Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Stock Solution Preparation
2.2. Fruit Juice Preparation
2.2.1. Fruit Juice Fermentation
2.2.2. Fruit Juice with Short and Long Chain Inulin Fiber
2.3. Microbial Analysis
2.4. Analysis of pH
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. Microbial Analysis
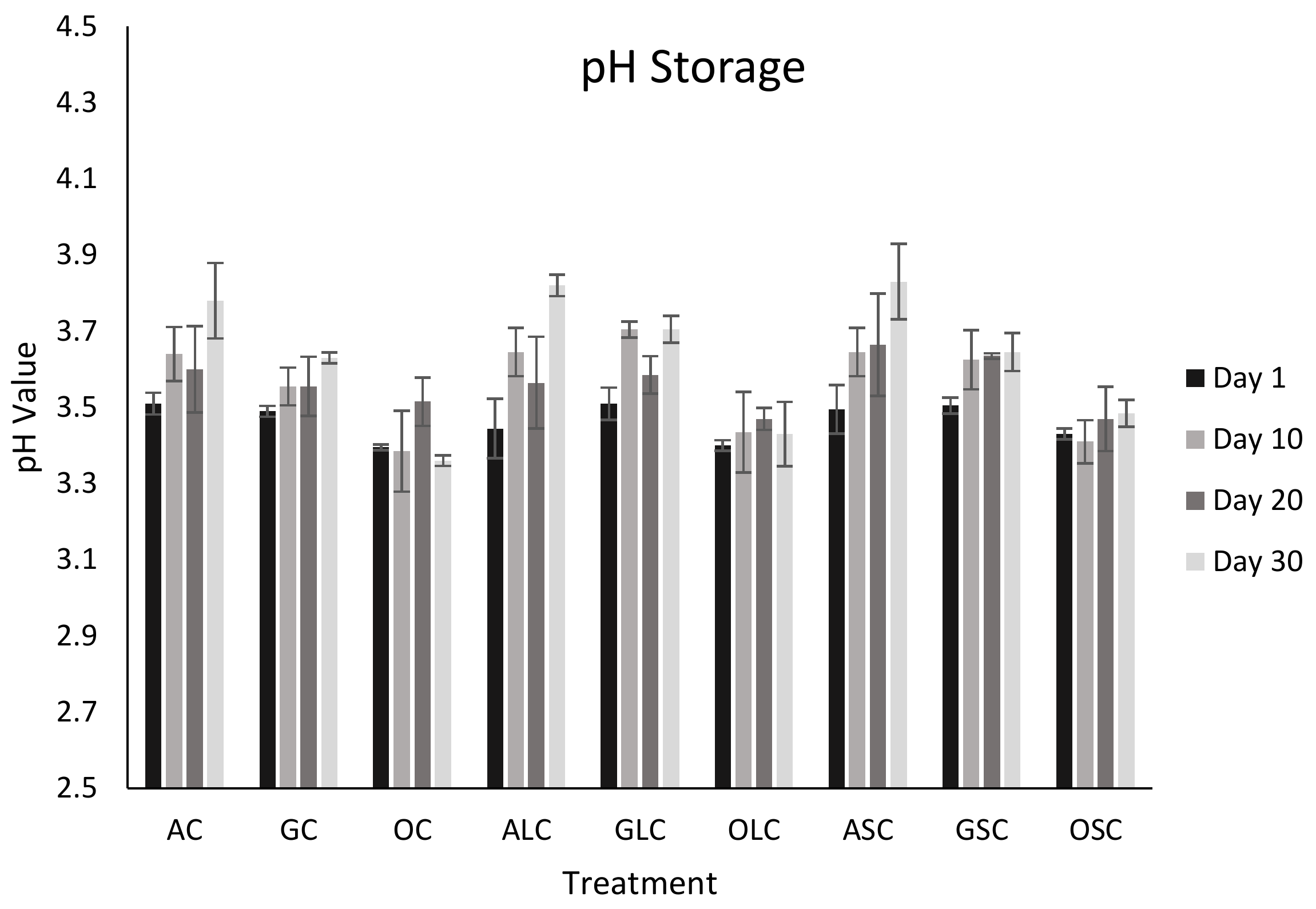
3.2. pH Analysis
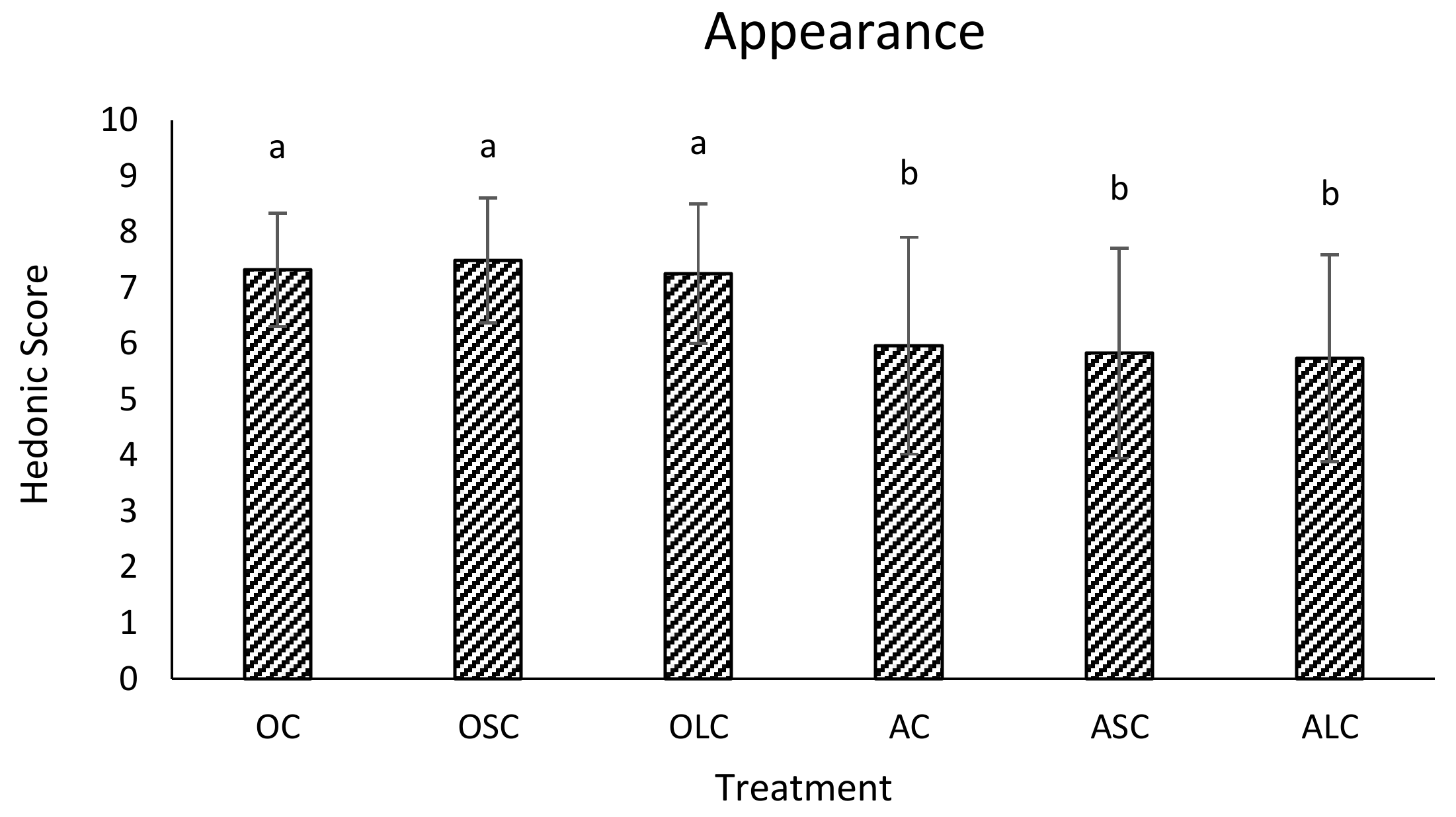
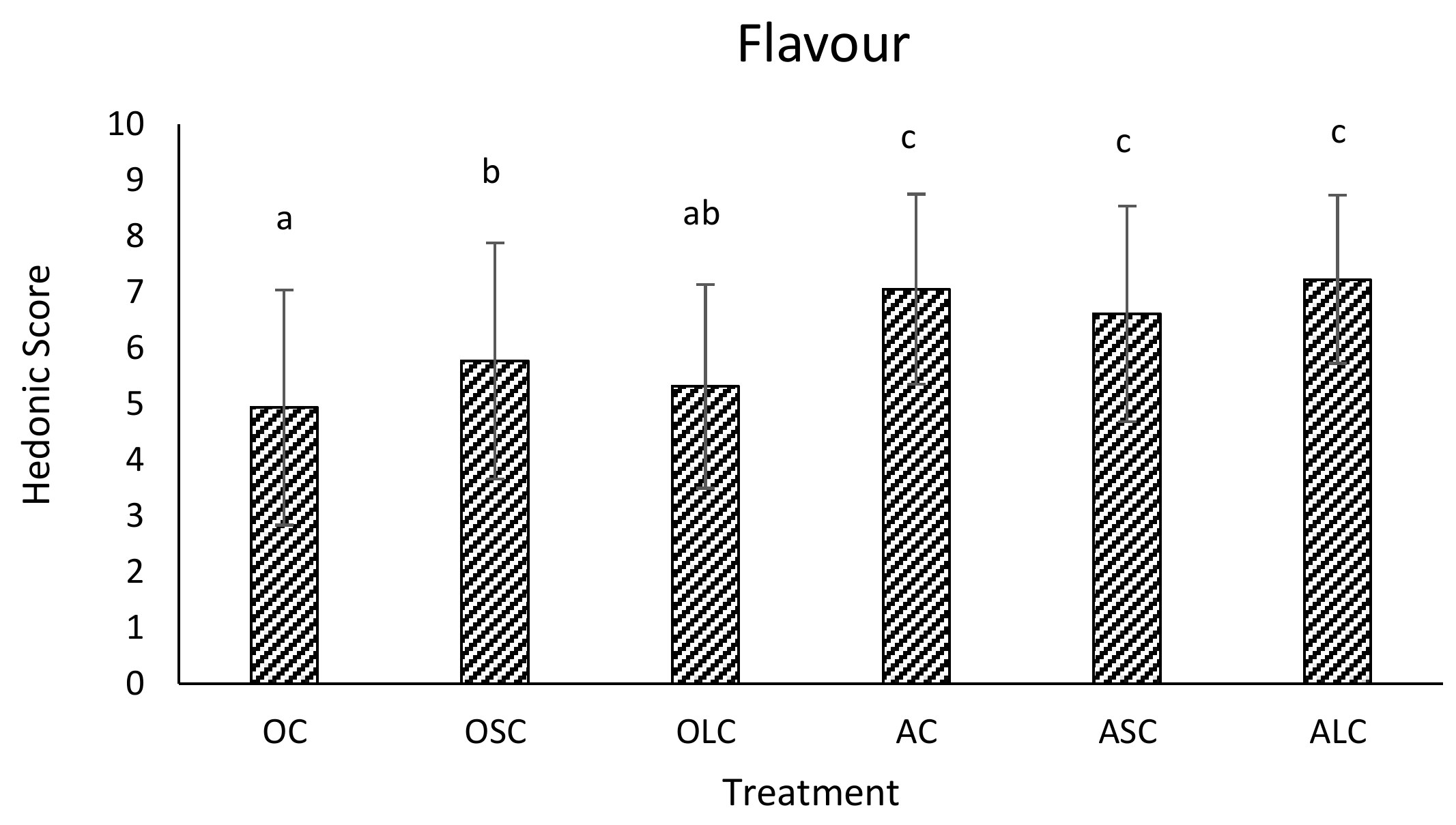
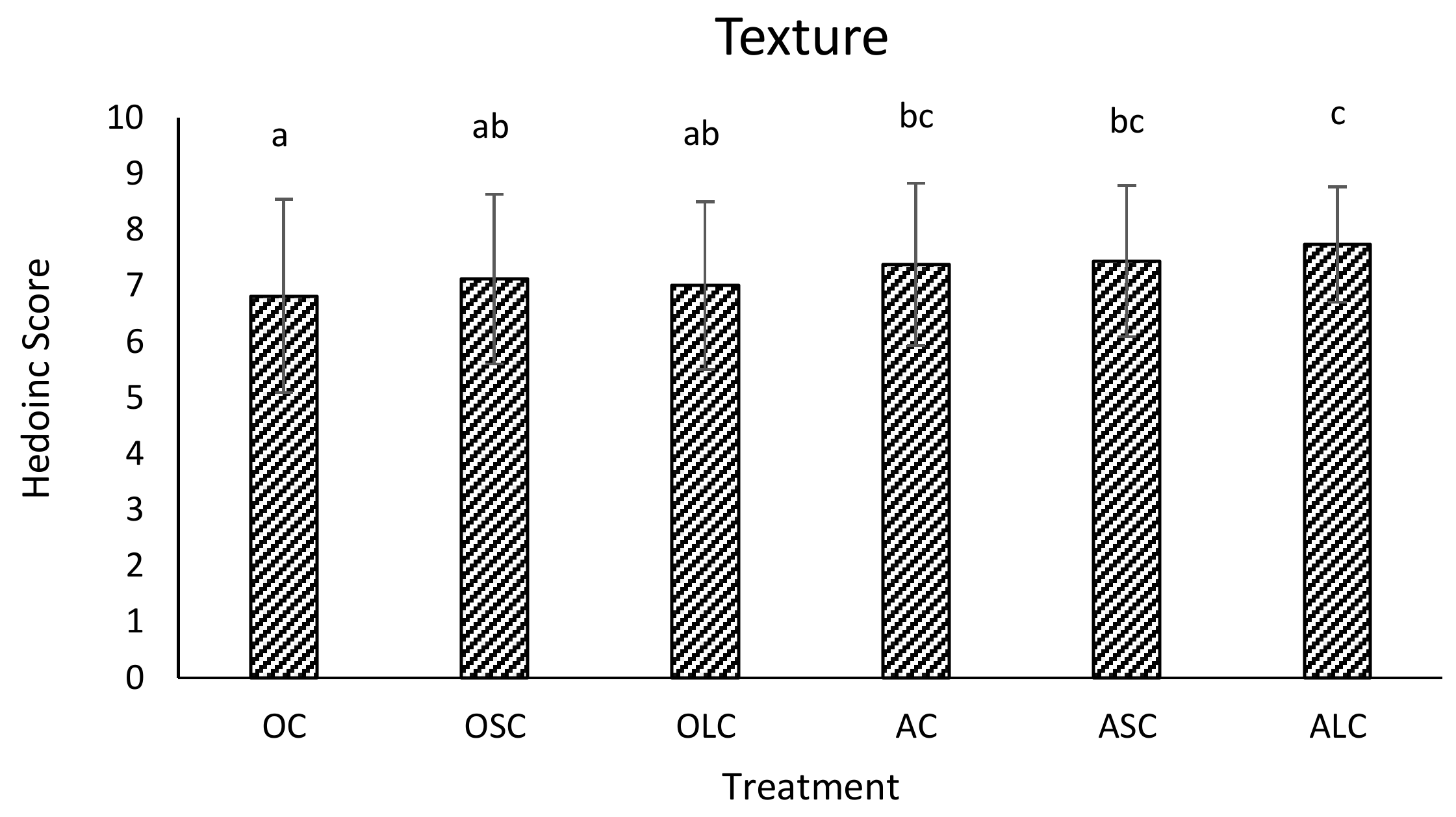
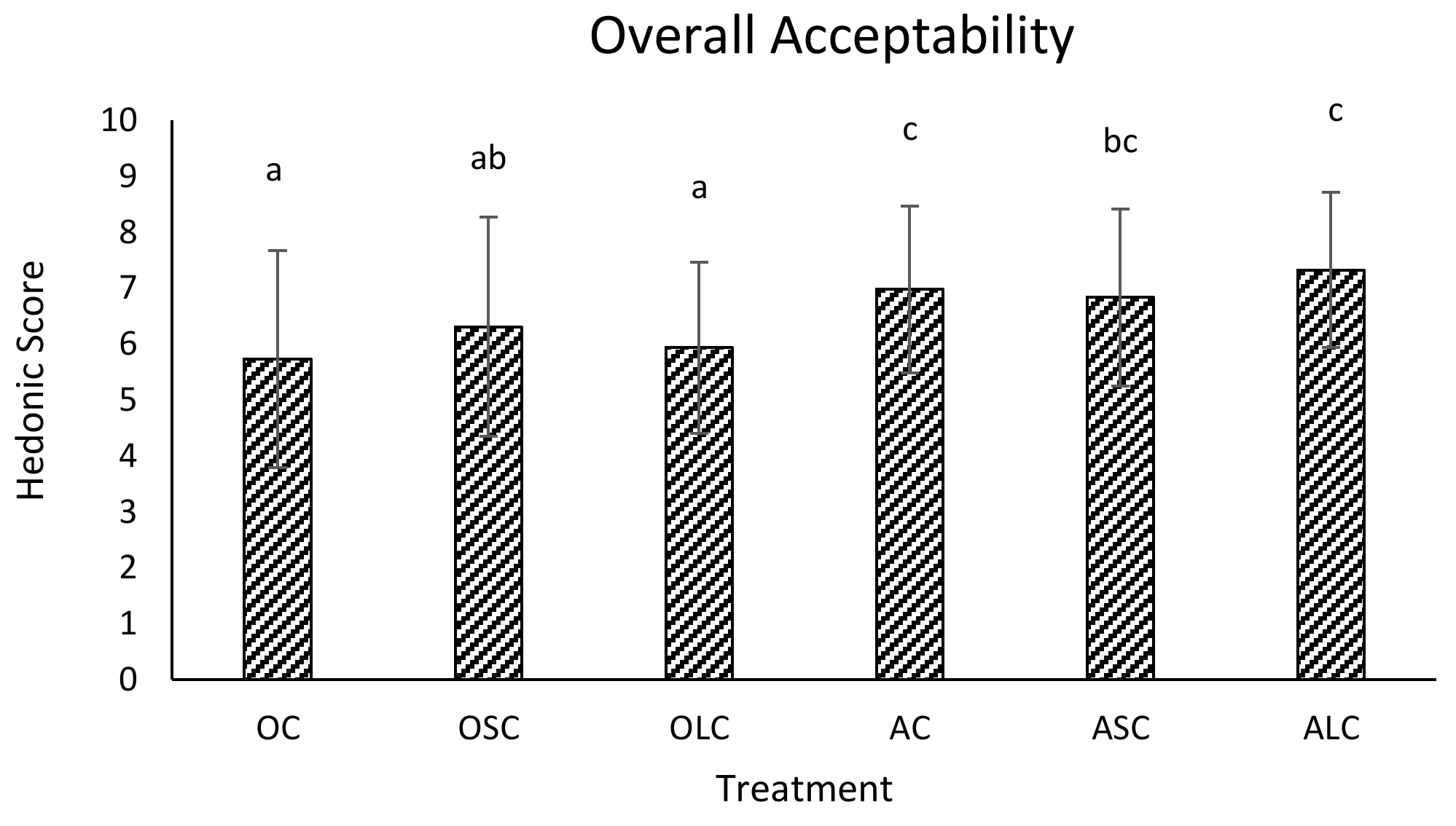
3.3. Sensory Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Shah, N. Probiotic Bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. The development of probiotics for women’s health. Can. J. Microbiol. 2017, 63, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Razavi, S.H.; Mahmoudi, R.; Gajarbeygi, P. Producing Probiotic Peach Juice. Biotechnol. Health Sci. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Madrona, G.S.; Garcia, S.; Prudencio, S.H. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT Food Sci. Technol. 2015, 63, 415–422. [Google Scholar] [CrossRef]
- De Souza Neves Ellendersen, L.; Granato, D.; Guergoletto, K.B.; Wosiacki, G. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M. Fortification and fermentation of fruit juices with probiotic lactobacilli. Ann. Microbiol. 2012, 62, 1573–1578. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Rodrigues, S. Optimization of the Fermentation of Cantaloupe Juice by Lactobacillus casei NRRL B-442. Food Bioprocess Technol. 2011, 1–8. [Google Scholar] [CrossRef]
- Mousavi, Z.; Mousavi, S.; Razavi, S.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Paula Espirito-Santo, A.; Carlin, F.; Renard, C.M.G. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth?—A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res. Int. 2015, 78, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, G.M.; de Carvalho Silva, J.V.; Mingotti, J.D.; Barao, C.E.; Klososki, S.J.; Pimentel, T.C. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT Food Sci. Technol. 2017, 75, 195–201. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Corbo, M.R.; Maddalena, L.; Sinigaglia, M. Suitability of Bifidobacterium spp. and Lactobacillus plantarum as Probiotics Intended for Fruit Juices Containing Citrus Extracts. J. Food Sci. 2013, 78, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gardner, N.J. Effect of storage in a fruit drink on subsequent survival of probiotic lactobacilli to gastro-intestinal stresses. Food Res. Int. 2008, 41, 539–543. [Google Scholar] [CrossRef]
- Giang, N.T.T.; Kieu, N.T.; Nam, T.N.; Dao, D.T.A.; Minh, N.P. Cashew Apple Juice Anacardium Occidentale L Probiotic Fermented from Lactobacillus acidophilus. Eur. J. Sustain. Dev. 2013, 2, 99–108. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Athmaselvi, K. Stress Tolerance and Physicochemical Properties of Encapsulation Processes for Lactobacillus rhamnosus in Pomegranate (Punica granatum L.) Fruit Juice. Food Sci. Biotechnol. 2016, 25, 125–129. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Mokhtari, S.; Khomeiri, M.; Jafari, S.M.; Maghsoudlou, Y.; Ghorbani, M. Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. Int. J. Food Sci. Technol. 2017, 52, 1042–1048. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Chooruk, A.; Kampoo, T. Survival of free and microencapsulated human-derived oral probiotic Lactobacillus paracasei SD1 in orange and aloe vera juices. Songklanakarin J. Sci. Technol. 2015, 37, 265–270. [Google Scholar]
- Ying, D.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J. Funct. Foods 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Helm, L.; Macdonald, I.A. Impact of beverage intake on metabolic and cardiovascular health. Nutr. Rev. 2017, 73 (Suppl. S2), 120–129. [Google Scholar] [CrossRef]
- De Souza Oliveira, R.P.; Perego, P.; de Oliveira, M.N.; Converti, A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT Food Sci. Technol. 2012, 47, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Di Stefano, E.; White, J.; Seney, S.; Hekmat, S.; Mcdowell, T.; Sumarah, M.; Reid, G. A Novel Millet-Based Probiotic Fermented Food for the Developing World. Nutrients 2017, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Newgent, J. Prebiotics and Probiotics: The Dynamic Duo; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2013; pp. 1–2. [Google Scholar]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Canbulat, Z.; Ozcan, T. Effects of short-chain and long-chain inulin on the quality of probiotic yogurt containing Lactobacillus rhamnosus. J. Food Process. Preserv. 2015, 39, 1251–1260. [Google Scholar] [CrossRef]
- Hekmat, S.; Morgan, K.; Soltani, M.; Gough, R. Sensory Evaluation of Locally-grown Fruit Purees and Inulin Fibre on Probiotic Yogurt in Mwanza, Tanzania and the Microbial Analysis of Probiotic Yogurt Fortified with Moringa oleifera. J. Health Popul. Nutr. 2015, 33, 60–67. [Google Scholar]
- Williams, M.; Hekmat, S. Lactobacillus rhamnosus GR-1 in Fermented Rice Pudding Supplemented with Short Chain Inulin, Long Chain Inulin, and Oat as a Novel Functional Food. Fermentation 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Nikmaram, P.; Mousavi, S.M.; Emam-Djomeh, Z.; Kiani, H.; Razavi, S.H. Evaluation and Prediction of Metabolite Production, Antioxidant Activities, and Survival of Lactobacillus casei 431 in a Pomegranate Juice Supplemented Yogurt Drink Using Support Vector Regression. Food Sci. Biotechnol. 2015, 24, 2105–2112. [Google Scholar] [CrossRef]
- Anvari, M.; Khayati, G.; Rostami, S. Optimisation of medium composition for probiotic biomass production using response surface methodology. J. Dairy Res. 2014, 81, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Luckow, T.; Sheehan, V.; Fitzgerald, G.; Delahunty, C. Exposure, health information and flavour-masking strategies for improving the sensory quality of probiotic juice. Appetite 2006, 47, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.F.; Almeida, F.D.L.; de Jesus, A.L.T.; da Costa, J.M.C.; Rodrigues, S. Storage Stability and Acceptance of Probiotic Beverage from Cashew Apple Juice. Food Bioprocess Technol. 2013, 6, 3155–3165. [Google Scholar] [CrossRef]

| Mean Counts (× CFU/mL) of L. rhamnosus GR-1 | |||||
|---|---|---|---|---|---|
| Treatment | 0 h | 24 h | 48 h | 72 h | p-Value |
| AC | 4.85 3.79 | 2.86 1.95 | 8.55 7.42 | 3.00 2.23 | >0.05 |
| ALC | 5.86 4.75 | 1.18 0.87 | 3.96 2.35 | 7.58 5.12 | >0.05 |
| ASC | 1.91 0.84 | 2.38 1.92 | 3.47 1.63 | 1.67 0.49 | >0.05 |
| GC | 1.06 0.24 | 1.72 1.58 | 2.66 2.14 | 2.37 1.68 | >0.05 |
| GLC | 0.62 0.11 | 4.25 1.07 | 0.28 0.15 | 0.50 0.33 | >0.05 |
| GSC | 1.06 0.10 | 1.99 1.79 | 0.37 0.00 | 1.73 1.72 | >0.05 |
| OC | 1.07 0.27 | 7.35 7.28 | 13.0 4.00 | 6.45 0.87 | >0.05 |
| OLC | 2.83 2.05 | 6.60 2.70 | 5.53 1.25 | 5.91 4.69 | >0.05 |
| OSC | 2.27 1.73 | 4.49 3.68 | 6.58 1.27 | 5.64 1.65 | >0.05 |
| Mean Counts (× CFU/mL) of L. rhamnosus GR-1 | |||||
|---|---|---|---|---|---|
| Treatment | 1 day | 10 days | 20 days | 30 days | p-Value |
| AC | 3.00 2.23 | 1.46 0.89 | 2.18 1.38 | 0.45 0.40 | <0.05 |
| ALC | 7.58 5.12 | 1.35 0.55 | 0.67 0.37 | 0.74 0.32 | <0.05 |
| ASC | 1.67 0.49 | 1.96 1.63 | 1.66 1.47 | 1.16 0.81 | <0.05 |
| GC | 2.37 1.68 | 0.76 0.50 | 0.05 0.04 | 0.01 0.01 | <0.05 |
| GLC | 0.50 0.33 | 0.34 0.01 | ND | ND | <0.05 |
| GSC | 1.73 1.72 | 0.69 0.44 | 0.14 0.09 | ND | <0.05 |
| OC | 6.45 0.87 | 6.02 1.40 | 8.01 2.69 | 10.7 2.34 | >0.05 |
| OLC | 5.91 4.69 | 9.25 0.42 | 5.86 0.20 | 14.3 4.03 | >0.05 |
| OSC | 5.64 1.65 | 8.05 5.87 | 9.84 2.07 | 8.20 2.55 | >0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, J.; Hekmat, S. Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber. Fermentation 2018, 4, 27. https://doi.org/10.3390/fermentation4020027
White J, Hekmat S. Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber. Fermentation. 2018; 4(2):27. https://doi.org/10.3390/fermentation4020027
Chicago/Turabian StyleWhite, Jessica, and Sharareh Hekmat. 2018. "Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber" Fermentation 4, no. 2: 27. https://doi.org/10.3390/fermentation4020027




