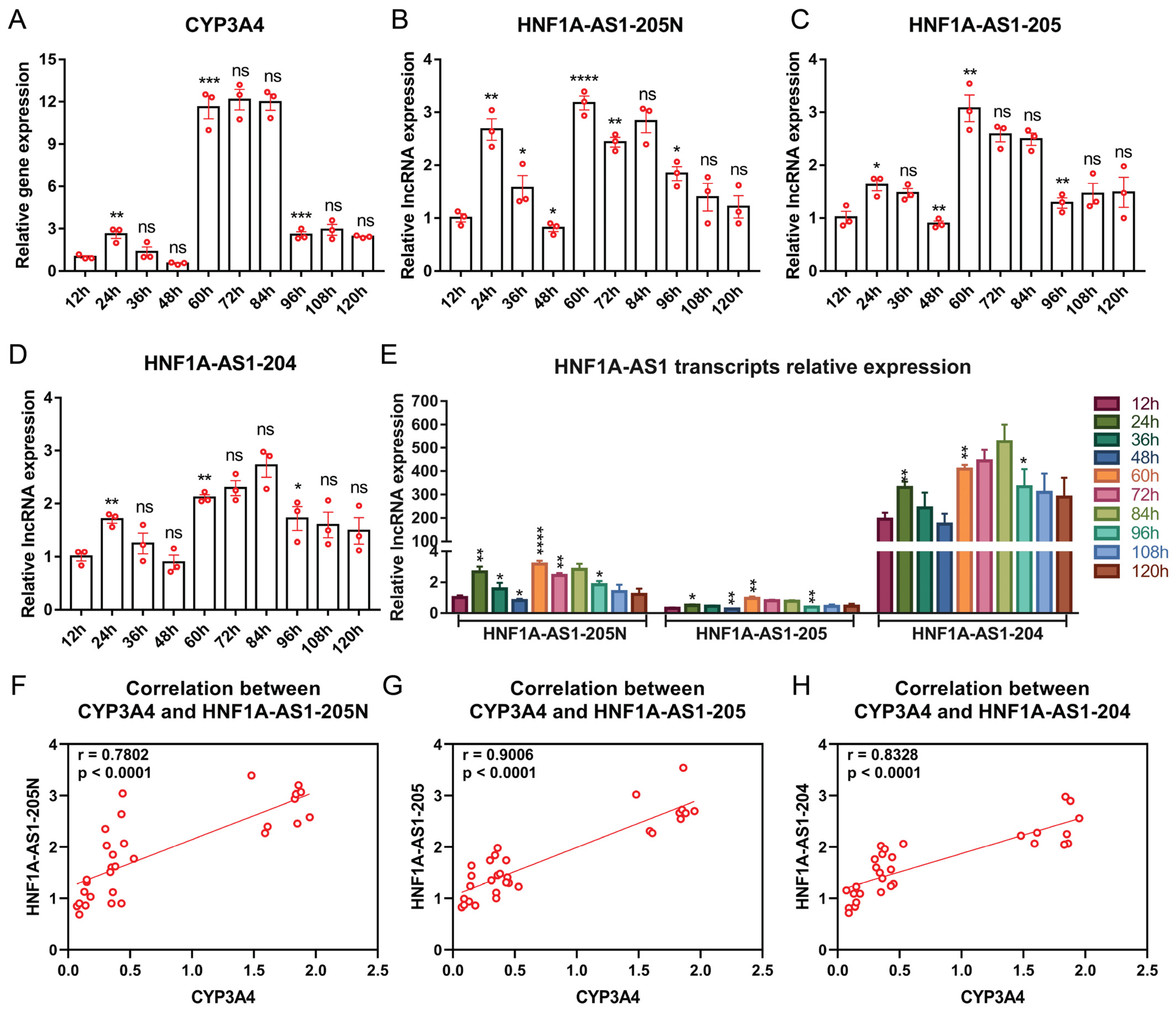
2.5. Expression Patterns of HNF1A-AS1 Transcripts during Rifampicin Treatment
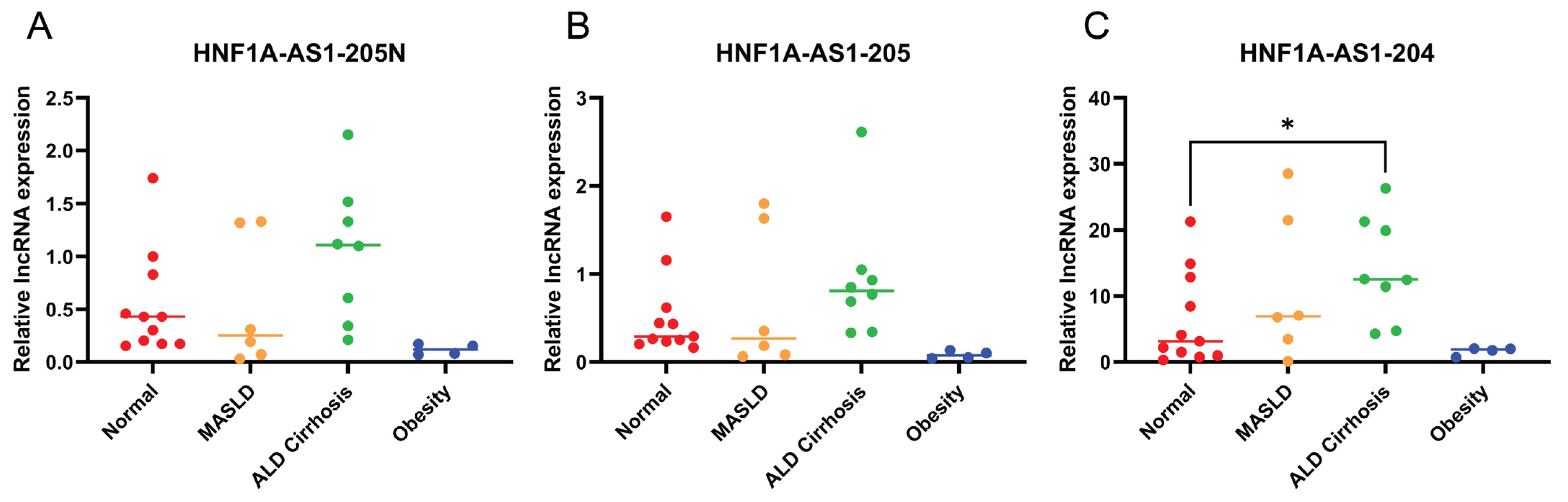
2.6. Expression Patterns of the Identified HNF1A-AS1 Transcripts in Different Liver Disease Conditions
3. Discussion
4. Materials and Methods
4.1. Human Liver Samples Collection
4.2. Differentiation of hESC to Hepatocytes In Vitro
4.3. RNA Extraction and Reverse Transcription
4.4. Primer Design for PCR and Quantitative RT-qPCR
4.5. PCR
4.6. Gel Electrophoresis
4.7. Purification of PCR Products and Sanger Sequence
4.8. RT-qPCR
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Song, J.H.; Cheng, Y.; Wu, W.; Bhagat, T.; Yu, Y.; Abraham, J.M.; Ibrahim, S.; Ravich, W.; Roland, B.C.; et al. Long Non-Coding RNA HNF1A-AS1 Regulates Proliferation and Migration in Oesophageal Adenocarcinoma Cells. Gut 2014, 63, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Shi, X.; Yao, Y.; Yang, W.; Song, Y. The Long Non-Coding RNA HNF1A-AS1 Regulates Proliferation and Metastasis in Lung Adenocarcinoma. Oncotarget 2015, 6, 9160–9172. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Liang, T.; Li, C.-R.; Li, Y.-J.; Jin, S.; Liu, Y. Long Non-Coding RNA HNF1A-AS1 up-Regulation in Non-Small Cell Lung Cancer Correlates to Poor Survival. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4858–4863. [Google Scholar]
- Liu, H.-T.; Liu, S.; Liu, L.; Ma, R.-R.; Gao, P. EGR1-Mediated Transcription of LncRNA-HNF1A-AS1 Promotes Cell-Cycle Progression in Gastric Cancer. Cancer Res. 2018, 78, 5877–5890. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Lin, L.; Ouyang, X.; Zhang, F.; Chen, S.; Wang, B.; Zhang, Z.; Chen, S.; Deng, L.; Wang, W.; et al. LncRNA Expression Profiling in Advanced Resected Gastric Adenocarcinoma Tissues. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, X.; Zhang, A.; Li, C.; Bai, J.; Dong, J. Long Non-Coding RNA HNF1A-AS1 Functioned as an Oncogene and Autophagy Promoter in Hepatocellular Carcinoma through Sponging Hsa-MiR-30b-5p. Biochem. Biophys. Res. Commun. 2016, 473, 1268–1275. [Google Scholar] [CrossRef]
- Wang, C.; Mou, L.; Chai, H.-X.; Wang, F.; Yin, Y.-Z.; Zhang, X.-Y. Long Non-Coding RNA HNF1A-AS1 Promotes Hepatocellular Carcinoma Cell Proliferation by Repressing NKD1 and P21 Expression. Biomed. Pharmacother. 2017, 89, 926–932. [Google Scholar] [CrossRef]
- Fang, C.; Qiu, S.; Sun, F.; Li, W.; Wang, Z.; Yue, B.; Wu, X.; Yan, D. Long Non-Coding RNA HNF1A-AS1 Mediated Repression of MiR-34a/SIRT1/P53 Feedback Loop Promotes the Metastatic Progression of Colon Cancer by Functioning as a Competing Endogenous RNA. Cancer Lett. 2017, 410, 50–62. [Google Scholar] [CrossRef]
- Zhu, W.; Zhuang, P.; Song, W.; Duan, S.; Xu, Q.; Peng, M.; Zhou, J. Knockdown of LncRNA HNF1A-AS1 Inhibits Oncogenic Phenotypes in Colorectal Carcinoma. Mol. Med. Rep. 2017, 16, 4694–4700. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Q.; Xie, M.; Feng, Y.; Ma, S.; Yi, C.; Wang, Z.; Li, Y.; Liu, X.; Liu, H.; et al. Aberrant Methylation-mediated Decrease of LncRNA HNF1A-AS1 Contributes to Malignant Progression of Laryngeal Squamous Cell Carcinoma via EMT. Oncol. Rep. 2020, 44, 2503–2516. [Google Scholar] [CrossRef]
- Xu, J.; Zou, J.; Wu, L.; Lu, W. Transcriptome Analysis Uncovers the Diagnostic Value of MiR-192-5p/HNF1A-AS1/VIL1 Panel in Cervical Adenocarcinoma. Sci. Rep. 2020, 10, 16584. [Google Scholar] [CrossRef]
- Chen, L.; Bao, Y.; Piekos, S.C.; Zhu, K.; Zhang, L.; Zhong, X.-B. A Transcriptional Regulatory Network Containing Nuclear Receptors and Long Noncoding RNAs Controls Basal and Drug-Induced Expression of Cytochrome P450s in HepaRG Cells. Mol. Pharmacol. 2018, 94, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, L.; Liu, J.; Chen, S.; Liu, G.; Nie, Y.; Wang, P.; Yang, W.; Chen, L.; Zhong, X.; et al. The HNF1 α-Regulated LncRNA HNF1 α-AS1 Is Involved in the Regulation of Cytochrome P450 Expression in Human Liver Tissues and Huh7 Cells. J. Pharmacol. Exp. Ther. 2019, 368, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, P.; Manautou, J.E.; Zhong, X. Knockdown of Long Noncoding RNAs Hepatocyte Nuclear Factor 1 α Antisense RNA 1 and Hepatocyte Nuclear Factor 4 α Antisense RNA 1 Alters Susceptibility of Acetaminophen-Induced Cytotoxicity in HepaRG Cells. Mol. Pharmacol. 2020, 97, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Wang, P.; Yang, K.; Wang, Y.; Yan, L.; Zhong, X.; Zhang, L. Long Noncoding RNAs Hepatocyte Nuclear Factor 4A Antisense RNA 1 and Hepatocyte Nuclear Factor 1A Antisense RNA 1 Are Involved in Ritonavir-Induced Cytotoxicity in Hepatoma Cells. Drug Metab. Dispos. 2022, 50, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Wang, Q.; Chen, S.; Wang, X.; Zhong, X.; Hu, W.; Thorne, R.F.; Han, S.; Wu, M.; et al. The Long Noncoding RNA HNF1A-AS1 with Dual Functions in the Regulation of Cytochrome P450 3A4. Biochem. Pharmacol. 2024, 220, 116016. [Google Scholar] [CrossRef] [PubMed]
- Chretien, S.; Dubart, A.; Beaupain, D.; Raich, N.; Grandchamp, B.; Rosa, J.; Goossens, M.; Romeo, P.H. Alternative Transcription and Splicing of the Human Porphobilinogen Deaminase Gene Result Either in Tissue-Specific or in Housekeeping Expression. Proc. Natl. Acad. Sci. USA 1988, 85, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Smith, C.W.J.; Jiggins, C.D. Alternative Splicing as a Source of Phenotypic Diversity. Nat. Rev. Genet. 2022, 23, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Gupta, R.; Davuluri, R.V. Alternative Transcription and Alternative Splicing in Cancer. Pharmacol. Ther. 2012, 136, 283–294. [Google Scholar] [CrossRef]
- de Klerk, E.; ’t Hoen, P.A.C. Alternative MRNA Transcription, Processing, and Translation: Insights from RNA Sequencing. Trends Genet. 2015, 31, 128–139. [Google Scholar] [CrossRef]
- Shabalina, S.A.; Ogurtsov, A.Y.; Spiridonov, N.A.; Koonin, E.V. Evolution at Protein Ends: Major Contribution of Alternative Transcription Initiation and Termination to the Transcriptome and Proteome Diversity in Mammals. Nucleic Acids Res. 2014, 42, 7132–7144. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep Surveying of Alternative Splicing Complexity in the Human Transcriptome by High-Throughput Sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative Isoform Regulation in Human Tissue Transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Wang, X.; Hou, J.; Quedenau, C.; Chen, W. Pervasive Isoform-specific Translational Regulation via Alternative Transcription Start Sites in Mammals. Mol. Syst. Biol. 2016, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Naftelberg, S.; Schor, I.E.; Ast, G.; Kornblihtt, A.R. Regulation of Alternative Splicing through Coupling with Transcription and Chromatin Structure. Annu. Rev. Biochem. 2015, 84, 165–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Park, J.K.; Zhang, J. Evidence That Alternative Transcriptional Initiation Is Largely Nonadaptive. PLoS Biol. 2019, 17, e3000197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Huang, B.; Xu, Y.-M.; Li, J.; Huang, L.-F.; Lin, J.; Zhang, J.; Min, Q.-H.; Yang, W.-M.; et al. Mechanism of Alternative Splicing and Its Regulation. Biomed. Rep. 2015, 3, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative Splicing and Cancer: A Systematic Review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative Splicing as a Regulator of Development and Tissue Identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437–451. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Zhang, W.; Chen, D.; Wang, Y. Aberrant Alternative Splicing in Breast Cancer. J. Mol. Cell Biol. 2019, 11, 920–929. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.; Lee, J.; Park, D.; Kim, Y.J.; Park, W.Y.; Hong, D.; Park, P.J.; Lee, E. Intron Retention Is a Widespread Mechanism of Tumor-Suppressor Inactivation. Nat. Genet. 2015, 47, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Dvinge, H.; Bradley, R.K. Widespread Intron Retention Diversifies Most Cancer Transcriptomes. Genome Med. 2015, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tovar-Corona, J.M.; Urrutia, A.O. Increased Levels of Noisy Splicing in Cancers, but Not for Oncogene-Derived Transcripts. Hum. Mol. Genet. 2011, 20, 4422–4429. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Lin, C.; Jia, X.; Zhu, H.; Song, J.; Zhang, Y. Noncoding RNAs Regulate Alternative Splicing in Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Liu, X.N.; Wang, T.T.; Pan, W.; Tao, Q.F.; Zhou, W.P.; Wang, F.; Sun, S.H. The MBNL3 Splicing Factor Promotes Hepatocellular Carcinoma by Increasing PXN Expression through the Alternative Splicing of LncRNA-PXN-AS1. Nat. Cell Biol. 2017, 19, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhou, H.; Liu, P.; Yan, L.; Yao, W.; Chen, K.; Zeng, J.; Li, H.; Hu, J.; Xu, H.; et al. LncRNA PVT1 and Its Splicing Variant Function as Competing Endogenous RNA to Regulate Clear Cell Renal Cell Carcinoma Progression. Oncotarget 2017, 8, 85353. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, Y.; Liu, Y.; Ye, F.; Song, Z.; Qin, H.; Meng, S.; Chen, Y.; Zhou, R.; Song, X.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Hepatic Cells. Hepatology 2007, 45, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Krueger, W.H.; Tanasijevic, B.; Barber, V.; Flamier, A.; Gu, X.; Manautou, J.; Rasmussen, T.P. Cholesterol-Secreting and Statin-Responsive Hepatocytes from Human ES and IPS Cells to Model Hepatic Involvement in Cardiovascular Health. PLoS ONE 2013, 8, e67296. [Google Scholar] [CrossRef]
- Young, C.K.J.; Young, M.J. Comparison of HepaRG Cells Following Growth in Proliferative and Differentiated Culture Conditions Reveals Distinct Bioenergetic Profiles. Cell Cycle 2019, 18, 476–499. [Google Scholar] [CrossRef]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and Induction of CYP Enzymes in Humans: An Update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Chen, J.; Raymond, K. Roles of Rifampicin in Drug-Drug Interactions: Underlying Molecular Mechanisms Involving the Nuclear Pregnane X Receptor. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, F.; Tan, F.; Tang, L.; Du, Z.; Mou, J.; Zhou, G.; Yuan, C. HNF1A-AS1: A Tumor-Associated Long Non-Coding RNA. Curr. Pharm. Des. 2022, 28, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Luo, J.; Liu, C.; Zhu, L. Regulatory Mechanisms and Potential Medical Applications of HNF1A-AS1 in Cancers. Am. J. Transl. Res. 2022, 14, 4154–4168. [Google Scholar] [PubMed]
- Zhan, Y.; Li, Y.; Guan, B.; Wang, Z.; Peng, D.; Chen, Z.; He, A.; He, S.; Gong, Y.; Li, X.; et al. Long Non-Coding RNA HNF1A-AS1 Promotes Proliferation and Suppresses Apoptosis of Bladder Cancer Cells through Upregulating Bcl-2. Oncotarget 2017, 8, 76656–76665. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.F.; Yu, L.; Jiao, Y. Sh-HNF1A-AS1 Reduces the Epithelial-Mesenchymal Transition and Stemness of Esophageal Cancer Cells. Neoplasma 2022, 69, 560–570. [Google Scholar] [CrossRef]


| Transcript ID * | Name | Length (bp) | Exon Number |
|---|---|---|---|
| ENST00000619441.1 | HNF1A-AS1-205 | 343 | 2 |
| ENST00000647473.1 | HNF1A-AS1-207 | 1144 | 5 |
| ENST00000701967.1 | HNF1A-AS1-209 | 357 | 2 |
| ENST00000701238.1 | HNF1A-AS1-208 | 582 | 2 |
| ENST00000646404.1 | HNF1A-AS1-206 | 557 | 3 |
| ENST00000537361.1 | HNF1A-AS1-203 | 659 | 2 |
| ENST00000539163.1 | HNF1A-AS1-204 | 2455 | 1 |
| ENST00000433033.3 | HNF1A-AS1-201 | 718 | 4 |
| ENST00000535301.2 | HNF1A-AS1-202 | 546 | 2 |
| Transcript | Primer (Forward) 5′–3′ | Primer (Reverse) 5′–3′ | Amplicon Size (bp) |
|---|---|---|---|
| HNF1A-AS1-205 | AGGAAGCACTTTGACCTCTG * | CCTACCCCACAGAGTCTGAT | 300 |
| HNF1A-AS1-207 | ATCCCACCAAAGGGGGCT | TCACAGAAAAGAATCTGTTC | 731 |
| HNF1A-AS1-209 | ATACAGGAAAGGGGAGCAGC ** | TCCACATCAGGTCCCATG *** | 220 |
| HNF1A-AS1-208 | AGGAAGCACTTTGACCTCTG * | TCCACATCAGGTCCCATG *** | 280 |
| HNF1A-AS1-206 | ATACAGGAAAGGGGAGCAGC ** | TCCACATCAGGTCCCATG *** | 387 |
| HNF1A-AS1-203 | AGGAAGCACTTTGACCTCTG * | TGTTACAAGGTTCAGGGCTC | 477 |
| HNF1A-AS1-204 | AGTTCCCTCCATCTAACATTCA | TTGTCTGGACTGAAGGACAA | 321 |
| HNF1A-AS1-201 | GCCTGTGGCCATACAGGA | GACAGGAGCAAAACTGCT | 501 |
| HNF1A-AS1-202 | AGGAAGCACTTTGACCTCTG * | ACAGAAGGAGACCCTGTC | 298 |
| Transcript | Primer (Forward) 5′–3′ | Primer (Reverse) 5′–3′ | Amplicon Size (bp) |
|---|---|---|---|
| HNF1A-AS1-205N | ATGCTGTTTGGGCTGGTC | TGGGATTGGGTTCCTTTG | 138 |
| HNF1A-AS1-205 | CACTCAGCCAGGATGAGG | TTTCCCTACCCCACAGAG | 207 |
| HNF1A-AS1-208 | AGGAAGCACTTTGACCTCTG | TCCACATCAGGTCCCATG | 280 |
| HNF1A-AS1-203N | TAAACACTAGCCAACACCC | AGCAGCAGCTTGACAAAT | 104 |
| HNF1A-AS1-203 | ACTCAGCCAGCTGCTCCCTCTA | GCTGTGGGTGGCAGAAGAGGAC | 109 |
| HNF1A-AS1-204 | AGTTCCCTCCATCTAACATTCA | TTGTCTGGACTGAAGGACAA | 321 |
| CYP3A4 | GATTGACTCTCAGAATTCAAAAGAAACTGA | GGTGAGTGGCCAGTTCATACATAATG | 148 |
| β-actin | GGACTTCGAGCAAGAGATGG | AGCACTGTGTTGGCGTACAG | 234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Nguyen, L.T.G.; Wassef, A.; Sadek, R.; Schmitt, T.M.; Guo, G.L.; Rasmussen, T.P.; Zhong, X.-b. Identification and Functional Characterization of Alternative Transcripts of LncRNA HNF1A-AS1 and Their Impacts on Cell Growth, Differentiation, Liver Diseases, and in Response to Drug Induction. Non-Coding RNA 2024, 10, 28. https://doi.org/10.3390/ncrna10020028
Jin J, Nguyen LTG, Wassef A, Sadek R, Schmitt TM, Guo GL, Rasmussen TP, Zhong X-b. Identification and Functional Characterization of Alternative Transcripts of LncRNA HNF1A-AS1 and Their Impacts on Cell Growth, Differentiation, Liver Diseases, and in Response to Drug Induction. Non-Coding RNA. 2024; 10(2):28. https://doi.org/10.3390/ncrna10020028
Chicago/Turabian StyleJin, Jing, Le Tra Giang Nguyen, Andrew Wassef, Ragui Sadek, Timothy M. Schmitt, Grace L. Guo, Theodore P. Rasmussen, and Xiao-bo Zhong. 2024. "Identification and Functional Characterization of Alternative Transcripts of LncRNA HNF1A-AS1 and Their Impacts on Cell Growth, Differentiation, Liver Diseases, and in Response to Drug Induction" Non-Coding RNA 10, no. 2: 28. https://doi.org/10.3390/ncrna10020028








