Abstract
Water contamination is a pervasive global crisis, affecting over 2 billion people worldwide, with pharmaceutical contaminants emerging as a significant concern due to their persistence and mobility in aquatic ecosystems. This review explores the potential of activated hydrochars, sustainable materials produced through biomass pyrolysis, to revolutionize the removal of pharmaceutical contaminants from water sources. These materials possess high surface area, porous structure, and exceptional adsorption capabilities, making them a promising solution. The impact of pharmaceutical contaminants on aquatic ecosystems and human health is far-reaching, affecting biodiversity, water quality, and public health. To address this complex issue, a diverse range of techniques, including adsorption, biodegradation, and advanced oxidation processes, are employed in the pharmaceutical industry. Activated hydrochars offer substantial adsorption capacity, sustainable feedstock origins, and a minimal carbon footprint. This review highlights their potential in pharmaceutical contaminant removal and their broader applications in improving soil and air quality, resource recovery, and sustainable waste management. Interdisciplinary collaboration and the development of intelligent treatment systems are essential to fully unlock the potential of activated hydrochars. Regulatory support and policy frameworks will facilitate their responsible and widespread application, promising a cleaner and more sustainable future. This paper aims to inform scientists, environmental experts, policymakers, and industry stakeholders about the promising role of activated hydrochars in addressing pharmaceutical contaminant challenges.
1. Introduction
Water contamination is a global environmental crisis that affects over 2 billion people worldwide [1]. This pervasive issue is primarily driven by pollutants stemming from various sources, such as sewage and leachate laden with pathogens, giving rise to profound concerns regarding public health and environmental well-being. Exposure to contaminants in water sources poses a severe threat, leading to waterborne diseases and long-term health issues [2]. Among the diverse array of water pollutants, pharmaceutical contaminants have emerged as a major concern, encompassing prescription and over-the-counter medications, personal care products, hormones, antibiotics, cytostatic drugs, antipyretics and analgesics, beta blockers, psychotropic medications, nonsteroidal anti-inflammatory drugs (NSAIDs), X-ray contrast media, herbicides, pesticides, and veterinary medications [3,4,5]. These pharmaceutical contaminants find their way into aquatic environments through various pathways, including human and animal wastewater, agricultural runoff, and industrial effluents [6].
What sets pharmaceutical contaminants apart is their exceptional persistence and mobility in aquatic ecosystems. They traverse substantial distances, affecting surface water, groundwater, and even potable water supplies, necessitating urgent measures to control and mitigate their presence. Effective disposal, advanced wastewater treatment, and rigorous monitoring are essential to tackle this multifaceted issue.
The escalated usage of pharmaceuticals, coupled with advances in detection methods and increased awareness of the associated risks, underlines the gravity of pharmaceutical contaminants as a distinct challenge. These contaminants are introduced into water bodies via numerous routes, leading to their prolonged presence and substantial threats to both public health and the environment. As such, effective disposal measures, advanced wastewater treatment, and rigorous monitoring are indispensable in addressing this complex problem.
The impact of pharmaceutical contaminants is far-reaching, affecting aquatic ecosystems and human populations alike. They disrupt aquatic life, causing population declines and accumulating in organisms, which can result in biomagnification and altered growth and development [7,8,9]. Furthermore, the presence of antibiotic-resistant bacteria in aquatic environments contributes to antibiotic resistance, while toxicological effects lead to increased mortality rates and decreased biodiversity [10,11]. Algal blooms triggered by pharmaceutical contaminants further disrupt aquatic ecosystems and produce harmful toxins. For humans, the risks include drinking water contamination, chronic exposure, direct health effects, and potential drug interactions [12,13]. The concept of “One Health” acknowledges the interconnectedness of human, animal, and environmental health, underscoring the necessity of water treatment and monitoring to mitigate these risks [14].
Efforts to address this multifaceted issue are centered on improving wastewater treatment, reducing pharmaceutical waste, and implementing regulatory measures. Pharmaceutical contaminant remediation plays a pivotal role in ensuring the safety and quality of pharmaceutical products. A diverse range of techniques, such as filtration, chromatography, distillation, crystallization, extraction, adsorption, membrane filtration, biodegradation, advanced oxidation processes, chemical precipitation, enzyme-based remediation, complexation, chelation, pH adjustment, advanced data analysis, and quality by design, are employed [15,16]. Each technique serves a unique purpose, from eliminating particulate pollutants through filtration to separating impurities by size using chromatography. The removal of volatile impurities is achieved through distillation, while crystallization isolates impurities from the target compound. Adsorption relies on specific adsorbents, whereas membrane filtration utilizes characteristics such as size, charge, and molecular weight to filter out impurities. Biodegradation addresses organic pollutants, while advanced oxidation processes generate reactive radicals for degradation [17]. Chemical precipitation leads to the formation of insoluble precipitates containing contaminants, while enzyme-based remediation catalyzes breakdown or modification. Adjusting the pH can prevent the precipitation of pollutants. Typically, a combination of these techniques is employed to ensure effective contaminant remediation within the pharmaceutical industry.
In this context, hydrochar, a sustainable and carbon-rich material produced through the pyrolysis of biomass, and its use emerges as a promising and environmentally friendly approach to address the complex issue of pharmaceutical contaminant removal. Hydrochar’s unique properties, such as its high surface area, porous structure, and adsorption capabilities, make it a compelling candidate for the remediation of pharmaceutical-contaminated water sources.
The overarching objective of this review paper is to inform and educate the scientific community, environmental experts, policymakers, and industry stakeholders about the promising approach of using hydrochars to mitigate the challenges posed by pharmaceutical contaminants in water sources. As we conclude the introduction, it is crucial to contextualize this review within the broader landscape of existing literature on pharmaceutical contaminants in water. While numerous review papers have addressed the removal of emerging contaminants, including pharmaceuticals, through adsorption methods, they often provide a generalized overview without a specific focus on hydrochar [18,19,20,21]. It is noteworthy that existing works typically discuss the broader category of adsorbents rather than exclusively delving into the unique attributes of hydrochar for pharmaceutical contaminant removal. In this vein, our review distinguishes itself by offering an exclusive exploration of hydrochar as a specialized adsorbent for the removal of pharmaceutical contaminants. By narrowing the focus to hydrochar, we aim to provide a comprehensive and in-depth analysis of its distinct properties—such as high surface area and exceptional adsorption capabilities—that render it a promising and environmentally friendly solution for addressing the complex challenges posed by pharmaceutical contaminants in water sources. This deliberate focus serves to contribute novel insights to the existing body of literature and positions our review as a specialized and valuable resource for those interested in the application of hydrochar in pharmaceutical contaminant remediation.
2. Methodology
The investigation was performed throughout the years by focusing on hydrochar and pharmaceutical contaminants and involved a systematic search on Scopus databases utilizing the combined keywords “hydrochar” and “pharmaceutical contaminants” on a single day, specifically 2 December 2023, to minimize potential fluctuations. The initial search yielded a solitary paper associated with the specified keywords. Subsequent modifications to the search, incorporating the keywords “biochar” and “pharmaceutical contaminants”, revealed 12 relevant papers. Further exploration was conducted on the ScienceDirect database using the combined keywords “hydrochar” and “pharmaceutical contaminants”, yielding 571 results. Subsequently, a meticulous filtering process was implemented, resulting in the exclusion of 123 articles deemed irrelevant or unrelated. This left a total of 136 articles. Among these, 39 were initially categorized as review papers, and their inclusion was subtracted, culminating in the identification of 84 research papers. Throughout the manuscript preparation, the synthesis of information drew not only from these 84 selected research papers but also from additional pertinent content obtained from Google Scholar.
3. The Emergence of Hydrochar as a Sustainable Adsorbent
Hydrochar, a carbonaceous material, is derived through the process of hydrothermal carbonization (HTC) applied to biomass feedstocks [22]. HTC is a thermochemical procedure that converts biomass into a carbon-rich substance under conditions of 180–250 °C and 2–20 MPa pressure in the presence of water [23]. Hydrochar possesses a suite of attributes that render it a promising and sustainable adsorbent for the removal of various water and wastewater pollutants. These attributes encompass a high surface area, a porous structure, a diverse array of surface functional groups, hydrophobic characteristics, and chemical and mechanical stability. Notably, hydrochar has demonstrated efficacy in the removal of a wide array of pollutants from water and wastewater, including heavy metals, organic contaminants, pharmaceutical residues, dyes, and nutrients [24,25].
Hydrochar generally exhibits non-toxicity and hydrophobicity. The characteristics of hydrochar, including surface chemistry, porosity, particle size, and specific surface area, are contingent upon the temperature and reaction duration applied during hydrothermal carbonization (HTC). The hydrochar surface is typically endowed with numerous oxygen-containing functional groups that manifest favorable adsorption affinities toward both polar and non-polar functional groups, thereby resulting in elevated adsorption capacity. It is noteworthy, however, that such advantageous properties may be compromised during gas-phase activation aimed at augmenting specific surface area. Consequently, judicious selection of processing conditions is imperative to preserve the desired attributes of hydrochar [26].
The hydrochar synthesis process occurs in an aqueous environment, typically employing a stainless steel autoclave loaded with biomass and a specified quantity of water (typically within the range of 1:3 to 1:10 ratios of biomass to water). In comparison to biochar, hydrochar exhibits a slightly acidic nature attributed to a higher presence of oxygenated functional groups. Pyrolysis-induced loss of carboxyl and hydroxyl groups renders biochar alkaline, with alkalinity influenced by inorganic and metal compounds like Ca and Mg. Hydrothermal carbonization (HTC) results in the removal of some inorganic components in the aqueous medium, contributing to the acidic pH of hydrochar [27].
Due to the lower temperature of the HTC process, carbon conversion is reduced compared to pyrolysis, yielding higher atomic ratios of H/C and O/C in hydrochar. Consequently, hydrochar demonstrates elevated atomic ratios of hydrogen to carbon and oxygen to carbon in contrast to biochar [23]. The increased hydrogen content in hydrochar, known for its involvement in polar interactions, may enhance its adsorption capacity for pharmaceutical compounds exhibiting polar or hydrogen-bonding functionalities. Additionally, the oxygen-containing functional groups in hydrochar can engage in various chemical interactions, including hydrogen bonding and Lewis acid–base interactions, potentially influencing the adsorption of pharmaceutical compounds with oxygen-binding sites or those susceptible to such interactions.
Hydrochar’s selectivity for pharmaceutical compounds over other organics in waters and wastewaters can be attributed to its porous structure, chemical functional groups, surface chemistry, electrostatic interactions, aromaticity, and the potential for specific affinity. The porous nature of hydrochar provides an effective medium for adsorption, while its surface features and chemical composition may favor interactions with pharmaceutical molecules. The presence of aromatic structures in hydrochar aligns with the aromatic rings often found in pharmaceutical compounds. Additionally, tailored modifications to the hydrochar surface can enhance its selectivity for pharmaceuticals. These combined factors contribute to the effectiveness of hydrochar as a selective adsorbent for pharmaceutical compounds in water treatment applications.
The sustainability of hydrochar as an adsorbent arises from its ability to be derived from a variety of biomass feedstocks, such as agricultural residues, forestry byproducts, and municipal solid waste [28,29]. Furthermore, the production of hydrochar can be integrated into other bioenergy processes like anaerobic digestion and biodiesel production [30]. Importantly, hydrochar can be regenerated and reused multiple times, reducing the demand for new adsorbent materials. The emergence of hydrochar as a sustainable adsorbent represents a promising development in the realm of water and wastewater treatment and stands as a cost-effective and environmentally friendly alternative to traditional adsorbent materials, including activated carbon.
Noteworthy examples of hydrochar’s use as a sustainable adsorbent in water and wastewater treatment include its effectiveness in arsenic removal from groundwater [31], elimination of organic pollutants (e.g., pesticides and herbicides) from agricultural runoff, the extraction of pharmaceutical contaminants from wastewater [26], removal of dyes from industrial wastewater [32], and nutrient retention (e.g., phosphorus and nitrogen) from wastewater.
Hydrochar, while still a relatively recent innovation, holds significant potential for revolutionizing the sustainable treatment of water and wastewater. Its emergence as a sustainable adsorbent represents a substantial advancement in the fields of environmental science and water treatment [28]. Hydrochar, originating from the hydrothermal carbonization of organic feedstocks like agricultural residues, sewage sludge, and organic waste, brings several salient advantages to the forefront: the inherently sustainable nature of hydrochar production, wherein it employs organic waste materials that would otherwise be discarded or landfilled, consequently reducing waste while adding value to these materials; the ability to derive hydrochar from a wide spectrum of renewable feedstocks, rendering it versatile and adaptable to diverse regional contexts and less reliant on fossil-based adsorbents; and the carbon-sequestering attributes of the production process, contributing to climate change mitigation by converting organic carbon into a stable, long-lasting form [33]. The tunable properties of hydrochar, such as surface area and functional groups, during its production enable optimization for specific contaminant adsorption. Moreover, the environmental impact of hydrochar production in terms of energy consumption and emissions tends to be lower than some traditional adsorbents. The broad effectiveness of hydrochar in adsorbing a wide variety of contaminants, including heavy metals, organic pollutants, and pharmaceuticals, makes it suitable for diverse applications in water treatment, soil remediation, and beyond. The regenerability and reusability of hydrochar further enhance its economic and environmental efficiency. Ongoing research continues to refine synthesis methods and enhance the adsorption properties of hydrochar, opening up new possibilities for its application.
The rise of hydrochar as a sustainable adsorbent aligns with global objectives of waste reduction, climate change mitigation, and ensuring access to clean water resources. Its potential to contribute to environmental sustainability and remediation makes it a promising material for addressing challenges related to water and soil contamination.
Hydrochars exhibit several unique properties that render them suitable for a multitude of applications. These properties include a typical surface area ranging from 100 to 500 m2/g, which makes them effective adsorbents for a broad spectrum of pollutants. Their porous structure allows for the adsorption of a wide range of contaminants, spanning both organic and inorganic compounds. Hydrochars feature various surface functional groups, such as hydroxyl, carboxyl, and carbonyl groups, which can be tailored to suit specific applications. Their inherent hydrophobicity, repelling water, proves advantageous in applications where water contact is undesirable, such as water filtration and soil improvement [34]. Furthermore, hydrochars demonstrate both chemical and mechanical stability, rendering them durable and reusable materials [23].
The properties of hydrochars can be influenced by several factors, including the type of biomass feedstock utilized in their production, with woody biomass yielding hydrochars with higher surface area and porosity compared to non-woody-biomass-derived hydrochars [35]. Additionally, the HTC process parameters, such as temperature, pressure, and reaction time, can impact the properties of hydrochars; for instance, higher temperatures during production tend to yield hydrochars with lower surface area and porosity than those produced at lower temperatures [36].
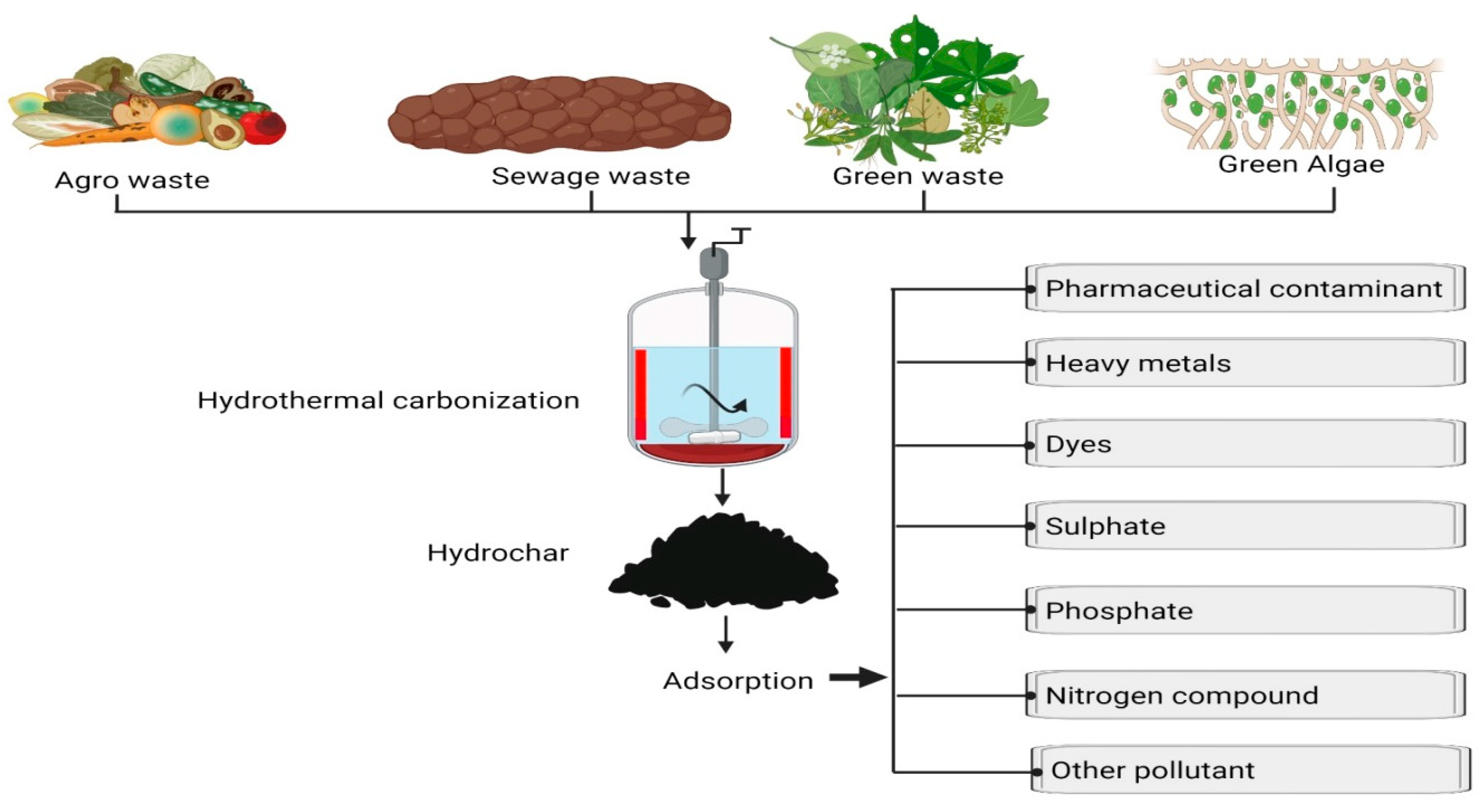
In sum, hydrochars hold promise as materials with diverse potential applications due to their unique attributes, encompassing high surface area, porous structure, diverse surface functional groups, hydrophobicity, and chemical and mechanical stability. These characteristics make them amenable to applications in water and wastewater treatment, soil improvement, energy storage, and the development of value-added products. Figure 1 provides an illustration of the preparation of waste biomass hydrochar and its various potential applications.
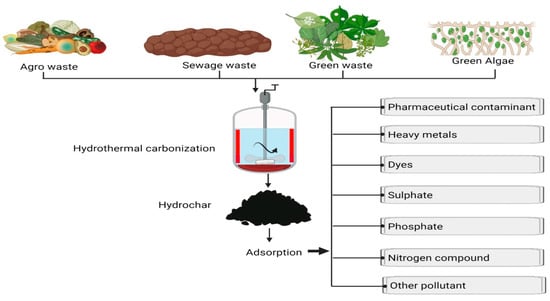
Figure 1.
Waste biomass hydrochar production—preparation techniques and multifaceted applications for sustainable resource utilization.
3.1. Production Methods for Hydrochars
Hydrochars are generated through the hydrothermal carbonization (HTC) process, which entails subjecting organic feedstocks to controlled conditions involving elevated temperature and pressure while in the presence of water [36,37]. The specific methodologies employed in hydrochar production may vary contingent on the chosen feedstock and the targeted properties of the resultant hydrochar. The primary steps encompassed in hydrochar production are as follows:
3.1.1. Feedstock Selection
The initial phase involves the selection of an appropriate feedstock. Organic materials such as agricultural residues (e.g., crop residues, wood), sewage sludge, algae, or organic waste are commonly employed. The selected feedstock is often subjected to pre-processing measures to enhance uniformity and its suitability for hydrothermal carbonization. This pre-processing can encompass actions such as shredding, drying, or size reduction to yield a more homogenous material.
3.1.2. Hydrothermal Reactor Utilization
The prepared feedstock is loaded into a specialized hydrothermal reactor, designed to endure elevated temperatures and pressures. The reactor is meticulously sealed to prevent gas escape. Water is introduced into the reactor to create a saturated or supercritical water environment. The temperature is then elevated, typically within the range of 180–250 °C, with a simultaneous increase in pressure, often ranging from 10 to 50 bar. The feedstock is subjected to hydrothermal treatment for a designated duration, usually spanning from several hours to a day or more. This process involves the rupture of chemical bonds within the organic materials, the polymerization of carbon compounds, and the eventual formation of hydrochar.
3.1.3. Cooling and Depressurization
Subsequent to the hydrothermal treatment, the reactor is meticulously cooled and depressurized, facilitating the safe removal of the formed hydrochar [37,38].
3.1.4. Collection and Post-Treatment
The resultant hydrochar, manifesting as a solid carbonaceous product, is gathered and separated from the aqueous phase. It may subsequently undergo a washing process to eliminate water-soluble impurities and is often subjected to drying to reduce moisture content. Depending on the aspired properties and the intended applications of the hydrochar, post-treatment processes may be administered. These may encompass activation, physical or chemical modification, and further drying. The resultant hydrochar is frequently characterized to evaluate properties such as surface area, porosity, and functional group composition, which serve to determine its suitability for specific applications.
3.1.5. Versatility and Tailoring
The production of hydrochars is highly versatile, and the operational conditions can be customized to achieve desired properties and performance characteristics. As such, the process parameters may fluctuate contingent on factors such as the type of feedstock, temperature, pressure, and treatment duration. This adaptability makes hydrothermal carbonization a valuable technology for the conversion of organic waste materials into a diverse array of sustainable and valuable products [38].
3.2. Activation Techniques for Enhancing Adsorption Properties
Activation techniques play a pivotal role in augmenting the adsorption properties of materials, including hydrochars, through the augmentation of their surface area, pore volume, and reactivity. These techniques are employed to optimize the adsorption capacity of hydrochars for particular contaminants. The following elucidates several common activation methods: Physical activation entails subjecting hydrochars to elevated temperatures in an oxygen-depleted environment, a process that expels volatile compounds and augments porosity. This results in activated hydrochars endowed with enhanced adsorption properties [25]. Chemical activation methods involve treating hydrochars with potent acids (e.g., phosphoric acid) or bases (e.g., potassium hydroxide). This treatment fosters the creation of pores and augments surface area, thus amplifying the hydrochars’ adsorption capacity. Subsequently, the activated hydrochars are meticulously washed to eliminate residual chemicals [39]. Chemical modification encompasses the introduction of functional groups (e.g., amino or thiol groups) through chemical processes, which enhances the hydrochar’s affinity for specific contaminants and improves adsorption efficiency [40]. The application of microwave irradiation to hydrochars promotes the release of volatile compounds, heightens porosity, and augments adsorption properties due to localized and rapid heating effects [41]. Steam activation involves the introduction of steam to the hydrochar under controlled conditions. This procedure engenders additional porosity and ameliorates adsorption properties [42]. Electrochemical methods are utilized to create activated hydrochars by applying an electric current or potential. This application induces the formation of pores and surface functional groups, thereby enhancing adsorption capabilities [43]. In situ activation occurs within the hydrothermal carbonization process through adjustments in reaction conditions, such as temperature, duration, and pressure. These modifications are tailored to the properties of the resulting hydrochars, aligning them with specific adsorption applications. Certain research endeavors employ a combination of multiple activation techniques to maximize adsorption properties. For instance, a hydrochar may undergo chemical activation followed by pyrolysis or steam activation [42]. The integration of other materials, such as nanoparticles or metal oxides, into hydrochars enhances their adsorption properties for specific contaminants. This process, referred to as composite formation, may also involve doping with substances like nitrogen or sulfur [44].
Activation techniques are instrumental in customizing hydrochars to meet the precise requirements of adsorption applications, whether these pertain to the removal of heavy metals, organic pollutants, or pharmaceuticals from water and wastewater. The selection of the most suitable activation method hinges on the target contaminants, feedstock characteristics, and intended properties of the hydrochar. Table 1 provides a comparative analysis of the physical properties of activated hydrochar in relation to other commonly used adsorbents.

Table 1.
Physical properties of activated hydrochar with other common adsorbents.
4. Sustainable Aspects of Hydrochar Production
Hydrochar production is distinguished by its notable sustainable attributes, rendering it an environmentally conscientious and resource-efficient process. The following delineates key sustainable facets of hydrochar production: Hydrochar production predominantly relies on organic waste materials, including agricultural residues, sewage sludge, and organic waste, which are often considered waste or byproducts. This transformative process converts these materials into valuable and reusable products, alleviating the burden of waste disposal [48]. Hydrothermal carbonization effectively captures carbon from organic feedstocks and converts it into a stable form integrated into the hydrochar. This carbon sequestration serves to mitigate climate change by removing carbon from the atmosphere and storing it in a more stable, long-lasting format. By diverting organic waste from landfills and incineration, hydrochar production diminishes the emission of methane, a potent greenhouse gas, from the decomposition of organic matter. It also mitigates emissions stemming from waste transportation and incineration [49]. Hydrochar production is characterized by resource efficiency, as it employs water as the reaction medium, often recycled, thereby minimizing water consumption. Additionally, the energy required for hydrothermal carbonization can be derived from renewable sources, further elevating its sustainability. Hydrochars find application as soil conditioners or fertilizers, augmenting soil fertility, water retention, and crop productivity. This advancement fosters sustainable agricultural practices by reducing the reliance on synthetic fertilizers and enhancing soil quality. When employed as adsorbents, hydrochars proffer a sustainable solution for water and wastewater treatment. Their effectiveness in eliminating contaminants from water reduces the dependence on energy-intensive treatment methods and diminishes the environmental repercussions of water pollution. Hydrochars, when used as a fuel in combustion, pyrolysis, or gasification processes, function as a renewable energy source. This alternative to fossil fuels aids in curbing greenhouse gas emissions. Hydrochar production aligns with the principles of a circular economy by repurposing organic waste materials and reintegrating them into productive utilization. This approach counters the conventional linear “take-make-dispose” model, thus fostering sustainability. Continual research and development endeavors within the sphere of hydrochar production and application methods strive to amplify its sustainability. Innovations, including the optimization of process conditions, post-treatment techniques, and feedstock selection, continue to bolster the environmental and economic efficiency of hydrochar production. The sustainable dimensions of hydrochar production render it an appealing and environmentally responsible solution for converting organic waste into valuable materials that actively contribute to waste reduction, climate change mitigation, and resource efficiency.
5. Characterization of Activated Hydrochars
Characterization of activated hydrochars plays a pivotal role in comprehending their properties and evaluating their suitability for a myriad of adsorption applications. To this end, it is imperative to consider key characterization methods and properties. These methods encompass the following: The Brunauer–Emmett–Teller (BET) analysis is employed to quantify the specific surface area of activated hydrochars, thereby yielding valuable insights into their adsorption capacity [50]. The Pore Size Distribution Analysis utilizes techniques such as Barrett–Joyner–Halenda (BJH) or Horvath–Kawazoe (HK) methods to ascertain the pore size distribution, encompassing micropores, mesopores, and macropores [51]. Scanning electron microscopy (SEM) provides visual data concerning surface morphology and pore structure [52]. The elemental analysis (CHN) determines the carbon, hydrogen, and nitrogen content, revealing alterations in composition resulting from activation [53]. Fourier transform infrared spectroscopy (FTIR) is utilized to identify functional groups on the surface, offering insights into the chemical composition [54]. X-ray photoelectron spectroscopy (XPS) plays a crucial role in analyzing the elemental composition and chemical states of elements on the surface of activated hydrochars [55]. The thermogravimetric analysis (TGA) quantifies weight changes as the activated hydrochar is heated, thereby furnishing information about its thermal stability and organic content [56]. Batch Adsorption Studies evaluate the adsorption capacity of activated hydrochars for specific contaminants by analyzing equilibrium and kinetic adsorption data [57]. Isotherm modelling involves fitting adsorption data to isotherm models, such as Langmuir and Freundlich, to elucidate adsorption behavior [58]. Kinetic studies are designed to assess the rate at which adsorption occurs, facilitating an understanding of the adsorption mechanism [59]. X-ray diffraction (XRD) is instrumental in determining the crystalline structure of activated hydrochars [60]. N2 Adsorption–Desorption Isotherms serve a dual purpose by aiding the BET analysis and providing insights into surface area and pore size [61]. Solid-state nuclear magnetic resonance (NMR) offers insights into the distribution of carbon species and functional groups on the surface [62]. Transmission electron microscopy (TEM) provides detailed imagery of the nanoscale structure and morphology of activated hydrochars [63]. Atomic force microscopy (AFM) quantifies surface roughness and topography [64].
It is crucial to assess the stability of activated hydrochars under various conditions and ascertain their potential for regeneration and reusability in adsorption applications. Characterization of activated hydrochars stands as a cornerstone for researchers and engineers in tailoring their properties to meet specific adsorption applications and gaining profound insights into the underlying adsorption mechanisms. The selection of characterization techniques should be governed by the study’s objectives and the particular properties of interest.
6. Adsorption Mechanisms of Hydrochars
6.1. Surface Chemistry and Functional Groups
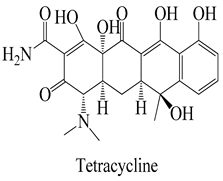
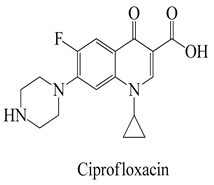
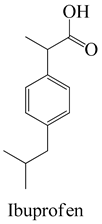
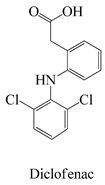
The surface chemistry of hydrochar is subject to the influence of a diverse array of functional groups, which fulfill a pivotal role in the process of adsorption and the consequent removal of pharmaceutical contaminants from water [26]. In this context, an array of key functional groups emerges as integral components of hydrochar surfaces, and their interactions with pharmaceutical contaminants assume significance. The following provides a delineation of such functional groups, along with examples of pharmaceutical contaminants and their associated interactions: Hydroxyl groups, manifesting on hydrochar surfaces, partake in interactions with polar pharmaceuticals such as antibiotics (e.g., tetracycline), primarily via the formation of hydrogen bonds. This mechanism serves to facilitate the adsorption of pharmaceutical contaminants [65]. Carboxyl groups actively engage in ion-exchange interactions with basic pharmaceuticals, including amines or cationic drugs (e.g., ciprofloxacin). In this context, the carboxyl groups found on hydrochar surfaces are instrumental in attracting and binding with positively charged species [66]. Phenolic groups on the surface of hydrochar intermingle with pharmaceutical contaminants characterized by aromatic rings, typified by nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen. The interactions predominantly transpire through π–π interactions and hydrogen bonding [67].
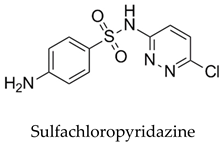
While relatively less common, amine-functionalized hydrochars are capable of establishing interactions with acidic pharmaceutical contaminants (e.g., aspirin) through acid–base interactions. In this scenario, the amine groups operate as proton donors [68]. Exemplar interaction is as follows: Oxygen-containing functional groups such as carbonyl (C=O) and ether (C-O-C) can engage with a diverse array of pharmaceutical contaminants, encompassing organic acids, esters, and other polar compounds [69]. The π-electron systems inherent in the aromatic structure of hydrochar can engage in interactions with aromatic pharmaceuticals [70], including phenolic compounds and polycyclic aromatic hydrocarbons (PAHs), primarily through π–π interactions. Further exemplar interaction is as follows: Electron-donating and electron-withdrawing groups situated on hydrochar surfaces hold the potential to influence the adsorption of pharmaceuticals harboring specific functional groups. In this context, electron-donating groups may engage with electron-withdrawing functional groups present in pharmaceutical contaminants [71]. The surface charge characterizing hydrochar, an attribute influenced by the presence of functional groups, assumes a role in electrostatic interactions with ionic pharmaceutical contaminants. Such interactions manifest in the attraction of anionic pharmaceuticals (e.g., sulfonamides) to positively charged surfaces, and vice versa [72].
The acid–base properties inherent to hydrochar surfaces possess the capacity to influence the adsorption of pharmaceutical contaminants characterized by specific acid–base characteristics. An illustrative example involves the acidic surface of hydrochar interacting with basic pharmaceuticals [73]. Functional groups on hydrochar surfaces that exhibit redox activity may partake in redox reactions, potentially leading to the transformation or degradation of pharmaceutical contaminants that are susceptible to oxidation or reduction. This phenomenon may be particularly relevant to pharmaceuticals with chemically sensitive structures [74].
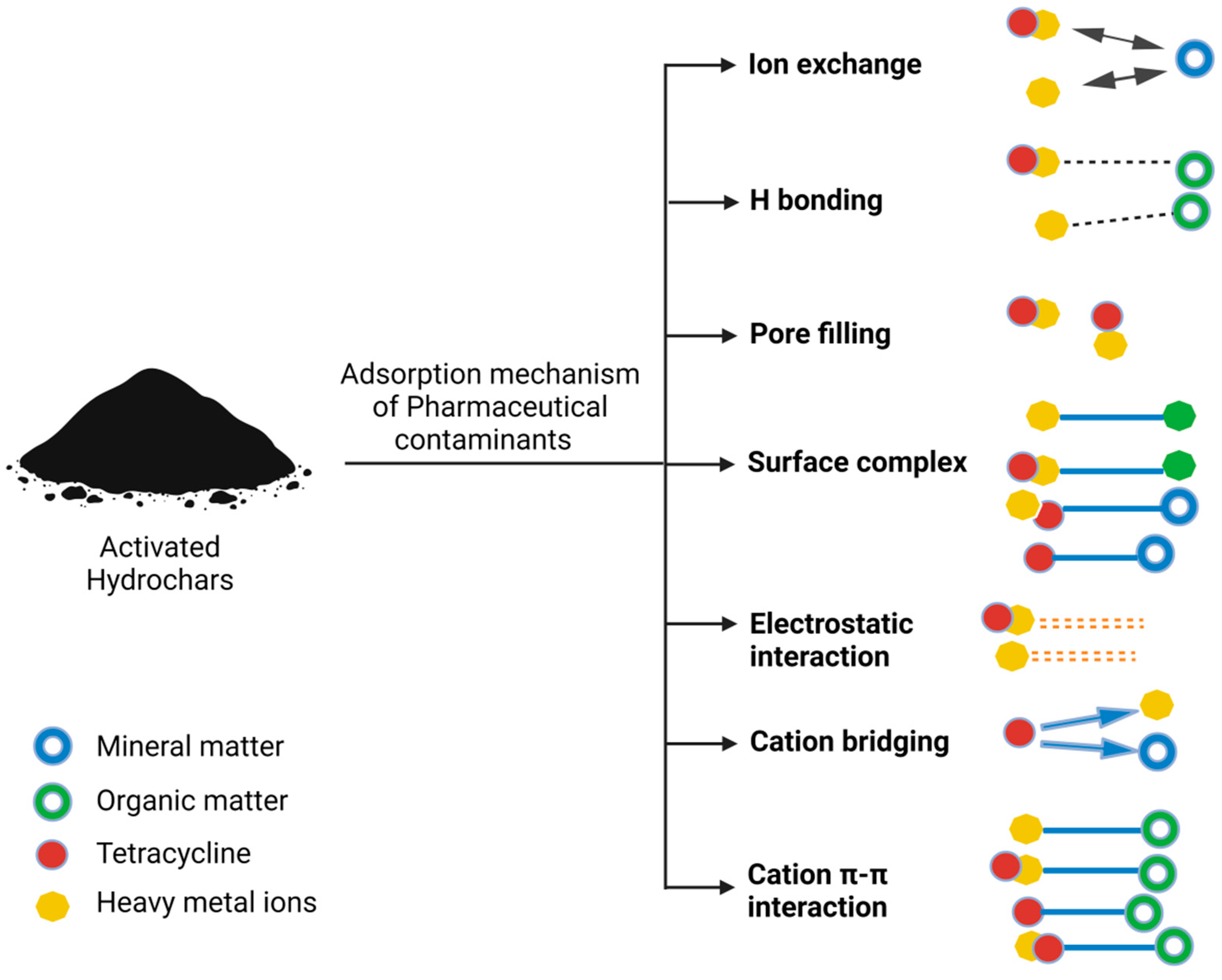
It is essential to acknowledge that the presence and distribution of these functional groups on hydrochar surfaces are subject to variation contingent on factors like the feedstock used, the production process, and post-treatment methods. These diverse functional groups collectively serve as the bedrock for the capacity of hydrochar to adsorb a wide spectrum of pharmaceutical contaminants through a multifaceted array of mechanisms, rendering it an effective and versatile adsorbent with pronounced relevance in the domain of water treatment applications. Figure 2 presents a comprehensive mechanism for the adsorption of various contaminant types, encompassing pharmaceutical contaminants and others.
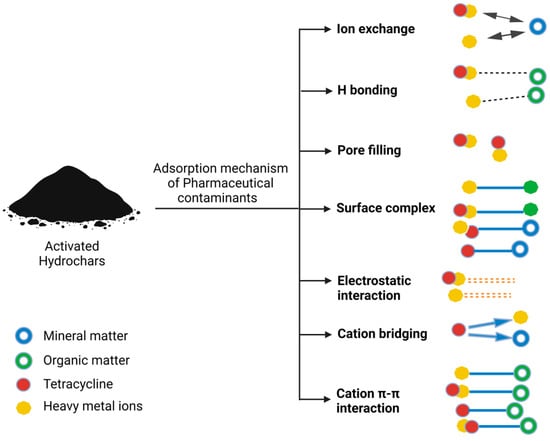
Figure 2.
Comprehensive mechanism for adsorption of various contaminant types, including pharmaceutical contaminants, and others.
6.2. Interaction between Pharmaceutical Contaminants and Hydrochars
The interaction between hydrochars and pharmaceutical contaminants constitutes a multifaceted process characterized by several intricate mechanisms, including adsorption, complexation, electrostatic interactions, and chemical reactions. These interactions hold paramount importance in the context of pharmaceutical contaminant removal from water. The following provides an in-depth overview of the intricacies inherent to these interactions: Hydrochars exhibit a porous structure replete with a substantial surface area. Pharmaceutical contaminants become physically adsorbed onto the hydrochar surface, a process mediated by van der Waals forces, hydrophobic interactions, and hydrogen bonding [75]. This mode of adsorption is typically reversible. Some pharmaceutical contaminants are capable of engaging in chemical reactions with the functional groups residing on the surface of hydrochars [76]. These interactions may culminate in covalent bonding, a feature that renders the adsorption process more stable and less prone to reversibility.
Specific pharmaceutical contaminants, particularly metal ions often present in pharmaceutical formulations, have the propensity to form complexes with the functional groups available on hydrochar surfaces. These complexation reactions can lead to the successful removal of metal contaminants from water [77]. The surface charge carried by hydrochars, influenced by their functional groups and the prevailing pH conditions, can be either positively or negatively charged. Pharmaceutical contaminants, bearing opposite charges, may be drawn toward the charged surface of hydrochars. This phenomenon engenders electrostatic interactions, thereby facilitating adsorption [78]. Hydrogen bonding emerges as a prevalent interaction mechanism between hydrochars and polar pharmaceutical contaminants. The hydroxyl (-OH) and carboxyl (-COOH) functional groups found on hydrochars are adept at forming hydrogen bonds with pharmaceutical compounds characterized by oxygen and hydrogen atoms [70].
The π-electron systems intrinsic to the aromatic structure of hydrochars have the capacity to engage in interactions with aromatic pharmaceutical contaminants, including those featuring benzene rings. These π–π interactions contribute substantively to the adsorption process [79]. The chemical architecture of pharmaceutical contaminants and their potential interaction mechanisms with hydrochar are delineated in Table 2.

Table 2.
Characteristics of BET surface area of hydrochar, and probable interaction with pharmaceutical contaminants.
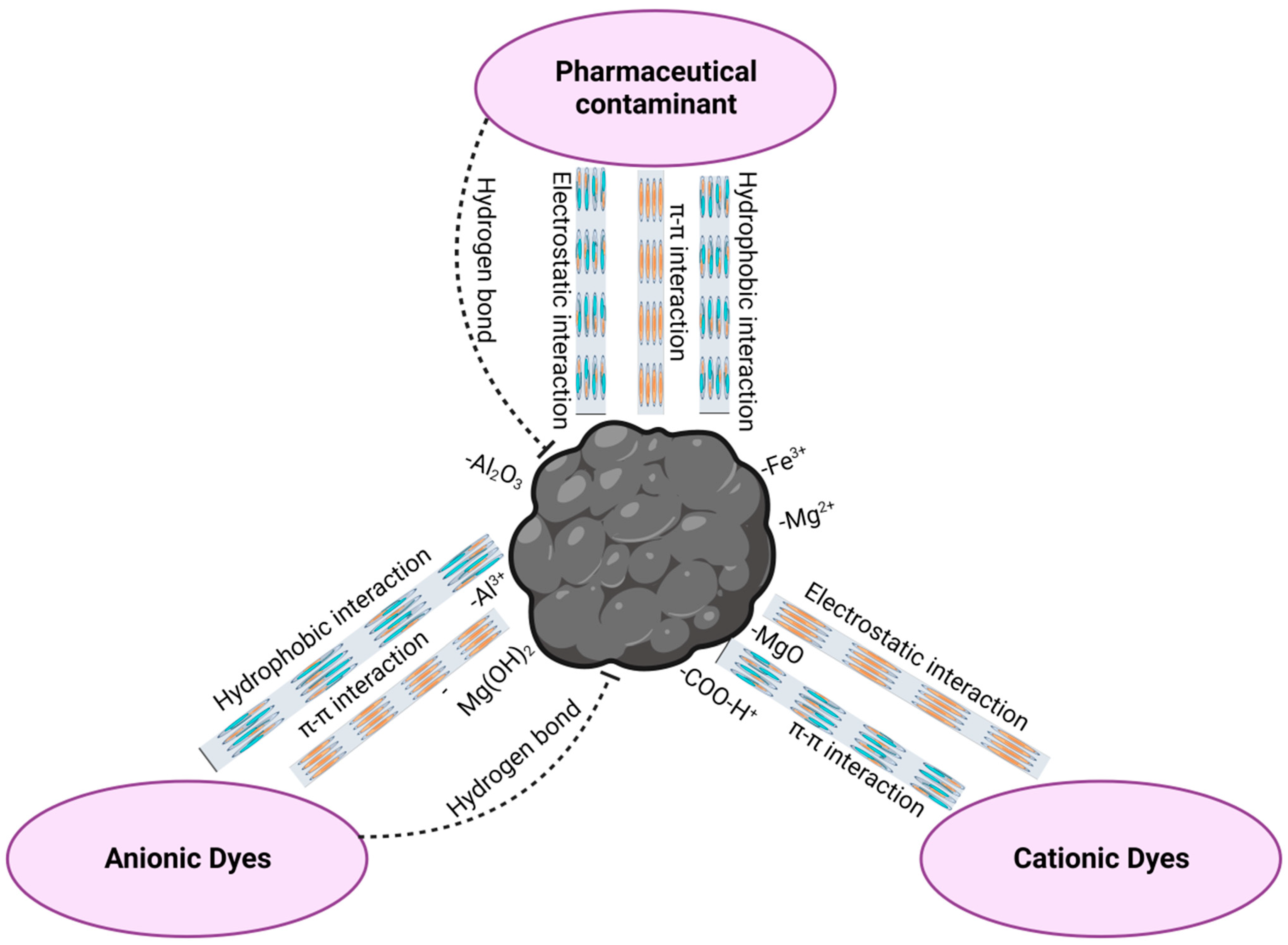
It is important to underscore that the specific mechanism and the extent of interaction between pharmaceutical contaminants and hydrochars are contingent upon a plethora of factors, including the chemical nature of the contaminants, the inherent properties of the hydrochar, solution pH, and the duration of contact. These interactions collectively render hydrochars efficacious adsorbents, well suited for the removal of a diverse spectrum of pharmaceutical contaminants from water and wastewater. The selection of the appropriate hydrochar and the judicious optimization of adsorption conditions assume critical significance in achieving efficacious removal outcomes. A depiction of the adsorption mechanisms of pharmaceutical and other contaminants onto hydrochar can be observed in Figure 3.

Figure 3.
Mechanistic insights into hydrochar adsorption of pharmaceutical and environmental contaminants.
6.3. Adsorption Modeling: Isotherms, Kinetics, and Thermodynamic Analysis
In the context of adsorption processes involving pharmaceutical contaminants on activated hydrochars, various kinetic and equilibrium models have been applied to provide a systematic understanding of the phenomena. These models serve as valuable tools for characterizing and predicting the adsorption behavior of such contaminants.
6.3.1. Kinetics Models
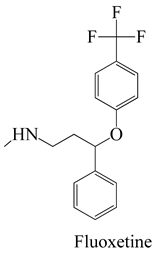
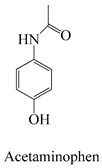
A prime example is the utilization of the Pseudo-First-Order Kinetics model, as demonstrated in a study focusing on the adsorption of antibiotics like ciprofloxacin onto activated hydrochar. This model enables the determination of the rate constant and estimation of the equilibrium adsorption capacity [85]. Similarly, the Pseudo-Second-Order Kinetics model finds application in scenarios such as the adsorption of antidepressants like fluoxetine onto activated hydrochar. It aids in assessing the rate constant and predicting the equilibrium adsorption capacity [83]. In cases involving the adsorption of antipyretics, like acetaminophen, the Intraparticle Diffusion Model is pertinent. This model offers insights into the rate of intraparticle diffusion and considers the effect of the boundary layer, represented by the constant [86].
6.3.2. Equilibrium Models
Equilibrium models play a pivotal role in understanding the final adsorption state of pharmaceutical contaminants. The Langmuir Isotherm, for instance, is employed when investigating the adsorption of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen on activated hydrochar. This model allows for the determination of the maximum monolayer adsorption capacity and the Langmuir adsorption constant [67]. The Freundlich Isotherm model is useful in the adsorption of hormones like estradiol onto activated hydrochar. It helps in assessing the Freundlich adsorption constant and the Freundlich exponent, which characterizes the adsorption intensity [87]. For the adsorption of compounds such as acetaminophen, the Sips Isotherm model can be applied. This model assists in determining the Sips adsorption constant and the Sips Isotherm exponent (n), which provides insights into surface heterogeneity and saturation [88].
6.3.3. Thermodynamic Analysis
In addition to kinetic and equilibrium models, a thermodynamic analysis is crucial for understanding the energy changes associated with adsorption processes. Thermodynamic parameters, such as Gibbs free energy (∆G), enthalpy (∆H), and entropy (∆S), can shed light on the spontaneity and feasibility of adsorption. For instance, a negative ∆G indicates a thermodynamically favorable process, while ∆H and ∆S provide insights into the nature of adsorption (endothermic or exothermic) and the degree of randomness at the adsorbent–adsorbate interface, respectively. The combination of these parameters offers a deeper understanding of the thermodynamics of pharmaceutical adsorption onto activated hydrochar, aiding in the design and optimization of adsorption processes for water treatment and environmental remediation.
In summary, the integration of kinetic, equilibrium, and thermodynamic models enables a comprehensive analysis of the adsorption of pharmaceutical contaminants on activated hydrochar surfaces. These models collectively provide a valuable framework for optimizing adsorption processes in water treatment scenarios while considering the associated energy changes and thermodynamic feasibility.
6.4. Applications of Activated Hydrochars in Pharmaceutical Contaminant Removal
Activated hydrochars serve as versatile and efficacious agents for the removal of pharmaceutical contaminants from water and wastewater, underpinned by their robust adsorption properties and environmentally sustainable attributes. Within this purview, several key applications manifest, underscoring their utility: Activated hydrochars can be seamlessly integrated into the operational processes of municipal and industrial water treatment facilities. Their application contributes to the elimination of pharmaceutical contaminants from both drinking water and wastewater, thereby upholding the water quality standards requisite for public health [72].
Activated hydrochars find utility in household water filtration systems, wherein they serve as stalwart sentinels against pharmaceutical contaminants. These systems ensure the delivery of potable water at the point of consumption, essentially rendering it devoid of these contaminants. In wastewater treatment installations, activated hydrochars emerge as valuable assets for the abatement of pharmaceutical contaminants within domestic and industrial effluents. By doing so, they aid in the fulfillment of regulatory requirements concerning effluent quality standards. Agricultural runoff, often laden with pharmaceutical contaminants, can be effectively treated through the deployment of activated hydrochars. Their use thwarts the ingress of these contaminants into surface waters, thereby mitigating the attendant environmental and ecological repercussions. In instances wherein groundwater stands tainted by pharmaceutical contaminants, activated hydrochars can be incorporated into remediation systems. Their function is to treat and purify groundwater, rendering it suitable for consumption.
Pharmaceutical manufacturing facilities benefit from the utilization of activated hydrochars for the treatment of effluents housing pharmaceutical contaminants. This not only ensures compliance with environmental regulations but also curtails the discharge of pollutants into aquatic ecosystems. Activated hydrochars can be seamlessly integrated into urban stormwater management systems. Their role lies in capturing pharmaceutical contaminants from urban runoff before this runoff reaches natural water bodies [89]. Within the ambit of aquaculture, activated hydrochars fulfill a pivotal role in sustaining water quality. By expeditiously removing pharmaceutical contaminants, they bolster the health of aquatic organisms and, concomitantly, the safety of seafood production [90]. In environments perturbed by pharmaceutical contamination and its concomitant ecological disruptions, activated hydrochars can be instrumental in restoring ecological equilibrium through the removal of environmental contaminants from natural water bodies [24]. The deployment of activated hydrochars is ubiquitously observed in research and pilot-scale investigations. These endeavors are designed to gauge the efficacy of activated hydrochars in the context of pharmaceutical contaminant removal. Such studies further aim to devise treatment processes that are both cost-effective and sustainable.
In effect, the utilization of activated hydrochars in the realm of pharmaceutical contaminant removal resonates harmoniously with the tenets of the One Health concept, which inherently acknowledges the interconnectedness of environmental, human, and animal health. This holistic approach renders dual benefits, safeguarding ecosystems while concurrently enhancing the well-being of human populations. The specific application of activated hydrochars hinges on several determinants, including the nature and concentration of pharmaceutical contaminants, the requisite water quality standards, and the contextual intricacies of the environment. In sum, activated hydrochars underscore their utility as a versatile, eco-friendly solution for the multifaceted challenge of pharmaceutical contaminant removal, effectively contributing to the safeguarding of water resources and public health. Table 3 provides a comprehensive overview of various hydrochar and biochar types, their respective adsorbent properties, and the underlying adsorption mechanisms.

Table 3.
Comprehensive Overview of Hydrochar and Biochar Types, Adsorbent Properties, and Adsorption Mechanisms.
6.5. Removal Efficiency and Factors Influencing Adsorption
The effectiveness of pharmaceutical contaminant removal by activated hydrochars is a nuanced interplay of various factors. A comprehensive understanding of these factors is pivotal for optimizing the adsorption process and achieving an efficient removal strategy [117]. Here are the pivotal factors influencing the adsorption of pharmaceutical contaminants by activated hydrochars: Activated hydrochars featuring a greater surface area generally exhibit enhanced adsorption efficiency due to the availability of more adsorption sites [26]. The type and concentration of surface functional groups, such as hydroxyl (-OH) and carboxyl (-COOH) groups, have a pronounced influence on adsorption capacity and affinity for specific contaminants [118]. The distribution of pore sizes, including micropores, mesopores, and macropores, is instrumental in shaping adsorption kinetics and the removal of pharmaceutical compounds of varying sizes.
The chemical structure, charge, and presence of functional groups in pharmaceutical contaminants dictate their affinity for specific functional groups on the hydrochar surface. The size of pharmaceutical molecules significantly affects their ability to interact with adsorption sites on the hydrochar surface, influencing adsorption efficiency [73]. The solubility of pharmaceutical contaminants in water directly influences their availability for adsorption, with less soluble compounds often demonstrating higher removal efficiency. Solution pH plays a pivotal role by affecting the surface charge of both hydrochars and pharmaceutical contaminants, thereby modulating electrostatic interactions. The optimal pH conditions for adsorption may vary among different contaminants [26,119]. The ionic strength of the solution introduces a competitive dynamic in the adsorption process, as ions can vie for adsorption sites with pharmaceutical contaminants. Elevated ionic strength may curtail removal efficiency. Temperature exerts a significant influence on adsorption kinetics. In many cases, higher temperatures expedite the adsorption process; however, it is noteworthy that increased temperature does not invariably correlate with heightened removal efficiency.
The quantity of activated hydrochars added to the solution plays a pivotal role in the adsorption process. Determining an optimal dosage is essential to strike a balance between removal efficiency and cost-effectiveness. The duration of interaction between activated hydrochars and pharmaceutical contaminants is pivotal. Longer contact times typically result in enhanced removal efficiency [120]. Longer contact times generally lead to higher removal efficiency. The initial concentration of pharmaceutical contaminants within the solution holds considerable significance. Elevated initial concentrations may paradoxically lead to reduced removal efficiency, as adsorption sites may become saturated. In scenarios where co-existing ions or solutes are present within the solution, they can compete with pharmaceutical contaminants for adsorption sites, potentially diminishing the removal efficiency [121]. Real water samples often contain complex matrices, such as organic matter, which can interfere with the adsorption process. This interference must be duly acknowledged and managed [122].
The ability to regenerate and reuse activated hydrochars represents a pivotal consideration. Understanding the regeneration methods and ascertaining the number of cycles before a decline in removal efficiency are crucial. The size and shape of activated hydrochar particles have an impact on their settling characteristics and mixing within the solution, which in turn influences adsorption efficiency [72]. The source material employed for hydrochar production and the specific activation methods utilized can yield variations in surface chemistry, pore structure, and adsorption properties [123]. Natural organic matter inherent in water can also compete for adsorption sites with pharmaceutical contaminants, thereby affecting the overall removal efficiency.
It is imperative to recognize that the influence of these factors can be compound-specific, contingent on the nature of the pharmaceutical contaminants under consideration, as well as the prevailing water quality conditions. Consequently, achieving an effective pharmaceutical contaminant removal strategy using activated hydrochars necessitates a tailored approach, inclusive of thorough experimentation and pilot studies to optimize these parameters in pursuit of the desired removal efficiency.
6.6. Regulations and Guidelines for Pharmaceutical Contaminants
Regulations and guidelines for pharmaceutical contaminants in water vary significantly across countries and regions. Noteworthy regulatory bodies include the United States Environmental Protection Agency (EPA), which, under the Safe Drinking Water Act, establishes maximum contaminant levels (MCLs) for substances, including pharmaceuticals [124]. The World Health Organization (WHO) provides international guidance through its “Guidelines for Drinking-water Quality”, addressing the evaluation and control of pharmaceutical contaminants [125]. The European Union (EU) outlines parameters in the Drinking Water Directive, encompassing criteria for water quality, including pharmaceuticals [126]. Various countries, through their environmental agencies, set standards and regulations, discernible in thresholds for pharmaceutical contaminants in surface waters [127]. Additionally, pharmaceutical industry regulations, such as those from the U.S. Food and Drug Administration (FDA), indirectly impact contaminant prevalence by mitigating environmental impact [128]. Several international agreements and organizations are dedicated to addressing concerns associated with pharmaceutical contaminants in water. Among them, the United Nations Environment Programme (UNEP) and the Organization for Economic Co-operation and Development (OECD) are notable participants [129].
Alongside regulations and guidelines, research initiatives and monitoring programs aim to identify and understand pharmaceutical contaminants in water, forming the foundation for regulatory decisions. It is crucial to recognize substantial disparities in regulations contingent on specific pharmaceutical contaminants, concentrations, contamination sources, and intended water use. The field of pharmaceutical contaminant regulation is dynamic, evolving with advancements in research and an enhanced understanding of associated risks, leading to the emergence of new guidelines and standards. Local and regional authorities play a vital role in enforcing these regulations, emphasizing the importance of consulting relevant bodies for the latest information on pharmaceutical contaminant regulations and guidelines.
7. Advantages and Limitations
Activated hydrochars proffer several distinctive advantages in the realm of pharmaceutical contaminant removal from aqueous systems: The impressive surface area and rich assortment of functional groups within activated hydrochars equip them to effectively adsorb a diverse spectrum of pharmaceutical contaminants. Often derived from renewable sources like biomass and organic waste materials, hydrochars manifest as an eco-conscious and sustainable adsorbent, ameliorating environmental impact. The abundant and relatively inexpensive feedstock materials utilized in hydrochar production contribute to economically viable treatment solutions. In select cases, activated hydrochars can undergo regeneration, bolstering their operational longevity and economizing operational expenses [130]. Hydrochars offer the possibility of precise modifications, empowering their customization to target specific pharmaceutical contaminants, thereby amplifying their versatility across varied treatment scenarios. By harnessing hydrochars for water treatment, the release of pharmaceutical contaminants into natural water bodies is curtailed, effectively mitigating environmental harm. The realm of activated hydrochars operates as a nexus for cross-disciplinary collaboration, amalgamating insights from chemistry, environmental science, and materials science to facilitate the exploration of innovative solutions.
However, the utilization of activated hydrochars for pharmaceutical contaminant removal is not devoid of certain limitations: The efficiency of activated hydrochars in pharmaceutical contaminant removal is subject to fluctuation contingent upon diverse factors encompassing the type of contaminant, solution conditions, and specific characteristics of the hydrochar employed. Co-existing ions and solutes in the water matrix may engage in competition with pharmaceutical contaminants for adsorption sites, conceivably diminishing the removal efficiency. While some activated hydrochars can undergo regeneration, restoration of their adsorption capacity may not be consistently achieved, and the process may be energy-intensive.
Real-world water samples frequently harbor intricate matrices including natural organic matter, which can interfere with the adsorption process and consequently abate its efficiency. Hydrochars may exhibit limited selectivity for distinct pharmaceutical contaminants, and certain compounds may prove resistant to effective removal. The endeavor to scale up activated hydrochar production and application for large-scale water treatment facilities may present engineering and logistical challenges. Achieving optimal removal efficiency often necessitates the conduct of meticulous experimentation and the intricate fine-tuning of operational parameters, which may not invariably be straightforward. The production of hydrochars from specific feedstock materials may entail safety and environmental considerations, and certain agricultural residues, for example, might yield associated environmental impacts.
The domain of activated hydrochars confronts competition from an array of alternative adsorbents and treatment technologies, necessitating comprehensive performance evaluation relative to other options. In summation, activated hydrochars represent a promising and ecologically responsible avenue for the elimination of pharmaceutical contaminants from water systems. These advantages encompass high adsorption capacity, sustainability, and cost-effectiveness. However, their performance is inherently nuanced, influenced by diverse factors, and may not uniformly address all pharmaceutical contaminants. Careful consideration and meticulous optimization are imperative when integrating activated hydrochars into water treatment processes.
8. Sustainability and Economic Viability
8.1. Environmental Benefits of Activated Hydrochar
The utilization of activated hydrochar extends a multitude of environmental benefits, rendering it an environmentally sustainable and ecologically congenial solution across diverse water and wastewater treatment applications. These advantages significantly contribute to environmental safeguarding and the prudent utilization of resources. The following enumerates the key environmental merits associated with the application of activated hydrochar: Activated hydrochar is predominantly derived from organic waste materials, including agricultural residues, food waste, and sewage sludge [25]. By metamorphosing these organic residues into a valuable end-product, hydrochar facilitates the diversion of organic waste from landfills, thus substantially curtailing methane emissions and abating landfill pollution [131]. The process of producing hydrochar via hydrothermal carbonization (HTC) results in the sequestration of carbon from organic materials. This sequestered carbon remains in a stable form within the hydrochar, thus yielding a notable contribution to carbon storage while concurrently mitigating greenhouse gas emissions.
The production of hydrochar predominantly employs renewable feedstock sources, such as biomass, which can be obtained from sustainable harvesting or the utilization of agricultural and forestry residues. This serves to curtail the dependency on fossil-based adsorbents and materials, thereby advancing environmental conservation [30,132]. The deployment of hydrochar as a soil amendment offers the distinct advantage of improving soil quality and structure [133]. This augmentation, in turn, abates soil erosion and sediment runoff, culminating in an amelioration of aquatic ecosystem health. Hydrochar’s introduction to soil bolsters nutrient retention and augments water-holding capacity. This, in tandem, diminishes the necessity for chemical fertilizers, thereby fostering the principles of sustainable agriculture.
Within the context of wastewater treatment, hydrochar can be harnessed to recover the phosphorus-containing chemicals in sewage sludge [134]. This operationally minimizes the release of excessive phosphorus into water bodies, thereby averting eutrophication and preserving the health of aquatic ecosystems. The implementation of hydrochar in water treatment procedures significantly curtails the dependence on chemical coagulants and flocculants that are conventionally deployed. This, consequently, leads to a reduction in the generation of chemical residuals and their correlated environmental repercussions. The intervention of hydrochar in water treatment endeavors efficiently alleviates eutrophication, a prevalent environmental quandary precipitated by the inordinate runoff of nutrients and pollutants into aquatic environments.
Hydrothermal carbonization, as the process of hydrochar production, is inherently energy-efficient and can be fueled by renewable energy sources. This, in turn, contributes to the minimization of the carbon footprint associated with hydrochar production [135]. By extricating nutrients and pollutants from water bodies, hydrochar serves as a potent mitigator for the adverse impacts of eutrophication and the discharge of pharmaceutical contaminants. This, thereby, curtails the environmental repercussions and safeguards both aquatic ecosystems and human health. The research and implementation of hydrochar materials promote interdisciplinary research and cooperation, encompassing fields such as chemistry, environmental science, materials science, and agriculture. This interdisciplinary synergy culminates in the advancement of holistic environmental solutions. Moreover, the very concept of transforming waste materials into valuable products like hydrochar aligns seamlessly with the principles of a circular economy, whereby waste generation is minimized and resource utilization is conducted with maximal efficiency [136].
In summary, the environmentally congenial attributes and manifold applications of activated hydrochar underscore its immense value as a versatile tool for addressing a gamut of environmental challenges, all the while perpetuating sustainability and the astute conservation of resources.
8.2. Cost-Effectiveness and Scalability
The cost-effectiveness and scalability of activated hydrochar production and its applications hinge upon an array of intricate factors, encompassing feedstock selection, production methodologies, treatment objectives, and the specific operational conditions in place [137]. This section provides an overview of the nuanced considerations relating to cost-effectiveness and scalability for activated hydrochar: The cost-effectiveness of hydrochar production is intricately linked to the availability and cost of feedstock materials. The utilization of abundant, economically viable organic waste materials, such as agricultural residues or sewage sludge, can significantly amplify cost-effectiveness. The method employed for hydrochar production, whether it is hydrothermal carbonization (HTC) or pyrolysis, plays a pivotal role in shaping production costs. HTC, notable for its relatively mild processing conditions, can present a more cost-effective alternative when contrasted with high-temperature pyrolysis techniques.
The energy efficiency inherent to the hydrochar production process can exert a pronounced impact on costs. The strategic deployment of renewable energy sources for the production process, coupled with optimized energy utilization, can markedly bolster cost-effectiveness. A meticulous calibration of operational parameters, encompassing variables such as temperature, pressure, and residence time during hydrochar production, can induce tangible reductions in energy and resource consumption. This, in turn, augments the overall cost-effectiveness of the process. The viability of regenerating and reusing hydrochars for multiple cycles holds the potential to mitigate operational costs, hence rendering the technology more cost-effective. However, the complexities of the regeneration process should be scrutinized.
The labor and maintenance prerequisites associated with hydrochar production and application ought to be integrated into the overarching cost-effectiveness analysis. These operational outlays are integral to the comprehensive assessment [138]. The specific treatment objectives, such as the elimination of pharmaceutical contaminants, nutrients, or heavy metals, can wield a discernible influence on cost-effectiveness. Tailoring treatment for specific contaminants may necessitate supplementary processing steps, thereby impacting costs. The existence of a market for hydrochar products, such as soil amendments, can bolster cost-effectiveness by generating revenue through the sale of hydrochar. An effective market strategy is pivotal in this context.
8.3. Scalability
The attainment of scalability in the context of activated hydrochar hinges on several imperatives. The following expounds on the foundational aspects of scalability: Ensuring a dependable and uninterrupted supply of feedstock materials is an imperative foundation for scalability. This necessitates the establishment of collaborations with feedstock providers, coupled with the optimization of logistical operations. The scalability of hydrochar production is closely correlated with the capacity of production facilities [130]. Augmenting production capacity may entail investments in larger equipment and facilities. Modular production systems provide an agile framework for scalability, allowing for the expeditious expansion of operations by adding or duplicating modules as per a requirement.
Compliance with prevailing environmental regulations and permits stands as a cardinal precondition for the amplification of hydrochar production and application. A profound understanding of, and strict adherence to, local and national regulations is foundational. Scalability is intricately linked to the market demand for hydrochar products. The diversification of the product range and the exploration of new applications can open up novel markets and concomitantly foster scalability. Sustained research and development endeavors geared toward enhancing hydrochar production processes and broadening the scope of contaminants treated constitute a pivotal avenue for scalability.
Collaborative efforts encompassing researchers, engineers, and stakeholders from diverse domains can serve to identify new opportunities for hydrochar applications and, correspondingly, reinforce scalable solutions. The demonstration of the economic viability of hydrochar production and its applications is a seminal prerequisite for securing investments and underpinning scalability.
In synthesis, the achievement of scalability within the realm of activated hydrochar necessitates assiduous planning, adapted production processes, meticulous adherence to technical, logistical, and economic considerations, efficacious market engagement, and unwavering compliance with regulatory mandates. The ability to furnish reliable and consistent treatment outcomes as production scales is paramount for triumphant scalability.
8.4. Life Cycle Analysis
A life cycle analysis (LCA) of activated hydrochar necessitates the comprehensive evaluation of the environmental repercussions entailed across its entire life cycle, spanning from the initial stages of raw material extraction to production, utilization, and eventual disposal [139]. Herein lies a general exposition of the primary stages and considerations that underpin an LCA of activated hydrochar: The LCA initiates with the meticulous scrutiny of the environmental impact inherent to the sourcing and extraction of feedstock materials used for hydrochar production, which may encompass a spectrum of sources, such as agricultural residues, organic waste, or sewage sludge. This phase necessitates an in-depth exploration of factors including land use, water utilization, and energy consumption in the collection of feedstock materials.
The environmental footprint of the hydrochar production process, which may encompass methodologies such as hydrothermal carbonization (HTC) or pyrolysis, is subjected to rigorous examination. This entails the dissection of energy consumption, emissions, and resource utilization during the production process. The transport of feedstock materials to the production site and the subsequent conveyance of hydrochar to end-users or treatment facilities constitute vital junctures in the LCA [89]. Evaluate fuel consumption, emissions, and energy usage in transportation. The analysis encompasses the assessment of fuel consumption, emissions, and energy usage in transportation. An appraisal of the environmental benefits and impacts arising from the use of activated hydrochar across diverse applications, be it in the realm of water treatment, soil enhancement, or as an adsorbent for pharmaceutical contaminants, constitutes a significant component. This involves an evaluation of the efficiency and efficacy of hydrochar in realizing treatment objectives. If pertinent, the environmental implications of regenerating and reusing hydrochar materials for successive cycles within treatment processes warrant evaluation. Likewise, contemplation extends to the potential avenues for disposal or recycling of hydrochar products at the culmination of their utility [140].
Evaluate the environmental impact of waste management options, such as landfilling, incineration, or composting. The LCA framework entails a comparative dimension, wherein the environmental impacts of employing activated hydrochar are juxtaposed against alternative treatment modalities or materials, ranging from chemical coagulants to landfilling or traditional wastewater treatment. This contrast embraces a spectrum of environmental impact categories, encompassing greenhouse gas emissions, energy usage, water consumption, acidification, eutrophication, and ecotoxicity. The LCA hinges upon the meticulous accumulation of data pertaining to energy consumption, emissions, and resource utilization across each stage of the hydrochar life cycle [141]. The formulation of a comprehensive inventory of environmental inputs and outputs is a pivotal undertaking.
Specialized LCA software and methodologies are instrumental in deciphering the ramifications of each facet of the hydrochar life cycle on diverse environmental indicators. This entails an interdisciplinary and holistic approach to a data synthesis and analysis. The conclusions drawn from the LCA serve as the substrate for the interpretation of results and the formulation of recommendations. These recommendations are geared toward the attenuation of environmental impacts, process amelioration, and optimization of the sustainability quotient intrinsic to both the production and application of activated hydrochar.
A sensitivity analysis is a salient component, serving to scrutinize the influence of variable parameters and underlying assumptions on the LCA results. This aspect spans the contemplation of diverse scenarios and the mitigation of uncertainties. In synthesis, the outcomes arising from an LCA serve to demarcate hotspots within the hydrochar life cycle, proffer insights into potential process enhancements, and underpin informed decision making. The overarching objective revolves around the minimization of environmental impacts and the bolstering of sustainability [142]. It is imperative to conduct LCAs in consonance with established standards and guidelines, such as ISO 14040 and ISO 14044. This adherence ensures methodological rigor, precision, and congruity in assessments [143].
9. Challenges and Future Directions
9.1. Challenges
The development and application of activated hydrochars for a multitude of environmental and water treatment objectives are associated with several formidable challenges. Confronting these challenges is of paramount importance in order to fully exploit the potential of this sustainable adsorbent. The following highlights key challenges inherent to activated hydrochars:
The choice of feedstock material for hydrochar production exerts significant influence on the adsorbent’s properties and cost-effectiveness [144]. Identifying and sourcing appropriate feedstock materials, especially in substantial quantities, can prove to be a formidable task. Feedstock materials frequently manifest variability in terms of composition, quality, and characteristics, which can complicate the production of consistent and high-quality hydrochars. The hydrochar production process, whether it be hydrothermal carbonization (HTC) or pyrolysis, necessitates fine-tuning to cater to various feedstock materials and diverse treatment objectives [145]. Achieving the desired properties in hydrochar can be a complex undertaking. The regeneration of hydrochars for reuse can present technical challenges, and the effectiveness of regeneration may exhibit variability contingent on the types of contaminants and intended applications.
Hydrochars may exhibit limitations in terms of adsorption capacity and selectivity for specific contaminants. Tailoring hydrochars to address a broad spectrum of pharmaceutical contaminants can be a challenging endeavor. Efforts to scale up hydrochar production and implement large-scale treatment processes may encounter engineering complexities, particularly when transitioning from laboratory-scale to industrial applications.
Hydrochars face competition from other water treatment and adsorption technologies. Demonstrating the advantages and cost-effectiveness of hydrochars in comparison to alternative methods can be a daunting task. Real water samples often contain complex matrices replete with various ions, organic matter, and contaminants. Assessing the performance of hydrochars in such intricate environments can pose challenges. Complying with regulatory requirements and securing the necessary permits for hydrochar production and application can entail significant time and cost investments.
The development of markets for hydrochar products, such as soil amendments or water treatment adsorbents, may necessitate substantial marketing endeavors and the cultivation of awareness among potential users. Gaining public acceptance and trust in the safety and effectiveness of hydrochars in water treatment and environmental applications can be a formidable endeavor, particularly when compared to conventional treatment methods. Research and development within the realm of hydrochars may be constrained by limitations in funding and resources, potentially impeding progress and innovation. The need to tailor hydrochars for specific pharmaceutical contaminants can engender time-consuming and costly endeavors.
9.2. Future Directions
The future directions in the realm of activated hydrochars encompass endeavors to address these challenges through concerted research, innovation, and collaborative efforts among experts in various fields. These efforts will be indispensable in unlocking the full potential of hydrochars as a sustainable and effective solution for water treatment and environmental remediation.
Undertaking comprehensive life cycle assessments (LCAs) to gain an in-depth comprehension of the environmental ramifications of hydrochars can be a resource-intensive endeavor. Mitigating the inherent challenges stemming from the interdisciplinary aspects of hydrochar development and application represents a complex task, necessitating concerted cooperation among specialists across diverse domains, encompassing chemistry, engineering, environmental science, and policy. Strategies aimed at surmounting these challenges encompass the continual pursuit of research initiatives, optimization of processes, and facilitation of collaborative efforts among researchers, policymakers, and industry stakeholders. These endeavors collectively seek to foster the evolution of effective and sustainable applications for activated hydrochars in the domains of water treatment and environmental remediation.
Advancements and innovations within the realm of activated hydrochars bear the potential to engender more efficient and sustainable solutions for water treatment and environmental remediation. The following elucidates prospective domains for enhancement and innovation:
- Diversified Feedstock Utilization: The exploration and incorporation of an expanded spectrum of feedstock materials, including unconventional sources such as algae, waste plastics, and byproducts originating from various industries, to enrich the diversity and properties of hydrochars.
- Enhanced Pre-processing Techniques: The development of effective pre-processing methodologies aimed at mitigating feedstock variability and enhancing the uniformity of hydrochar properties.
- Advanced Production Methodologies: Research into advanced production techniques, encompassing approaches like microwave-assisted hydrothermal carbonization (MAHTC) and continuous-flow systems, designed to bolster the efficiency and scalability of hydrochar manufacturing.
- Tailored Hydrochars: Investigations into methods for customizing hydrochars to target specific pharmaceutical contaminants or other pollutants, potentially involving surface functional group modifications or the introduction of nanoparticles to enhance selectivity.
- Regeneration Technique Refinement: Improvement in regeneration techniques with the goal of extending the operational lifespan of hydrochars, thereby reducing the necessity for continuous production and diminishing waste generation.
- Scaling Challenges Addressed: Examination of engineering challenges related to the upscaling of hydrochar production from laboratory to industrial scales, covering aspects such as reactor design, energy efficiency, and cost-effectiveness.
- Integration with Conventional Treatment: Exploration of the integration of hydrochar-based treatment systems with conventional water treatment processes to optimize pharmaceutical contaminant removal.
- Real-time Monitoring and Control: Development of real-time monitoring and control systems capable of adapting treatment processes based on contaminant concentrations and feedstock properties to enhance operational efficiency.
- Resource Recovery Opportunities: Investigation of prospects for resource recovery from hydrochar production, such as the extraction of valuable compounds from feedstock or hydrochar materials.
- Cost–Benefit Analyses: Conducting comprehensive cost–benefit analyses to ascertain the economic feasibility of hydrochar-based treatment solutions compared to traditional methodologies, thereby substantiating the case for investment and adoption.
- Market Expansion: Expanding markets for hydrochar products, with a particular focus on its utility as a soil amendment, by educating agricultural stakeholders about the advantages of hydrochar in agriculture.
- Public Awareness and Acceptance: Elevating public awareness and acceptance through outreach programs and the provision of information regarding the safety and efficacy of hydrochar in water treatment and environmental applications.
- Interdisciplinary Collaboration: Fostering interdisciplinary collaboration among researchers, engineers, policymakers, and industry stakeholders to address intricate challenges and stimulate innovation.
- Comprehensive Environmental Impact Assessment: Continuation of comprehensive life cycle assessments (LCAs) and environmental impact studies to discern the complete environmental repercussions of hydrochar utilization.
- Pilot Studies and Demonstrations: Implementation of pilot studies and demonstration projects to validate the efficacy of hydrochar-based treatment solutions under real-world conditions.
- Waste Reduction Strategies: Exploration of methodologies to minimize waste generation during hydrochar production and consideration of waste utilization or valorization strategies.
- Advocacy for Regulatory Support: Advocacy for regulatory support and the establishment of standards that endorse the responsible utilization of hydrochars and acknowledge their potential benefits.
By channeling efforts into these domains for enhancement and innovation, researchers, engineers, and industry professionals can actively contribute to the progression of activated hydrochars as a sustainable and effective solution for water treatment and environmental remediation.
9.3. Future Research Prospects
The research outlook for activated hydrochar in the context of pharmaceutical contaminant removal from water and wastewater demonstrates considerable promise and holds the potential to make substantial contributions in addressing pressing environmental and public health challenges. The following represents a delineation of prospective research avenues and uncharted territories in this field:
- Exploration of Tailoring Techniques: Systematic investigations into methods for customizing the physicochemical properties of hydrochar, encompassing surface functional groups, porosity, and specific surface area, to augment its adsorption capacity and specificity for pharmaceutical contaminants.
- Nanomaterial Integration: Delving into the integration of nanoparticles and nanomaterials into hydrochar matrices to engineer nanostructured hydrochars with enhanced adsorption efficiency specifically tailored for targeted contaminants.
- Advanced Production Processes: Research into advanced hydrothermal and pyrolysis procedures aimed at producing hydrochars with optimized properties, potentially utilizing waste or biomass-derived feedstocks.
- Regeneration Techniques: Improvement and development of cost-effective hydrochar regeneration methodologies to extend its operational lifespan and curtail waste generation.
- Scaling Up Challenges: Addressing engineering and logistical challenges associated with upscaling hydrochar production for large-scale water treatment applications.
- Hybrid Treatment Systems: Exploration of the synergistic integration of hydrochar with other treatment technologies, including membrane filtration, activated carbon, or biological treatment, to forge hybrid systems that harness the strengths of each component for superior pharmaceutical contaminant removal.
- Adaptive Treatment Systems: Development of intelligent and adaptive treatment systems capable of dynamically adjusting hydrochar utilization based on real-time water quality monitoring and pharmaceutical contaminant concentrations.
- Contaminant Recovery Strategies: Investigation of techniques for desorbing and recovering pharmaceutical contaminants from loaded hydrochar materials, enabling their safe disposal or recycling.
- Environmental Fate and Impact Assessments: Conducting comprehensive environmental fate and impact studies to gauge the long-term repercussions of hydrochar application on soil, water, and ecosystems.
- Cost–Benefit Analyses: Undertaking cost–benefit analyses to discern the economic viability of hydrochar deployment in comparison to alternative treatment methods.
- Knowledge Dissemination: Promotion of the adoption of hydrochar-based solutions in water treatment, agriculture, and environmental remediation through concerted awareness campaigns and dissemination of knowledge.
- Regulatory Frameworks: Advocating for the establishment of regulatory frameworks and guidelines conducive to the responsible and widespread utilization of hydrochar in water and environmental applications.
- Interdisciplinary Collaboration: Encouraging interdisciplinary research collaborations involving experts in chemistry, engineering, environmental science, and public health to address multifaceted challenges at the interface of water quality and public health.
- Resource Mobilization: Securing resources and funding for research endeavors in the field and soliciting investment from both the public and private sectors to bolster the development of hydrochar technology.
By embarking on these research trajectories, the field of activated hydrochar for pharmaceutical contaminant removal can continue its evolution, furnishing sustainable, efficient, and cost-effective solutions for water and wastewater treatment. Simultaneously, it contributes to the safeguarding of the environment and the enhancement of public health.
10. Conclusions
In conclusion, this comprehensive review paper has cast light upon the substantial and promising role of activated hydrochars in addressing the pressing issue of pharmaceutical contaminant elimination from water sources. A thorough exploration of their attributes and applications reveals that activated hydrochars stand on the cusp of revolutionizing the field of water treatment. Their substantial adsorption capacity, stemming from sustainable feedstock origins and a minimal carbon footprint, underscores their pivotal role in mitigating the threats posed to aquatic ecosystems and human health by pharmaceutical contaminants. However, the potential utility of activated hydrochars extends well beyond pharmaceutical contaminant removal. These materials present innovative solutions to a diverse array of environmental challenges, encompassing enhancements in soil quality, air quality, resource recovery, and sustainable waste management. As we navigate the path forward in this domain, fostering interdisciplinary collaboration and the development of adaptable, intelligent treatment systems assumes paramount importance. Regulatory backing and policy frameworks will be instrumental in ensuring the responsible and widespread application of hydrochars. In summary, activated hydrochars represent an innovative and ecologically sustainable strategy that holds the potential to usher in a future marked by heightened environmental stewardship. The interdisciplinary nature of this field, amalgamating expertise in chemistry, engineering, environmental science, and public health, emphasizes the necessity for collaborative endeavors to unlock the full potential of hydrochars.
The journey ahead is replete with opportunities for further innovation, optimization, and upscaling, as well as the creation of intelligent, adaptive treatment systems. Regulatory frameworks and policy support will play a pivotal role in facilitating the responsible and widespread utilization of hydrochars. In summary, activated hydrochars proffer a compelling solution to the multifaceted quandary of pharmaceutical contaminant removal and have the potential to overhaul our approach to various environmental challenges. It is through persistent research, collaboration, and unwavering commitment that we shall fully harness the capabilities of activated hydrochars, contributing to a cleaner and more sustainable future for our planet.
Author Contributions
Conceptualization, S.M.; investigation, writing—review of original draft, M.K.G. and S.M.; formal analysis, S.M., M.K.G., T.M., R.N., B.M., M.C., A.B., D.D. and R.D.; supervision, S.M.; editing and project administration, S.M., M.K.G., T.M., R.N., B.M., M.C., A.B., D.D. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors express their sincere gratitude to the Adamas University, Kolkata, toward successful execution of this review work.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Berihun, G.; Abebe, M.; Hassen, S.; Gizeyatu, A.; Berhanu, L.; Teshome, D.; Walle, Z.; Desye, B.; Sewunet, B.; Keleb, A. Drinking water contamination potential and associated factors among households with under-five children in rural areas of Dessie Zuria District, Northeast Ethiopia. Front. Public Health 2023, 11, 1199314. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. 6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview; ACS Publications: Washington, DC, USA, 2001. [Google Scholar]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef]
- Mondal, S.; Xu, J.; Chen, G.; Huang, S.; Huang, C.; Yin, L.; Ouyang, G. Solid-phase microextraction of antibiotics from fish muscle by using MIL-101(Cr)NH2-polyacrylonitrile fiber and their identification by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1047, 62–70. [Google Scholar] [CrossRef]
- Mondal, S.; Jiang, J.; Li, Y.; Ouyang, G. Carbon and tin-based polyacrylonitrile hybrid architecture solid phase microextraction fiber for the detection and quantification of antibiotic compounds in aqueous environmental systems. Molecules 2019, 24, 1670. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Kock, A.; Glanville, H.; Law, A.; Stanton, T.; Carter, L.; Taylor, J. Emerging challenges of the impacts of pharmaceuticals on aquatic ecosystems: A diatom perspective. Sci. Total Environ. 2023, 878, 162939. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2020, 410, 124619. [Google Scholar] [CrossRef] [PubMed]
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.; Oliveira, C.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Neglected Trop. Dis. 2014, 8, e3257. [Google Scholar] [CrossRef] [PubMed]
- Imwene, K.; Ngumba, E.; Kairigo, P. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Aikat, K.; Halder, G. Sorptive uptake of Ranitidine hydrochloride by Parthenium hysterophorus based chemically treated N-biochar in static bed continuous flow system. Results Surf. Interfaces 2022, 8, 100071. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (aops) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. Chemengineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Zhou, Y.; Leong, S.Y.; Li, Q. Modified biochar for removal of antibiotics and antibiotic resistance genes in the aqueous environment: A review. J. Water Process. Eng. 2023, 55, 104222. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Z.; Liu, H.; Dong, S.; Nghiem, L.D.; Gao, L.; Chaves, A.V.; Zamyadi, A.; Li, X.; Wang, Q. A review on microalgae-mediated biotechnology for removing pharmaceutical contaminants in aqueous environments: Occurrence, fate, and removal mechanism. J. Hazard. Mater. 2023, 443, 130213. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Yaah, V.B.K.; Zbair, M.; de Oliveira, S.B.; Ojala, S. Hydrochar-derived adsorbent for the removal of diclofenac from aqueous solution. Nanotechnol. Environ. Eng. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: A review on challenges, commercialization, and future perspectives. Chemosphere 2022, 286, 131490. [Google Scholar] [CrossRef]
- Mestre, A.S.; Tyszko, E.; Andrade, M.A.; Galhetas, M.; Freire, C.; Carvalho, A.P. Sustainable activated carbons prepared from a sucrose-derived hydrochar: Remarkable adsorbents for pharmaceutical compounds. RSC Adv. 2015, 5, 19696–19707. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Lin, H.; Zhao, X.; Shang, J.; Liu, Z. Arsenic removal via a novel hydrochar from livestock waste co-activated with thiourea and γ-Fe2O3 nanoparticles. J. Hazard. Mater. 2021, 419, 126457. [Google Scholar] [CrossRef]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage sludge hydrochar: An option for removal of methylene blue from wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A recent advancement on hydrothermal carbonization of biomass to produce hydrochar for pollution control. Carbon Lett. 2023, 33, 1909–1933. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344. [Google Scholar] [CrossRef]
- Lima, E.C.; Naushad, M.; Reis, G.S.D.; Dotto, G.L.; Pavan, F.A.; Guleria, A.; Seliem, M.K.; Sher, F. Production of carbon-based adsorbents from lignocellulosic biomass. In Biomass-Derived Materials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 169–192. [Google Scholar]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M.; Vitolo, S. Activated carbon from hydrochar produced by hydrothermal carbonization of wastes. Chem. Eng. Trans. 2017, 57, 169–174. [Google Scholar]
- Liu, L.; Cai, W.; Dang, C.; Han, B.; Chen, Y.; Yi, R.; Fan, J.; Zhou, J.; Wei, J. One-step vapor-phase assisted hydrothermal synthesis of functionalized carbons: Effects of surface groups on their physicochemical properties and adsorption performance for Cr(VI). Appl. Surf. Sci. 2020, 528, 146984. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Congsomjit, D.; Areeprasert, C. Hydrochar-derived activated carbon from sugar cane bagasse employing hydrothermal carbonization and steam activation for syrup decolorization. Biomass Convers. Biorefinery 2021, 11, 2569–2584. [Google Scholar] [CrossRef]
- Tasca, A.L.; Clematis, D.; Marco, P.; Vitolo, S.; Puccini, M. Herbicide removal from water: Investigating the potential of electrochemistry and hydrochar-based activated carbon. Chem. Eng. Trans. 2019, 74, 841–846. [Google Scholar]
- Zhang, X.; Wang, Y.; Cai, J.; Wilson, K.; Lee, A.F. Bio/hydrochar sorbents for environmental remediation. Energy Environ. Mater. 2020, 3, 453–468. [Google Scholar] [CrossRef]
- Isemin, R.; Muratova, N.; Kuzmin, S.; Klimov, D.; Kokh-Tatarenko, V.; Mikhalev, A.; Milovanov, O.; Dalibard, A.; Ibitowa, O.A.; Nowotny, M.; et al. Characteristics of Hydrochar and Liquid Products Obtained by Hydrothermal Carbonization and Wet Torrefaction of Poultry Litter in Mixture with Wood Sawdust. Processes 2021, 9, 2082. [Google Scholar] [CrossRef]
- Islam, T.; Sultana, A.I.; Chambers, C.; Saha, S.; Saha, N.; Kirtania, K.; Reza, M.T. Recent Progress on Emerging Applications of Hydrochar. Energies 2022, 15, 9340. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Padhye, L.P.; Bandala, E.R.; Wijesiri, B.; Goonetilleke, A.; Bolan, N. Hydrochar: A promising step towards achieving a circular economy and sustainable development goals. Front. Chem. Eng. 2022, 4, 867228. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.; Antonietti, M.; Titirici, M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of high surface area sludge-based activated hydrochar via hydrothermal carbonization and application in the removal of basic dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Liu, S.; Wang, K.; Jiang, Y. Pore structure characterization of coal by NMR cryoporometry. Fuel 2017, 190, 359–369. [Google Scholar] [CrossRef]
- Achaw, O.-W. A study of the porosity of activated carbons using the scanning electron microscope. In Scanning Electron Microscopy; InTechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Apaydın-Varol, E.; Pütün, A.E. Preparation and characterization of pyrolytic chars from different biomass samples. J. Anal. Appl. Pyrolysis 2012, 98, 29–36. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Al Minshid, A.; Grassian, V.H. ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid–solid interface in environmentally and biologically relevant media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.; Dalai, A.K. Optimized production and characterization of highly porous activated carbon from algal-derived hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Thermogravimetric analysis and carbon stability of chars produced from slow pyrolysis and hydrothermal carbonization of manure waste. J. Anal. Appl. Pyrolysis 2019, 140, 434–443. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Niu, Y.; Xue, Q.; Ali, E.F.; Shaheen, S.M.; Tsang, D.C.; Rinklebe, J. Impact of catalytic hydrothermal treatment and Ca/Al-modified hydrochar on lability, sorption, and speciation of phosphorus in swine manure: Microscopic and spectroscopic investigations. Environ. Pollut. 2022, 299, 118877. [Google Scholar] [CrossRef]
- Yihunu, E.W.; Minale, M.; Abebe, S.; Limin, M. Preparation, characterization and cost analysis of activated biochar and hydrochar derived from agricultural waste: A comparative study. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Tu, W.; Liu, Y.; Xie, Z.; Chen, M.; Ma, L.; Du, G.; Zhu, M. A novel activation-hydrochar via hydrothermal carbonization and KOH activation of sewage sludge and coconut shell for biomass wastes: Preparation, characterization and adsorption properties. J. Colloid Interface Sci. 2021, 593, 390–407. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state 13C nuclear magnetic resonance (nmr) spectroscopy. Energy Fuels 2011, 25, 388–397. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Hoekman, S.K.; Liu, T.; Gai, C.; Peng, N. Homogeneously dispersed zerovalent iron nanoparticles supported on hydrochar-derived porous carbon: Simple, in situ synthesis and use for dechlorination of pcbs. ACS Sustain. Chem. Eng. 2016, 4, 3261–3267. [Google Scholar] [CrossRef]
- Fard, M.Y. Carbon nanotube network and interphase in buckypaper nanocomposites using atomic force microscopy. Int. J. Mech. Sci. 2021, 212, 106811. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Adsorptive removal of sulfonamides, tetracyclines and quinolones from wastewater and water using carbon-based materials: Recent developments and future directions. J. Clean. Prod. 2022, 349, 131421. [Google Scholar] [CrossRef]
- Adewuyi, A. Chemically modified biosorbents and their role in the removal of emerging pharmaceutical waste in the water system. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Lee, S.-C.; Jang, H.-Y.; Kim, S.-B. Characterization of ibuprofen removal by calcined spherical hydrochar through adsorption experiments, molecular modeling, and artificial neural network predictions. Chemosphere 2023, 311, 137074. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of emerging pollutants from water through adsorption onto carbon-based materials. In Emerging and Nanomaterial Contaminants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–213. [Google Scholar]
- Aghababaei, A.; Borugadda, V.B.; Dalai, A.; Niu, C.H. An investigation on adsorption of carbamazepine with adsorbents developed from flax shives: Kinetics, mechanisms, and desorption. Chem. Eng. Res. Des. 2023, 189, 138–155. [Google Scholar] [CrossRef]
- Liu, L.; Sim, S.F.; Lin, S.; Wan, J.; Zhang, W.; Li, Q.; Peng, C. Integrated structural and chemical analyses for HCl-supported hydrochar and their adsorption mechanisms for aqueous sulfachloropyridazine removal. J. Hazard. Mater. 2021, 417, 126009. [Google Scholar] [CrossRef]
- Su, F.; Peng, H.; Yin, H.; Luo, C.; Zhu, L.; Zhong, W.; Mao, L.; Yin, D. Biowaste-derived hydrochar microspheres: Realizing metal-free visible-light photocatalytic oxidation of amines. J. Catal. 2021, 404, 149–162. [Google Scholar] [CrossRef]
- Rocha, L.S.; Pereira, D.; Sousa, É; Otero, M.; Esteves, V.I.; Calisto, V. Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: A review. Sci. Total Environ. 2020, 718, 137272. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Ledesma, B.; Laginhas, C.; Titirici, M.-M. Surface interactions during the removal of emerging contaminants by hydrochar-based adsorbents. Molecules 2020, 25, 2264. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Z.; Guo, Z.; Chen, M.; Yan, J.; Qian, L.; Han, L.; Li, J.; Gu, M. Significant roles of surface functional groups and Fe/Co redox reactions on peroxymonosulfate activation by hydrochar-supported cobalt ferrite for simultaneous degradation of monochlorobenzene and p-chloroaniline. J. Hazard. Mater. 2023, 445, 130588. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Sun, K.; Sun, H.; Wang, Z.; Libra, J.A.; Xing, B. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Fu, B.; Zhai, Y.; Wang, W.; Maroney, A.; Keller, A.A.; Wang, H.; Chovelon, J.-M. Simultaneous removal of pharmaceuticals and heavy metals from aqueous phase via adsorptive strategy: A critical review. Water Res. 2023, 236, 119924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ji, Y.; Shao, Q. Facile modification of hydrochar derived from cotton straw with excellent sorption performance for antibiotics: Coupling DFT simulations with experiments. Sci. Total Environ. 2021, 760, 144124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumeren, I.; Hamid, N.A. Removal of chloramphenicol compounds using hydrochar from dried leaves. IIUM Eng. Congr. Proc. 2023, 1, 32–41. [Google Scholar]
- Chen, S.-Q.; Chen, Y.-L.; Jiang, H. Slow pyrolysis magnetization of hydrochar for effective and highly stable removal of tetracycline from aqueous solution. Ind. Eng. Chem. Res. 2017, 56, 3059–3066. [Google Scholar] [CrossRef]
- Qin, X.; Meng, W.; Cheng, S.; Xing, B.; Shi, C.; Nie, Y.; Wang, Q.; Xia, H. Efficient removal of heavy metal and antibiotics from wastewater by phosphate-modified hydrochar. Chemosphere 2023, 345, 140484. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Pazos, M.; Sanromán, A. Facile one-step synthesis of a versatile nitrogen-doped hydrochar from olive oil production waste, “alperujo”, for removing pharmaceuticals from wastewater. Environ. Pollut. 2023, 330, 121751. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; dos Santos, D.F.; Fonseca, B.C.d.S.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen removal by calcium alginate/activated hydrochar composite beads: Batch and fixed-bed studies. Int. J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef]
- Kim, D.G.; Choi, D.; Cheon, S.; Ko, S.-O.; Kang, S.; Oh, S. Addition of biochar into activated sludge improves removal of antibiotic ciprofloxacin. J. Water Process. Eng. 2020, 33, 101019. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-R.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Jiang, L.-H.; Tan, X.-F.; Huang, X.-X.; Yin, Z.-H.; Liu, N.; Li, J. Hydrothermal synthesis of montmorillonite/hydrochar nanocomposites and application for 17β-estradiol and 17α-ethynylestradiol removal. RSC Adv. 2018, 8, 4273–4283. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; Bergamasco, R.; Vareschini, D.T.; de Barros, M.A.S.D. Activated hydrochar produced from brewer's spent grain and its application in the removal of acetaminophen. Bioresour. Technol. 2020, 310, 123399. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Priya, A.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment—A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Méndez, A.; El Azzouzi, M.; Romero, E. New insights into the efficient removal of emerging contaminants by biochars and hydrochars derived from olive oil wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef]
- Ndoun, M.C.; Elliott, H.A.; Preisendanz, H.E.; Williams, C.F.; Knopf, A.; Watson, J.E. Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 2021, 3, 89–104. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Sep. Purif. Technol. 2021, 266, 118592. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis, E.A.S.; Porras, J.; Flórez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, X.; Xu, H.; Sun, Y.; Wu, J. Effects of temperature, solution pH, and ball-milling modification on the adsorption of non-steroidal anti-inflammatory drugs onto biochar. Bull. Environ. Contam. Toxicol. 2020, 105, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Tomul, F.; Ha, N.T.H.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.-T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Xie, S.; Wang, Y.; Ma, J.; Shang, X. Study of ciprofloxacin removal by biochar obtained from used tea leaves. J. Environ. Sci. 2018, 73, 20–30. [Google Scholar] [CrossRef]
- Dang, B.-T.; Gotore, O.; Ramaraj, R.; Unpaprom, Y.; Whangchai, N.; Bui, X.-T.; Maseda, H.; Itayama, T. Sustainability and application of corncob-derived biochar for removal of fluoroquinolones. Biomass Convers. Biorefinery 2022, 12, 913–923. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized biochar obtained from pine needles as a novel adsorbent to remove caffeine from aqueous solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- dos Santos Lins, P.V.; Henrique, D.C.; Ide, A.H.; de Paiva e Silva Zanta, C.L.; Meili, L. Evaluation of caffeine adsorption by MgAl-LDH/biochar composite. Environ. Sci. Pollut. Res. 2019, 26, 31804–31811. [Google Scholar] [CrossRef]
- Filipinas, J.Q.; Rivera, K.K.P.; Ong, D.C.; Pingul-Ong, S.M.B.; Abarca, R.R.M.; de Luna, M.D.G. Removal of sodium diclofenac from aqueous solutions by rice hull biochar. Biochar 2021, 3, 189–200. [Google Scholar] [CrossRef]
- Bagheri, A.; Abu-Danso, E.; Iqbal, J.; Bhatnagar, A. Modified biochar from Moringa seed powder for the removal of diclofenac from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 7318–7327. [Google Scholar] [CrossRef]
- Singh, V.; Srivastava, V.C. Self-engineered iron oxide nanoparticle incorporated on mesoporous biochar derived from textile mill sludge for the removal of an emerging pharmaceutical pollutant. Environ. Pollut. 2020, 259, 113822. [Google Scholar] [CrossRef]
- Anfar, Z.; Zbair, M.; Ahsiane, H.A.; Jada, A.; El Alem, N. Microwave assisted green synthesis of Fe 2 O 3/biochar for ultrasonic removal of nonsteroidal anti-inflammatory pharmaceuticals. RSC Adv. 2020, 10, 11371–11380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Du, Q.; Zhang, H.; Cui, Y. Antibiotic removal by agricultural waste biochars with different forms of iron oxide. RSC Adv. 2019, 9, 14143–14153. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Show, S.; Rahman, W.U.; Halder, G. Linearity and non-linearity analysis of isotherms and kinetics for ibuprofen remotion using superheated steam and acid modified biochar. Process. Saf. Environ. Prot. 2019, 126, 193–204. [Google Scholar] [CrossRef]
- Chakraborty, P.; Singh, S.D.; Gorai, I.; Singh, D.; Rahman, W.-U.; Halder, G. Explication of physically and chemically treated date stone biochar for sorptive remotion of ibuprofen from aqueous solution. J. Water Process. Eng. 2020, 33, 101022. [Google Scholar] [CrossRef]
- Chakraborty, P.; Banerjee, S.; Kumar, S.; Sadhukhan, S.; Halder, G. Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: Isotherm, kinetics, thermodynamics and cost estimation. Process. Saf. Environ. Prot. 2018, 118, 10–23. [Google Scholar] [CrossRef]
- Mondal, S.; Bobde, K.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: Equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manag. 2016, 182, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total Environ. 2021, 751, 141755. [Google Scholar] [CrossRef]
- Wang, H.; Fang, C.; Wang, Q.; Chu, Y.; Song, Y.; Chen, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv. 2018, 8, 16260–16268. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Ahmad, M.; Mohan, D.; Chang, S.X.; Ok, Y.S. Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour. Technol. 2014, 166, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.; Memon, N.; Memon, S.Q.; Yavuz, H.; Lachgar, A.; Denizli, A. Evaluation of hydrochar efficiency for simultaneous removal of diclofenac and ibuprofen from aqueous system using surface response methodology. Environ. Sci. Pollut. Res. 2019, 26, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hayoun, B.; Escudero-Curiel, S.; Bourouina, M.; Bourouina-Bacha, S.; Sanromán, M.A.; Pazos, M. Preparation and characterization of high performance hydrochar for efficient adsorption of drugs mixture. J. Mol. Liq. 2022, 353, 118797. [Google Scholar] [CrossRef]
- Oumabady, S.; Selvaraj, P.S.; Periasamy, K.; Veeraswamy, D.; Ramesh, P.T.; Palanisami, T.; Ramasamy, S.P. Kinetic and isotherm insights of Diclofenac removal by sludge derived hydrochar. Sci. Rep. 2022, 12, 2184. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N.; Yap, P.-S.; Iwuchukwu, F.U.; Liu, T.; de Souza, E.C.; Ighalo, J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 104, 117–135. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol. Sci. Total Environ. 2018, 628, 722–730. [Google Scholar] [CrossRef]
- Chatir, E.M.; El Hadrami, A.; Ojala, S.; Brahmi, R. Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere. Surf. Interfaces 2022, 30, 101849. [Google Scholar] [CrossRef]
- Foster, S.A.; Pennino, M.J.; Compton, J.E.; Leibowitz, S.G.; Kile, M.L. Arsenic drinking water violations decreased across the united states following revision of the maximum contaminant level. Environ. Sci. Technol. 2019, 53, 11478–11485. [Google Scholar] [CrossRef]
- WHO Organization. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; WHO Organization: Geneva, Switzerland, 2017.
- Dettori, M.; Arghittu, A.; Deiana, G.; Castiglia, P.; Azara, A. The revised European Directive 2020/2184 on the quality of water intended for human consumption. A step forward in risk assessment, consumer safety and informative communication. Environ. Res. 2022, 209, 112773. [Google Scholar] [CrossRef]
- Van Winckel, T.; Cools, J.; Vlaeminck, S.E.; Joos, P.; Van Meenen, E.; Borregán-Ochando, E.; Steen, K.V.D.; Geerts, R.; Vandermoere, F.; Blust, R. Towards harmonization of water quality management: A comparison of chemical drinking water and surface water quality standards around the globe. J. Environ. Manag. 2021, 298, 113447. [Google Scholar] [CrossRef]
- Lee, D.; Choi, K. Comparison of regulatory frameworks of environmental risk assessments for human pharmaceuticals in EU, USA, and Canada. Sci. Total Environ. 2019, 671, 1026–1035. [Google Scholar] [CrossRef]
- Miettinen, M.; Khan, S.A. Pharmaceutical pollution: A weakly regulated global environmental risk. Rev. Eur. Comp. Int. Environ. Law 2022, 31, 75–88. [Google Scholar] [CrossRef]
- Fernández-Sanromán, Á.; Lama, G.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Bridging the gap to hydrochar production and its application into frameworks of bioenergy, environmental and biocatalysis areas. Bioresour. Technol. 2021, 320, 124399. [Google Scholar] [CrossRef]
- Somorin, T.; Campos, L.; Kinobe, J.; Kulabako; Afolabi, O. Sustainable valorisation of agri-food waste from open-air markets in Kampala, Uganda via standalone and integrated waste conversion technologies. Biomass Bioenergy 2023, 172, 106752. [Google Scholar] [CrossRef]
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C. Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722. [Google Scholar] [CrossRef]
- Islam, M.A.; Limon, M.S.H.; Romić, M.; Islam, M.A. Hydrochar-based soil amendments for agriculture: A review of recent progress. Arab. J. Geosci. 2021, 14, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Hu, G.; Basar, I.A.; Li, J.; Lyczko, N.; Nzihou, A.; Eskicioglu, C. Phosphorus recovery from municipal sludge-derived ash and hydrochar through wet-chemical technology: A review towards sustainable waste management. Chem. Eng. J. 2021, 417, 129300. [Google Scholar] [CrossRef]
- Bardhan, M.; Novera, T.M.; Tabassum, M.; Islam, A.; Islam, A.; Hameed, B. Co-hydrothermal carbonization of different feedstocks to hydrochar as potential energy for the future world: A review. J. Clean. Prod. 2021, 298, 126734. [Google Scholar] [CrossRef]
- Munir, M.T.; Saqib, N.U.; Li, B.; Naqvi, M. Food waste hydrochar: An alternate clean fuel for steel industry. Fuel 2023, 346, 128395. [Google Scholar] [CrossRef]
- Hussin, F.; Hazani, N.N.; Khalil, M.; Aroua, M.K. Environmental life cycle assessment of biomass conversion using hydrothermal technology: A review. Fuel Process. Technol. 2023, 246, 107747. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Yacout, D.M.; Van Caneghem, J.; Jansson, S. Comparative environmental assessment of end-of-life carbonaceous water treatment adsorbents. Bioresour. Technol. 2020, 302, 122866. [Google Scholar] [CrossRef]
- Gievers, F.; Loewen, A.; Nelles, M. Hydrothermal carbonization (HTC) of sewage sludge: GHG emissions of various hydrochar applications. In Progress in Life Cycle Assessment; Springer: Cham, Switzerland, 2019; pp. 59–68. [Google Scholar]
- Liu, X.; Hoekman, S.K.; Farthing, W.; Felix, L. TC2015: Life cycle analysis of co-formed coal fines and hydrochar produced in twin-screw extruder (TSE). Environ. Prog. Sustain. Energy 2017, 36, 668–676. [Google Scholar] [CrossRef]
- Durak, H. Comprehensive assessment of thermochemical processes for sustainable waste management and resource recovery. Processes 2023, 11, 2092. [Google Scholar] [CrossRef]
- Esquiaqui, L.; Santos, S.D.F.d.O.M.; Ugaya, C.M.L. A systematic review of densified biomass products life cycle assessments. Int. J. Environ. Sci. Technol. 2023, 20, 9311–9334. [Google Scholar] [CrossRef]
- Nadarajah, K. Customized Hydrochar from Biomass Materials for Water Treatment. Ph.D. Thesis, Queensland University of Technology, Brisbane City, Australia, 2020. [Google Scholar]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).