Production and Quality Analysis of Wine from Honey and Coconut Milk Blend Using Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
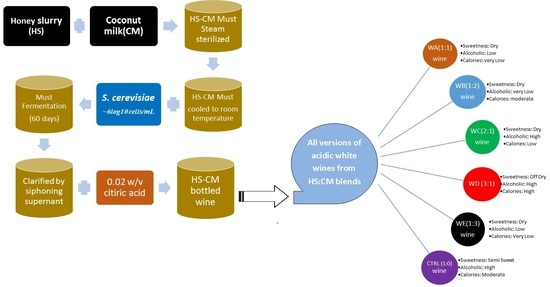
2.1. Sample Collection/Preparation
2.2. Preparation of Inoculum Starter Culture
2.3. Preparation and Fermentation Protocol
2.4. Specific Gravity
2.5. Proximate Compositionand Physicochemical Analysis
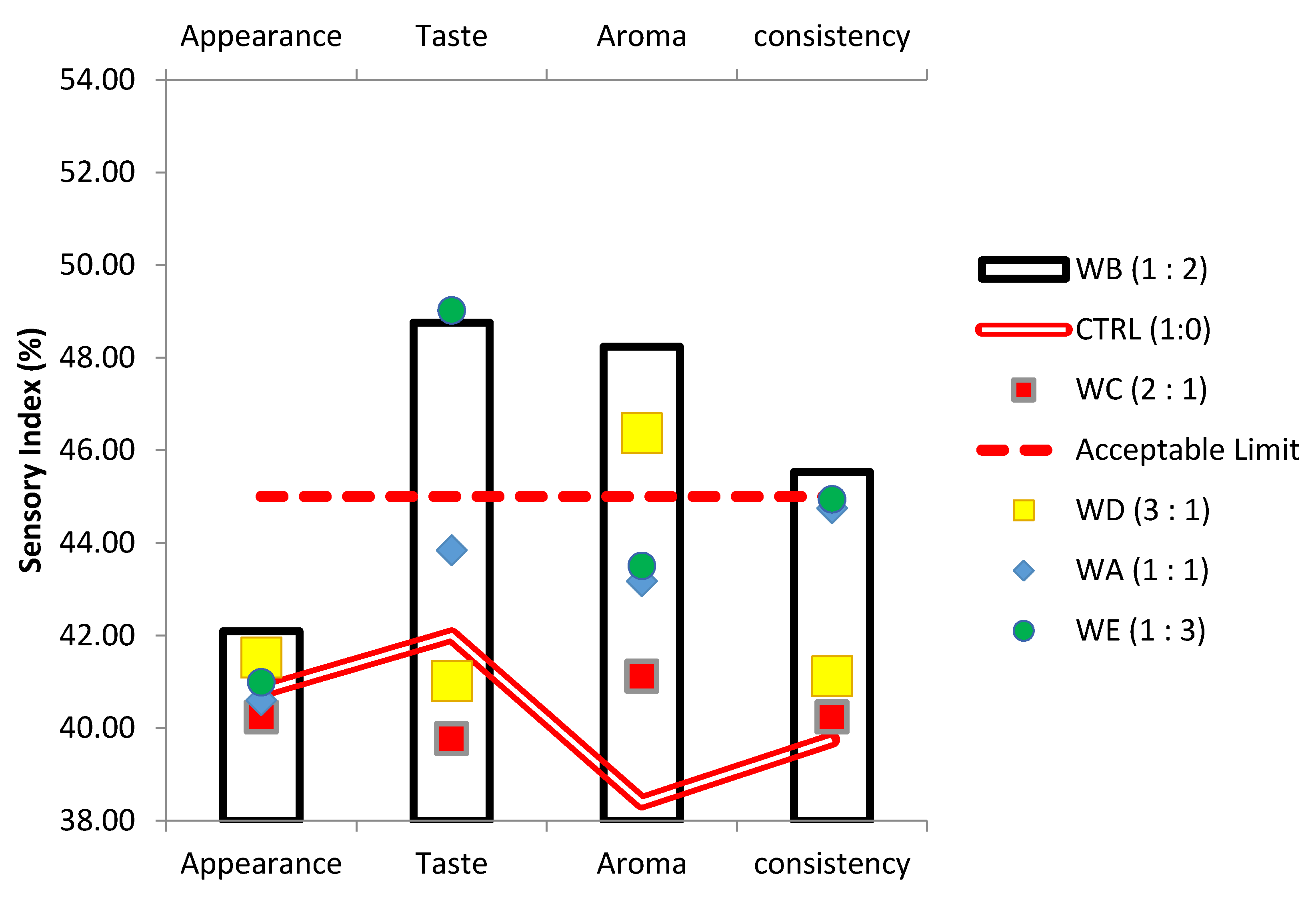
2.6. Sensory Evaluation
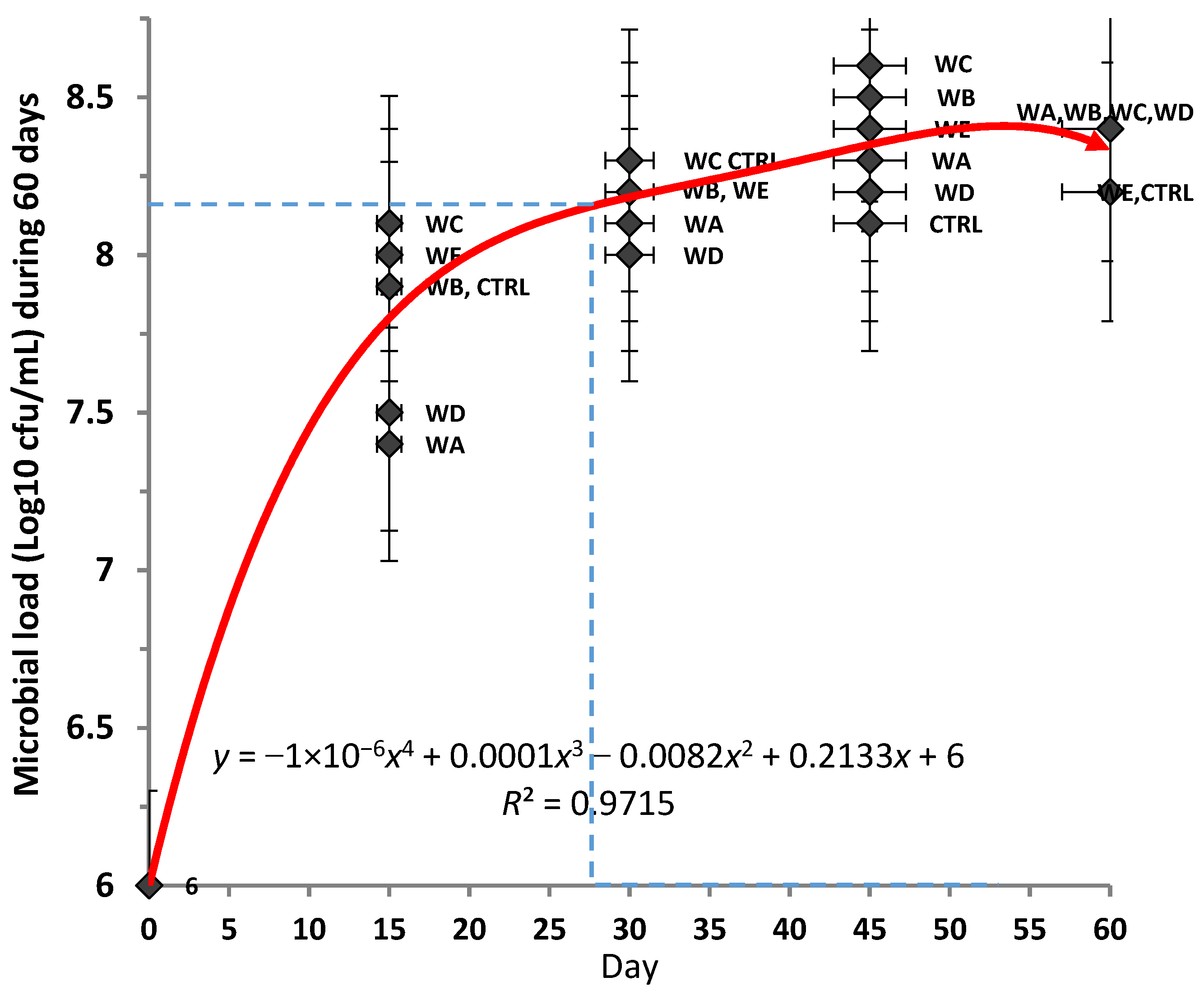
2.7. Microbial Analysis
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Steinkraus, K.H. Fermentation in World Food Processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–27. [Google Scholar] [CrossRef]
- Balogu, T.V.; Abdulkadir, A.; Ikegwu, M.T.; Akpadolu, B.; Akpadolu, K. Production and Sensory Evaluation of Non-Alcoholic Wine from Sugarcane and Tiger Nut Blend Using Saccharomyces cerevisiae. Int. J. BioSci. Agric. Technol. 2016, 7, 7–14. [Google Scholar]
- Gupta, J.K.; Sharma, R. Production Technology and quality characteristics of mead and fruit-honey wines. Nat. Prod. Radiance 2009, 8, 345–355. [Google Scholar]
- Kraus, E.C. Mead (Honey) Wine. Copyright of Home Wine & Beer Making Supplies. 2012. Available online: www.eckraus.com (accessed on 27 January 2017).
- Rhim, J.W.; Kim, D.H.; Jung, S.I. Production of Fermented Honey Wine. Korean J. Food Sci. Technol. 1997, 29, 337–342. [Google Scholar]
- Sarba, A.C. Obtaining Different Types of Wine Products from Honey. Unpublished. Ph.D. Thesis, Doctoral School of Engineering, Faculty of Animal Breeding and Biotechnologies, University of Agricultural Science and Veterinary Medicine, Cluj-Napoca, Romania, 2015. Available online: http://www.usamvcluj.ro/en/files/teze/en/2015/sarba.pdf (accessed on 22 March 2017).
- Tamang, J.P.; Tamang, B.; Schillinger, U.; Guigas, C.; Holzapfel, W.H. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int. J. Food Microbiol. 2009, 135, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, P.T.; Kasipathy, K. Fermented Food and Beverage of the World; CRC Press: London, UK; New York, NY, USA, 2010; pp. 114–116. [Google Scholar]
- Battcock, M.; Azam-Ali, S. Fermented Fruits and Vegetables: A Global Perspective; FAO Agricultural Services Bulletin No. 134; FAO: Roma, Italy, 1998; Available online: http://www.fao.org/docrep/x0560e/x0560e00.htm (accessed on 28 March 2017).
- American Society for Brewing Chemists (ASBC). Methods of Analysis of ABSC, 14th ed.; ASBC: Saint Paul, MN, USA, 2011. [Google Scholar]
- May, D.; Sharpe, A. The Only Wine Book You’ll Ever Need; Adams Media, F + W Publication Company: Cincinnati, OH, USA, 2004; p. 275. [Google Scholar]
- Delfini, C.; Formica, J.V. Wine Microbiology: Science and Technology (Food Science and Technology); CRC Press: Boca Raton, FL, USA, 2001; p. 496. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1999; Volume 11. [Google Scholar]
- OIV: Organization of Compendium of International Methods of Wine and Must Analysis, 2012 Edition. Available online: https://www.scribd.com/document/324259472/Metodos-Analisis-Vol-2-OIV (accessed on 15 March 2017).
- Coelho, J.M.; Howe, P.A.; Sacks, G.L. A Headspace Gas Detection Tube Method for Measurement of SO2 in Wine without Disruption of Sulfur Dioxide Equilibria. Am. J. Enol. Vitic. 2015, 66, 257–265. [Google Scholar] [CrossRef]
- International Standard Organization (ISO). Sensory Analysis Standards. 2017. Available online: https://www.iso.org (accessed on 22 March 2017).
- Cowan, S.T.; Steel, K.J. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: London, UK, 2004; p. 331. [Google Scholar]
- Buba, F.; Gidado, A.; Shugaba, A. Physicochemical and Microbiological Properties of Honey from North East Nigeria. Biochem. Anal. Biochem. 2013, 2, 142. [Google Scholar] [CrossRef]
- Awada, M.H.; Elgornazi, A.M. Physicochemical characterization of honey from KasrKhiar and Garaboli areas-Libya. Asian J. Plant Sci. Res. 2016, 6, 8–12. [Google Scholar]
- Zoecklein, B.Z.; Fugelsang, F.C.; Gump, B.H.; Nury, F.S. Wine analysis and production; Springer: New York, NY, USA, 2005. [Google Scholar]
- Gardner, D. Volatile Acidity in Wine: Wine Made Easy. Available online: http://extension.psu.edu/food/enology/wine-production/wine-made-easy-fact-sheets/volatile-acidity-in-wine/extension_publication_file (accessed on 15 March 2017).
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology: Practical Applications and Procedures, 2nd ed.; Springer: New York, NY, USA, 2007; p. 381. [Google Scholar]
- Cordero-Bueso, G.; Esteve-Zarzoso, B.; Gil-Díaz, M.; García, M.; Cabellos, J.M.; Arroyo, T. Improvement of Malvar Wine Quality by Use of Locally-Selected Saccharomyces cerevisiae Strains. Fermentation 2016, 2, 1–13. [Google Scholar] [CrossRef]
- Umeh, J.I.; Ejikeme, P.C.N.; Egbuna, S.O. Optimization of Process Conditions for Alcoholic Wine Production from Pineapple Using RSM. Int. J. Multidiscip. Sci. Eng. 2015, 6, 23–30. [Google Scholar]
- Reddy, L.V.; Reddy, O.V.S. Production, optimization and characterization of wine from Mango (Mangiferaindica Linn.). Nat. Prod. Radiance 2009, 8, 426–435. [Google Scholar]
- Sanchez, S.; Demain, A.L. Metabolic Regulation and Overproduction of Primary Metabolites. Microbiol. Biotechnol. 2008, 1, 283–319. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Liden, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Raw Honey | Raw Coconut CM (0:1) | HS (1:1) |
|---|---|---|---|
| Sugar content (g/L) | 68.74 | 33.41 | 36.63 |
| Moisture content (%) | 16.7 | 72.5 | 31.6 |
| Ash (%) | 0.35 | 0.85 | 0.16 |
| Crude fat content (%) | ND | 18.3 | ND |
| pH | 4.05 | 5.56 | 4.35 |
| Specific Gravity | 1.4231 | 0.995 | 1.1250 |
| Parameters | WA (1:1) | WB (1:2) | WC (2:1) | WD (3:1) | WE (1:3) | CTRL (1:0) |
|---|---|---|---|---|---|---|
| pH | 4.10 | 4.20 | 4.20 | 4.20 | 4.20 | 4.20 |
| Temperature (°C) | 26.0 | 27.0 | 26.0 | 26.0 | 26.0 | 27.0 |
| Total titratable acidity (g/L) | 12.9 | 12.5 | 13.1 | 13.7 | 19.3 | 14.3 |
| Attenuation (%) | 94.50 | 81.78 | 98.63 | 97.6 | 72.81 | 91.265 |
| Volatile acidity (g/L) | 7.74 | 7.5 | 7.86 | 8.22 | 11.58 | 8.58 |
| Residual sugar (g/L) | 3.93 | 3.24 | 3.01 | 12.72 | 5.01 | 25.20 |
| Fermentative capacity (g/L) | 72.59 | 15.49 | 247.15 | 295.24 | 14.10 | 263.80 |
| Apparent fermentative degree (%) | 3.04 | 0.67 | 9.65 | 11.29 | 0.61 | 10.14 |
| Fermentation velocity | 0.51 | 0.58 | 0.99 | 0.59 | 0.65 | 0.58 |
| Free SO2 (mg/L) | 39.34 | 7.47 | 4.77 | 4.31 | 8.04 | 4.30 |
| Initial Specific Gravity | 1.0331 | 1.0081 | 1.1082 | 1.1332 | 1.0083 | 1.1250 |
| Final Specific Gravity | 1.0017 | 1.0014 | 1.0013 | 1.0055 | 1.0022 | 1.0109 |
| Parameter | WA (1:1) | WB (1:2) | WC (2:1) | WD (3:1) | WE (1:3) |
|---|---|---|---|---|---|
| Moisture content (%) | 94.31 | 92.65 | 89.83 | 91.23 | 96.98 |
| Ash content (%) | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 |
| Total protein content (%) | ND | ND | ND | ND | ND |
| Crude fiber (%) | ND | ND | ND | ND | ND |
| Crude fat content (%) | ND | ND | ND | ND | ND |
| Total carbohydrate (%) | 5.67 | 7.32 | 10.16 | 8.75 | 3.01 |
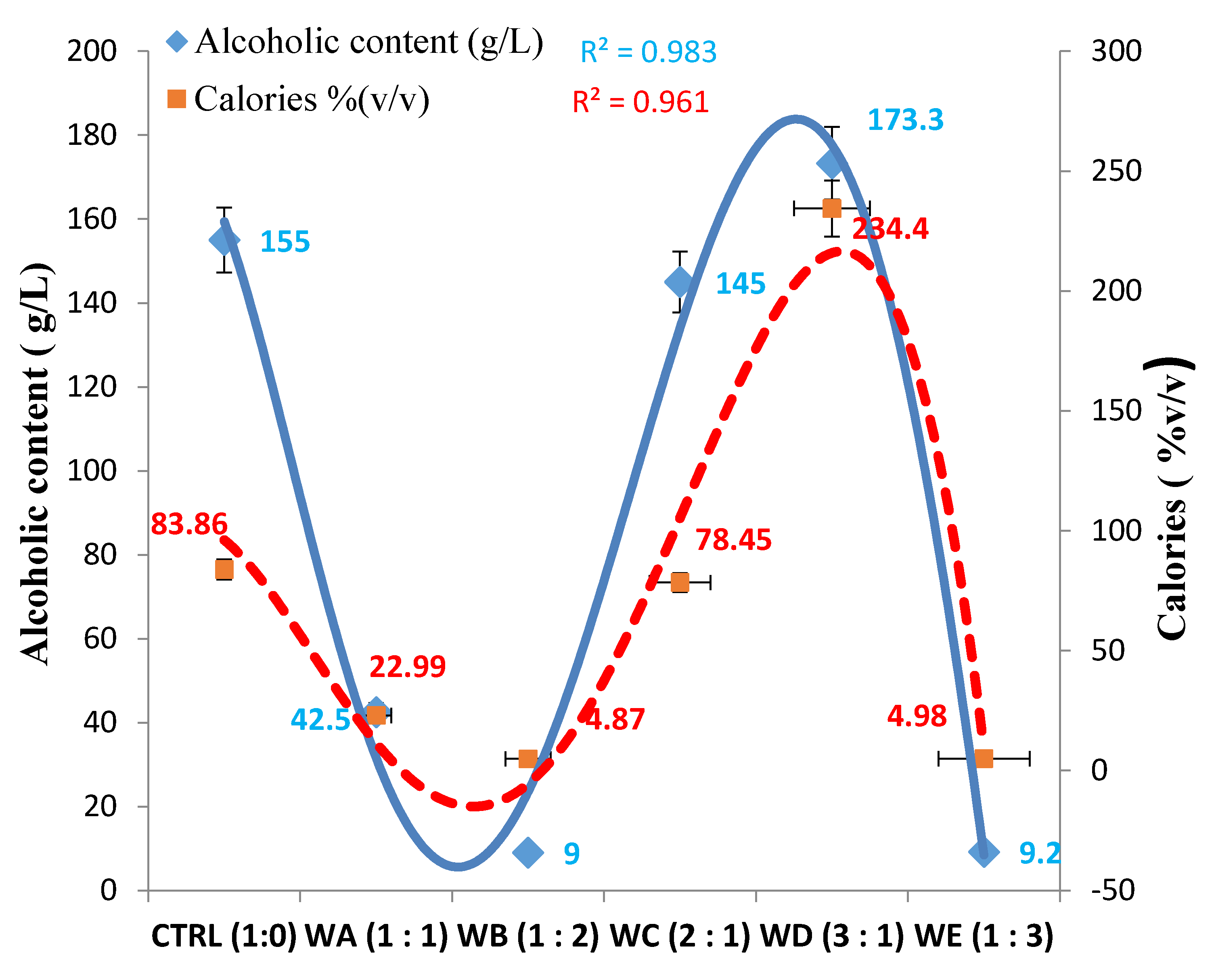
| Parameter | WA (1:1) | WB (1:2) | WC (2:1) | WD (3:1) | WE (1:3) | CTRL (1:0) |
|---|---|---|---|---|---|---|
| Sweetness | Dry | Dry | Dry | Off-Dry | Dry | Semi-Sweet |
| Calories | Very Low | Very Low | Low | High | Very Low | Moderate |
| Acidity/alkalinity | Acidic | Acidic | Acidic | Acidic | Acidic | Acidic |
| Alcoholic | Low | Moderate | High | High | Low | High |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogu, T.V.; Towobola, O. Production and Quality Analysis of Wine from Honey and Coconut Milk Blend Using Saccharomyces cerevisiae. Fermentation 2017, 3, 16. https://doi.org/10.3390/fermentation3020016
Balogu TV, Towobola O. Production and Quality Analysis of Wine from Honey and Coconut Milk Blend Using Saccharomyces cerevisiae. Fermentation. 2017; 3(2):16. https://doi.org/10.3390/fermentation3020016
Chicago/Turabian StyleBalogu, Tochukwu V., and Oyinloye Towobola. 2017. "Production and Quality Analysis of Wine from Honey and Coconut Milk Blend Using Saccharomyces cerevisiae" Fermentation 3, no. 2: 16. https://doi.org/10.3390/fermentation3020016