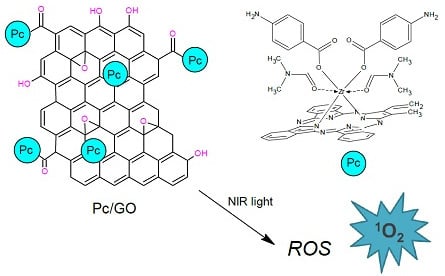
Synthesis, Spectroscopic Characterization and Photoactivity of Zr(IV) Phthalocyanines Functionalized with Aminobenzoic Acids and Their GO-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Syntheses
2.1.1. Pc Complexes
2.1.2. Pc Composites
2.2. Instrumentation
2.3. Materials Characterization
3. Results
3.1. Characterization of Pc Complexes and Composites
3.2. Spectroscopic Studies and Singlet Oxygen Generation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jones, R.; Davidson, K.; Krier, A. Structure, electrical conductivity and electrochromism in thin films of substituted and unsubstituted lanthanide bisphthalocyanines. Thin Solid Film 1997, 298, 228–236. [Google Scholar] [CrossRef]
- Duarte, J.P.; Vilao, R.C.; Gil, J.M.; Alberto, H.V.; de Campos, N.A.; Weidinger, A. Muoniated radicals in the organic semiconductor zinc-phthalocyanine. Physica B 2003, 326, 94–96. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Touda, H.; Takada, M.; Matsushige, K. Quasi-intrinsic semiconducting state of titanyl-phthalocyanine films obtained under ultrahigh vacuum conditions. Appl. Phys. Lett. 2000, 76, 873–875. [Google Scholar] [CrossRef] [Green Version]
- Peisert, H.; Knupfer, M.; Schwieger, T.; Auerhammer, J.M.; Golden, M.S.; Fink, J. Full characterization of the interface between the organic semiconductor copper phthalocyanine and gold. J. Appl. Phys. 2002, 91, 4872–4878. [Google Scholar] [CrossRef]
- Drobizhev, M.; Rebane, A.; Spahni, H.; Spangler, W.; Wolleb, H. Two Photon or Multiphoton Phthalocyanine Based Absorption Material for Optical Data Storage. European Patent Pub. No. WO/2007/014849, 8 February 2007. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007014849&tab=PCTBIBLIO (accessed on 22 December 2019).
- Li, X.; Ng, D.K.P. Synthesis and spectroscopic properties of the first phthalocyanine–nucleobase conjugates. Tetrahedron Lett. 2001, 42, 305–309. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer. Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Role of activated oxygen species in photodynamic therapy. Methods Enzymol. 2000, 319, 376–400. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyrins Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Allen, C.M.; Sharman, W.M.; van Lier, J.E.J. Current status of phthalocyanines in the photodynamic therapy of cancer. J. Porphyryns Phthalocyanines 2001, 5, 161–169. [Google Scholar] [CrossRef]
- Yslas, E.I.; Durantini, E.N.; Rivarola, V.A. Zinc-(II) 2,9,16,23-tetrakis (methoxy) phthalocyanine: Potential photosensitizer for use in photodynamic therapy in vitro. Bioorg. Med. Chem. 2007, 15, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Vittar, N.B.R.; Awruch, J.; Azizuddin, K.; Rivarola, V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int. J. Biochem. Cell Biol. 2010, 42, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Diao, J.; Wang, D.; Liu, J.; Zhang, J. Design, synthesis and biological evaluation of folate-porphyrin: A new photosensitizer for targeted photodynamic therapy. J. Porphyrins Phthalocyanines 2010, 14, 547–555. [Google Scholar] [CrossRef]
- Pereira, M.M.; Monteiro, C.J.P.; Simoes, A.V.C.; Pinto, S.M.A.; Arnaut, L.G.; Sa, G.F.F.; Silva, E.F.F.; Rocha, L.B.; Simoes, S.; Formosinho, S.J. Synthesis and photophysical properties of amphiphilic halogenated bacteriochlorins: New opportunities for photodynamic therapy of cancer. J. Porphyrins Phthalocyanines 2009, 13, 567–573. [Google Scholar] [CrossRef]
- Rani-Beeram, S.; Meyer, K.; McCrate, A.; Hong, Y.; Nielsen, M.; Swavey, S. A Fluorinated ruthenium porphyrin as a potential photodynamic therapy agent: Synthesis, characterization, DNA binding, and melanoma Cell Studies. Inorg. Chem. 2008, 47, 11278–11283. [Google Scholar] [CrossRef]
- Ejsmont, K.; Kubiak, R. (1,2-Benzenedicarbonitrile-kN)diiodo-(phthalocyaninato-k4N)zirconium(IV). Acta Cryst. C 1998, 54, 572–574. [Google Scholar] [CrossRef]
- Tomachynski, L.A.; Chernii, V.Y.; Volkov, S.V. Synthesis and properties of axially substituted zirconium(IV) and hafnium(IV) phthalocyanines with organic ligands. J. Porphyrins Phthalocyanines 2001, 5, 731–734. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Volkov, S.; Chernii, V.; Tomachynski, L.; Radzki, S. Synthesis and spectral properties of axially substituted zirconium(IV) and hafnium(IV) water soluble phthalocyanines in solutions. J. Alloys Compd. 2004, 380, 186–190. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Chernii, V.; Tomachynski, L.; Kowalska, M.; Legendziewicz, J.; Radzki, S. Correlation between computer models of structure of 5-sulfosalicylato Zr(IV) phthalocyanine with results obtained by NMR, ESI-MS and UV–Vis spectra. Opt. Mater. 2010, 32, 1193–1201. [Google Scholar] [CrossRef]
- Tomachynski, L.A.; Tretyakova, I.; Chernii, V.; Kowalska, M.; Volkov, S.V.; Gerasymchuk, Y.; Radzki, S. Synthesis and spectral properties of Zr(IV) and Hf(IV) phthalocyanines with β-diketonates as axial ligands. Inorg. Chim. Acta 2008, 361, 2569–2581. [Google Scholar] [CrossRef]
- Hao, X.; Chen, S.; Zhu, H.; Wang, L.; Zhang, Y.; Yin, Y. The synergy of graphene oxide and polydopamine assisted immobilization of lysozyme to improve antibacterial properties. Chem. Sel. 2017, 2, 2174–2182. [Google Scholar] [CrossRef]
- Hao, X.; Chen, S.; Yu, H.; Liu, D.; Sun, W. Metal ion-coordinated carboxymethylated chitosan grafted carbon nanotubes with enhanced antibacterial properties. RSC Adv. 2016, 6, 39–43. [Google Scholar] [CrossRef]
- Hao, X.; Chen, S.; Wang, W.; Yang, Z.; Yue, L.; Sun, H.; Cheng, F. Ag NP-coordinated glucosamine-grafted carbon nanotubes with enhanced antibacterial properties. New J. Chem. 2017, 41, 7045–7051. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Hu, W.B.; Peng, C.; Luo, W.J.; Lv, M.; Li, X.M.; Li, D.; Huang, Q.; Fan, C.H. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide-chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
- Karimi, L.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Mirjalili, M. Using graphene/TiO2 nanocomposite as a new route for preparation of electroconductive, self-cleaning, antibacterial and antifungal cotton fabric without toxicity. Cellulose 2014, 21, 3813–3827. [Google Scholar] [CrossRef]
- Mi, B.X. Graphene oxide membranes for ionic and molecular sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B.X. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.F.; Yuan, H.Y.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H.J. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Zou, J.L.; Chen, X.L. Using silica nanoparticles as a catalyst carrier to the highly sensitive determination of thiamine. Microchem. J. 2007, 86, 42–47. [Google Scholar] [CrossRef]
- Tomachynski, L.A.; Chernii, V.Y.; Volkov, S.V. Synthesis of dichlorophthalocyaninato complexes of titanium, zirconium, and hafnium. Russ. J. Inorg. Chem. 2002, 47, 254–257. [Google Scholar]
- Gerasymchuk, Y.; Lukowiak, A.; Wedzynska, A.; Kedziora, A.; Bugla-Ploskonska, G.; Piatek, D.; Bachanek, T.; Chernii, V.; Tomachynski, L.; Strek, W. New photosensitive nanometric graphite oxide composites as antimicrobial material with prolonged action. J. Inorg. Biochem. 2016, 159, 142–148. [Google Scholar] [CrossRef]
- Tomachynski, L.A.; Chernii, V.Y.; Gorbenko, H.N.; Filonenko, V.V.; Volkov, S.V. Synthesis, spectral properties, and antitumor activity of a new axially substituted phthalocyanine complex of zirconium(IV) with citric acid. Chem. Biodivers. 2004, 1, 862–867. [Google Scholar] [CrossRef]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Henderson, B.W.; Doougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–147. [Google Scholar] [CrossRef]
- Hanuza, J.; Godlewska, P.; Kadłubański, P.; Ptak, M.; Mączka, M.; Gerasymchuk, Y.S.; Legendziewicz, J. Molecular structure and vibrational properties of pyramidal MPc+ phthalocyanine cation in InPcI and LuPc(OAc) complexes. J. Mol. Struct. 2017, 1130, 699–710. [Google Scholar] [CrossRef]
- Song, M.; Tahershamsi, L.; Zhao, J.; Zhang, Z.; Grennberg, H. Efficient gelation of graphene oxide aqueous dispersion induced by sonication-promoted Leuckart reaction. ChemNanoMat 2018, 4, 1145–1152. [Google Scholar] [CrossRef]
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Table of Spectral Data for Structure Determination of Organic Compounds, 3rd ed.; Springer-Verlag: Berlin, Germany, 1998; pp. 238–305. [Google Scholar]
- Schafer, M.; Drayß, M.; Springer, A.; Zacharias, P.; Meerholz, K. Radical cations in electrospray mass spectrometry: Formation of open-shell species, examination of the fragmentation behaviour in ESI-MSn and reaction mechanism studies by detection of transient radical cations. Eur. J. Org. Chem. 2007, 31, 5162–5174. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Chernii, V.Y.; Tomachynski, L.A.; Legendziewicz, J.; Radzki, S. Spectroscopic characterization of zirconium(IV) and hafnium(IV) gallatephthalocyanines in monolithic silica gels obtained by sol-gel method. Opt. Mater. 2005, 27, 1484–1494. [Google Scholar] [CrossRef]
- Lukowiak, A.; Gerasymchuk, Y.; Wedzynska, A.; Tahershamsi, L.; Tomala, R.; Strek, W.; Piatek, D.; Krona-Glowniak, I.; Speruda, M.; Kedziora, A.; et al. Light-activated zirconium(IV) phthalocyanine derivatives linked to graphite oxide flakes and discussion on their antibacterial activity. Appl. Sci. 2019, 9, 4447. [Google Scholar] [CrossRef] [Green Version]
- Güzel, E.; Şaki, N.; Akın, M.; Nebioğlu, M.; Şişman, İ.; Erdoğmuş, A.; Koçak, M.B. Zinc and chloroindium complexes of furan-2-ylmethoxy substituted phthalocyanines: Preparation and investigation of aggregation, singlet oxygen generation, antioxidant and antimicrobial properties. Synth. Met. 2018, 245, 127–134. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahershamsi, L.; Gerasymchuk, Y.; Wedzynska, A.; Ptak, M.; Tretyakova, I.; Lukowiak, A. Synthesis, Spectroscopic Characterization and Photoactivity of Zr(IV) Phthalocyanines Functionalized with Aminobenzoic Acids and Their GO-Based Composites. C 2020, 6, 1. https://doi.org/10.3390/c6010001
Tahershamsi L, Gerasymchuk Y, Wedzynska A, Ptak M, Tretyakova I, Lukowiak A. Synthesis, Spectroscopic Characterization and Photoactivity of Zr(IV) Phthalocyanines Functionalized with Aminobenzoic Acids and Their GO-Based Composites. C. 2020; 6(1):1. https://doi.org/10.3390/c6010001
Chicago/Turabian StyleTahershamsi, Leili, Yuriy Gerasymchuk, Anna Wedzynska, Maciej Ptak, Iryna Tretyakova, and Anna Lukowiak. 2020. "Synthesis, Spectroscopic Characterization and Photoactivity of Zr(IV) Phthalocyanines Functionalized with Aminobenzoic Acids and Their GO-Based Composites" C 6, no. 1: 1. https://doi.org/10.3390/c6010001