Transcriptomic Database Analysis of Magnesium Transporter (MGT) Gene Family in Pear (Pyrus bretschneideri) Revealed Its Role in Reproductive Stage Development
Abstract
:1. Introduction
2. Material and Methods
2.1. RNA Sequencing of 5 Pear Cultivars
2.2. Identification of Isolation of MGT Genes from the Pear Genome
2.3. Physical Location and Synteny of MGT Genes
2.4. Phylogenetic Analysis of MGT Proteins
2.5. Gene Structure and Conserved Motif Analysis
2.6. Interactive Protein Partners
2.7. Gene Ontology Analysis of PbMGT Genes
2.8. Promoter Analysis of PbMGT Genes
2.9. Prediction of Targeted miRNAs
2.10. Prediction of 3D Protein Structures
2.11. Microarray Expression Analysis of PbMGT Gene Family
2.12. Statistical Analysis
3. Results
3.1. Domain Organization of PbMGT Proteins
3.2. Chromosomal Localization
3.3. Phylogenetic Analysis
3.4. Synteny Analysis of PbMGT Genes
3.5. Gene Structure and Conserved Motifs Analysis
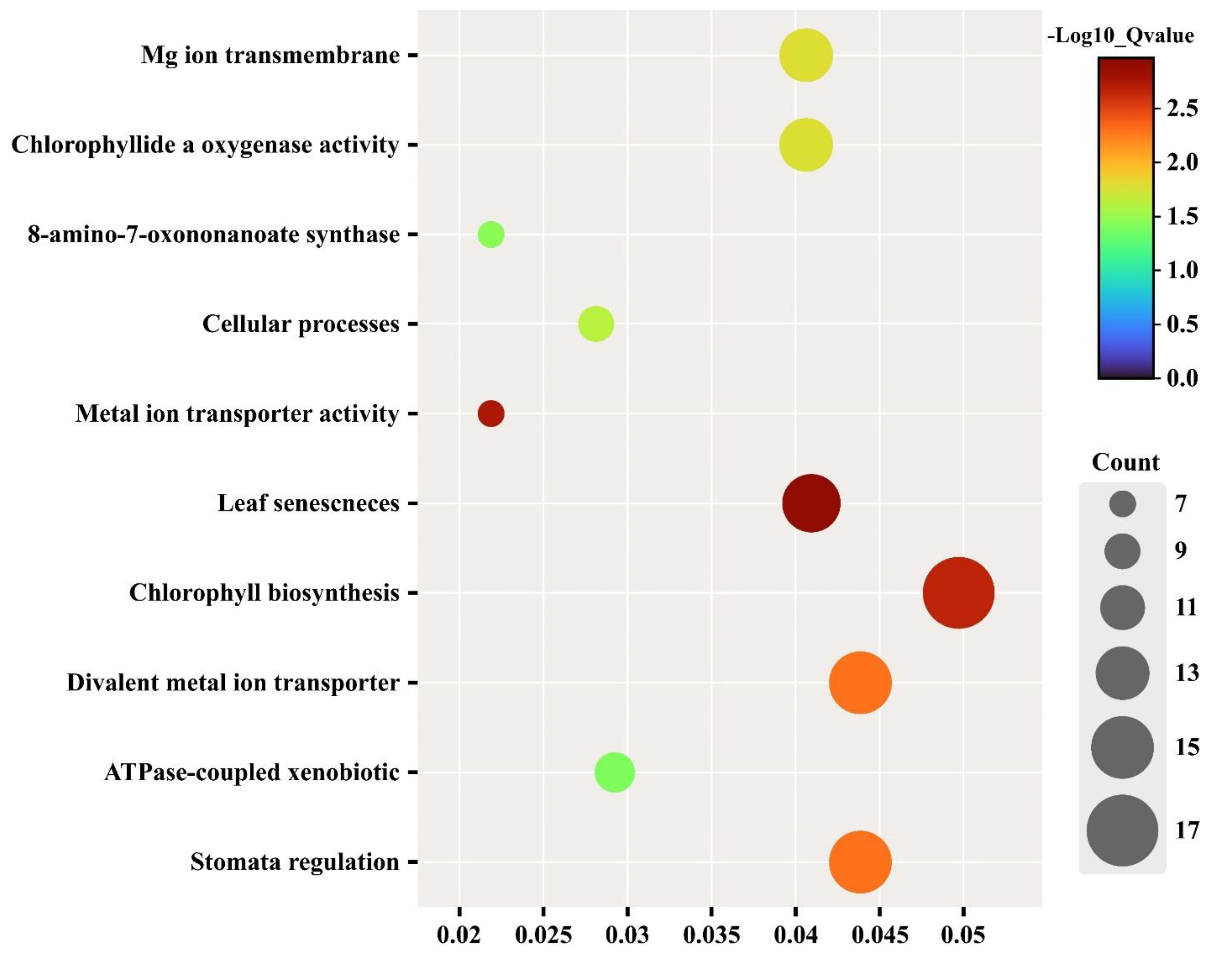
3.6. Gene ontology Enrichment Analysis
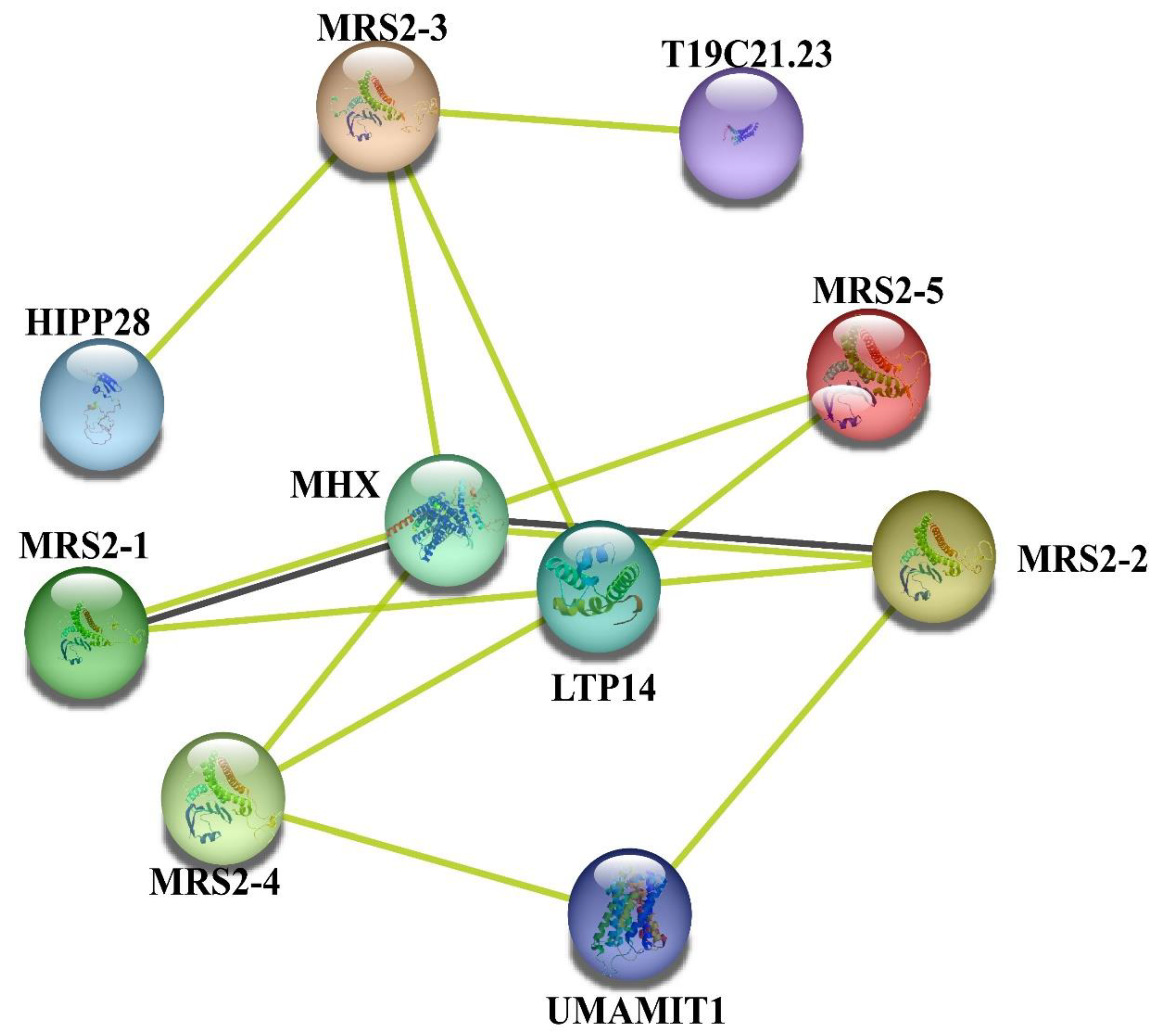
3.7. Protein–Protein Interaction
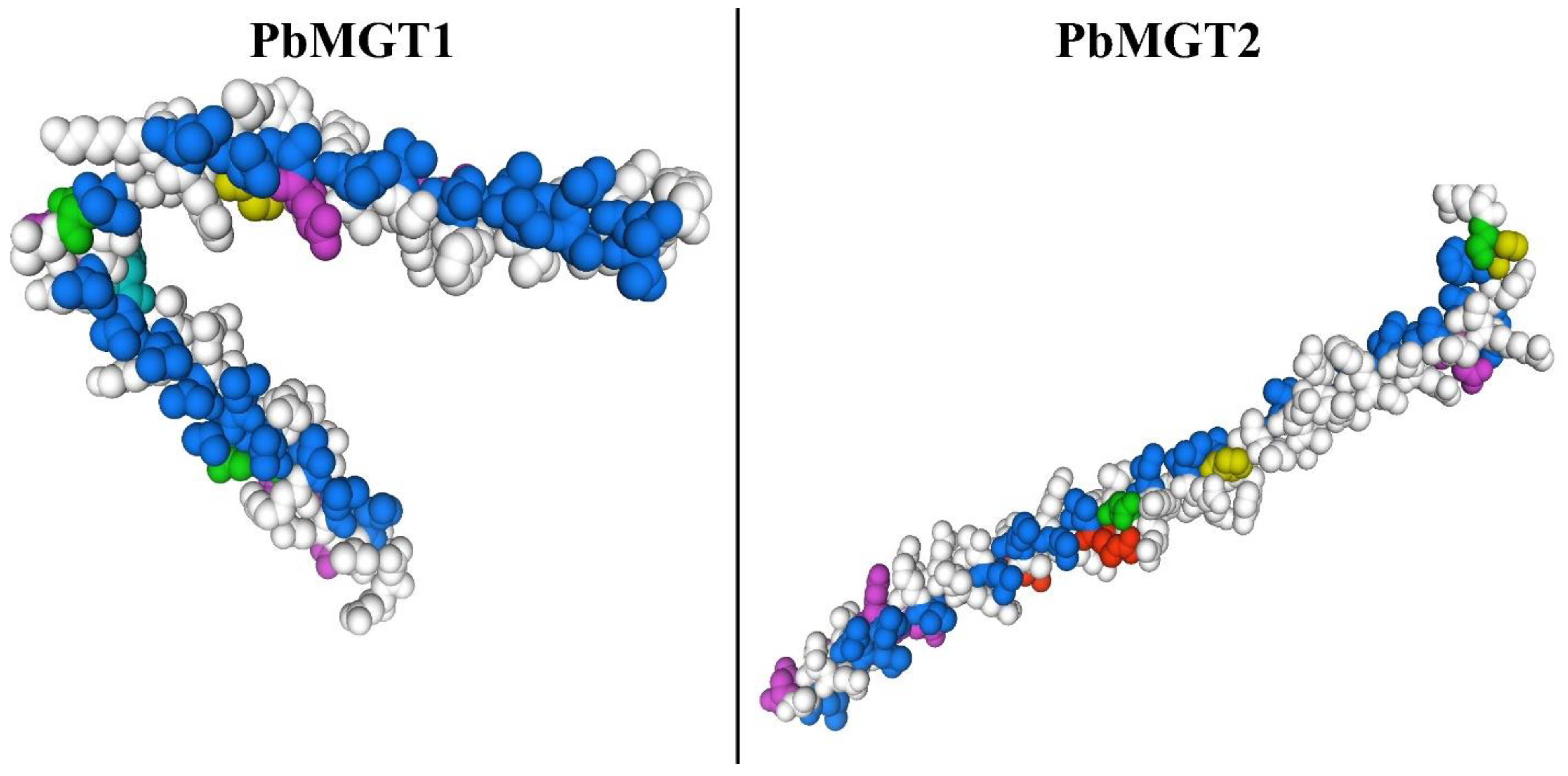
3.8. D Protein Structure of PbMGT
3.9. Cis-Acting Elements of Promoter Region PbMGT Genes
3.10. Predicted Targeted miRNAs by PbMGT Genes
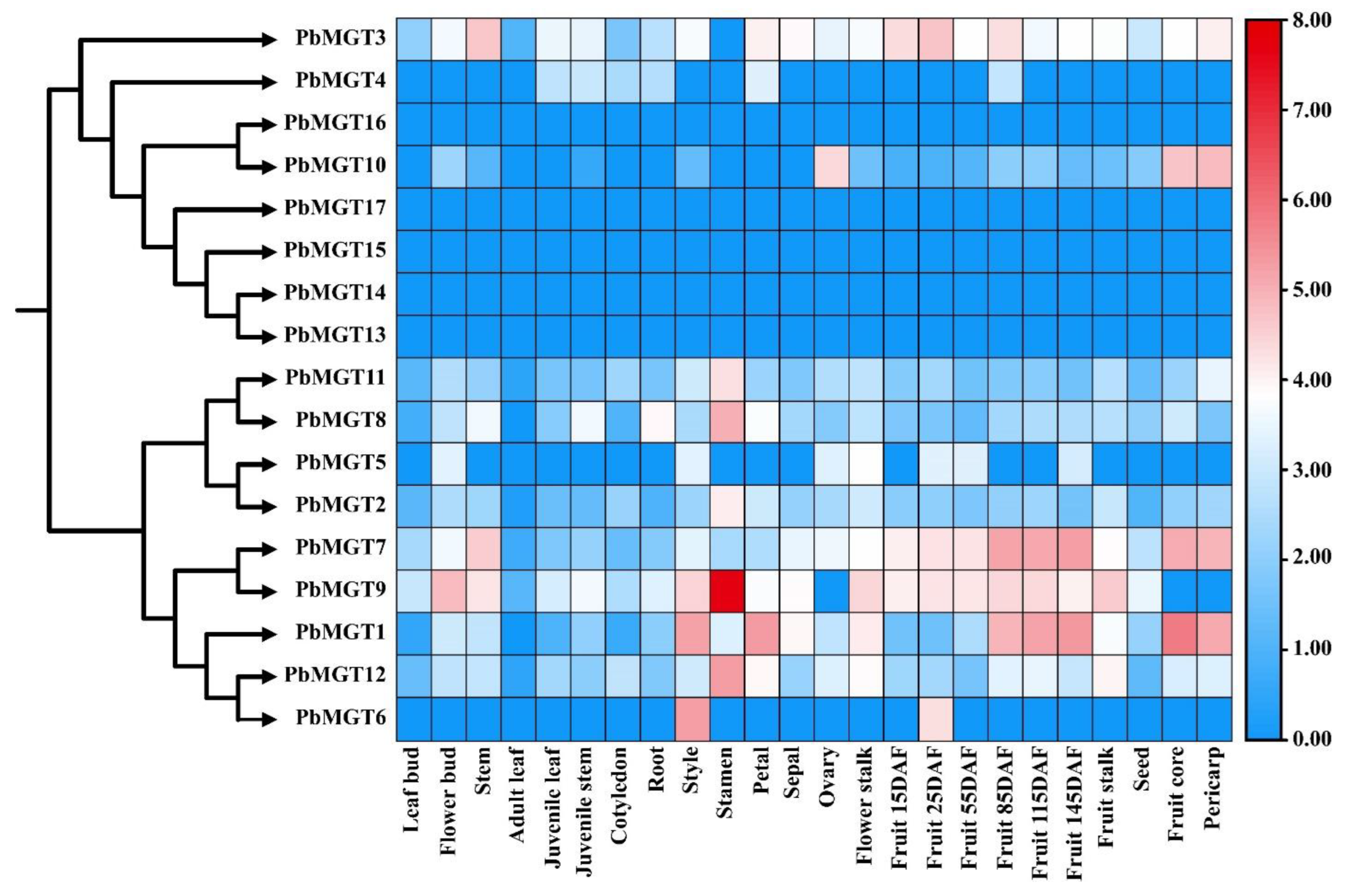
3.11. Spatial Expression of PbMGT Genes
3.12. Expression of PbMGT Gene Family in Five Pear Species
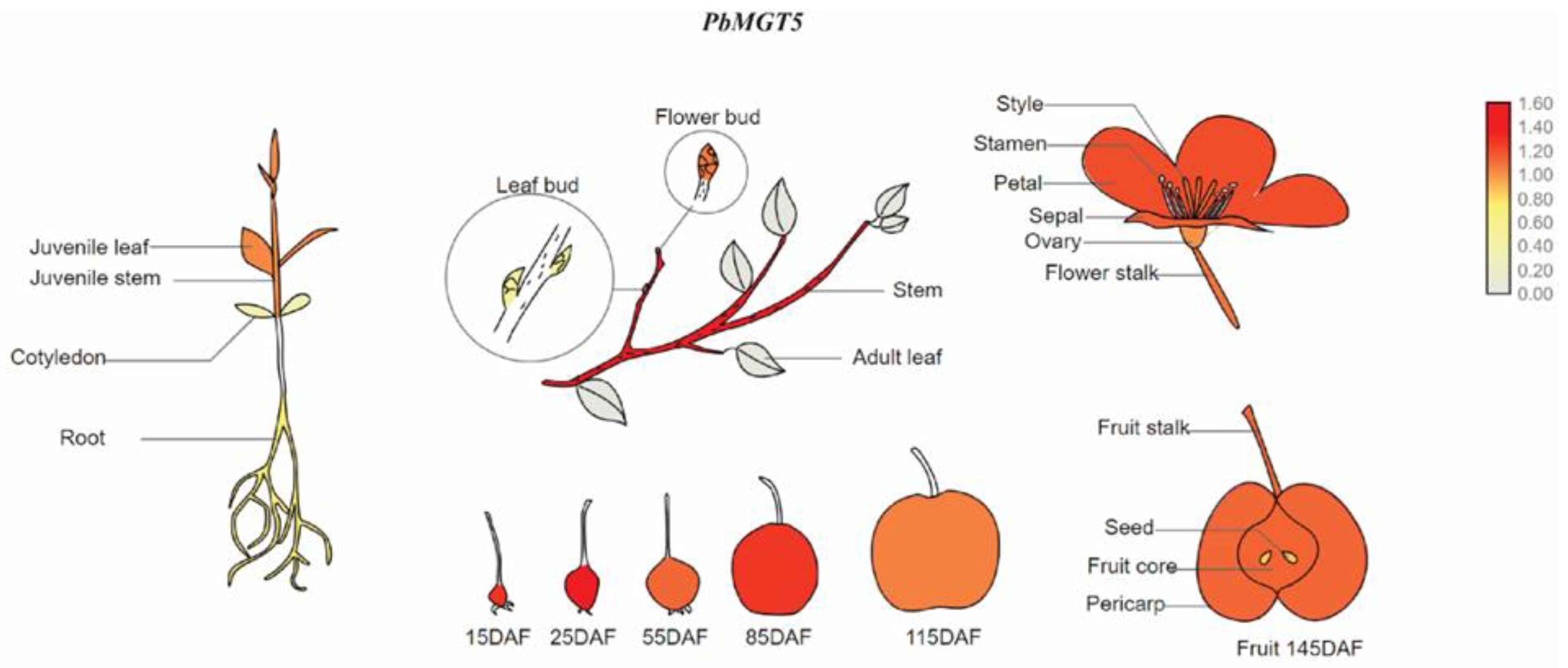
3.13. Graphical Representation of PbMGT5 in Pear Tissues
4. Discussion
4.1. PbMGT Are Widespread in Pears
4.2. PbMGT Participates in a Wide Range of Biological Activities
4.3. Pear Reproductive Biology Alters by PbMGT Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.A.; Liu, G. Application of biostimulants to improve tomato yield in Florida. Veg. Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- Heidari, P.; Abdullah; Faraji, S.; Poczai, P. Magnesium transporter Gene Family: Genome-Wide Identification and Characterization in Theobroma cacao, Corchorus capsularis, and Gossypium hirsutum of Family Malvaceae. Agronomy 2021, 11, 1651. [Google Scholar] [CrossRef]
- Bin, M.; Yi, G.; Zhang, X. Discovery and characterization of magnesium transporter (MGT) gene family in Citrus sinensis and their role in magnesium deficiency stress. Plant Growth Regul. 2023, 100, 733–746. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, X.; Xu, J.; Chen, Z.; Fan, T.; Zeng, Z.; Wang, H.; Hour, A.-L.; Yu, Q.; Ming, R.; et al. Comparative genomics revealed the gene evolution and functional divergence of magnesium transporter families in Saccharum. BMC Genom. 2019, 20, 83. [Google Scholar] [CrossRef]
- Ge, M.; Zhong, R.; Sadeghnezhad, E.; Hakeem, A.; Xiao, X.; Wang, P.; Fang, J. Genome-wide identification and expression analysis of magnesium transporter gene family in grape (Vitis vinifera). BMC Plant Biol. 2022, 22, 217. [Google Scholar] [CrossRef]
- Mohamadi, S.F.; Babaeian Jelodar, N.; Bagheri, N.; Nematzadeh, G.; Hashemipetroudi, S.H. New insights into comprehensive analysis of magnesium transporter (MGT) gene family in rice (Oryza sativa L.). 3 Biotech 2023, 13, 322. [Google Scholar] [CrossRef]
- Heidari, P.; Puresmaeli, F.; Mora-Poblete, F. Genome-Wide Identification and Molecular Evolution of the Magnesium Transporter (MGT) Gene Family in Citrullus lanatus and Cucumis sativus. Agronomy 2022, 12, 2253. [Google Scholar] [CrossRef]
- Gebert, M.; Meschenmoser, K.; Svidová, S.a.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A Root-Expressed Magnesium Transporter of the MRS2/MGT Gene Family in Arabidopsis thaliana Allows for Growth in Low-Mg2+ Environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef]
- Liu, G.; Yu, H.; Yuan, L.; Li, C.; Ye, J.; Chen, W.; Wang, Y.; Ge, P.; Zhang, J.; Ye, Z.; et al. SlRCM1, which encodes tomato Lutescent1, is required for chlorophyll synthesis and chloroplast development in fruits. Hortic. Res. 2021, 8, 128. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, M.; Zheng, H.; Jian, Y.; Huang, W.-L.; Yuan, Q.; Zheng, A.-H.; Chen, Q.; Zhang, Y.-T.; Lin, Y.-X.; et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020, 7, 161. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-Regulation of a Magnesium Transporter Gene OsMGT1 Is Required for Conferring Aluminum Tolerance in Rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.-G.; Liu, Z.-H.; Yuan, Y.-J.; Guo, L.-L.; Mao, D.-D.; Tian, L.-F.; Chen, L.-B.; Luan, S.; Li, D.-P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Horie, T.; Che, J.; Li, J.; An, G.; Ma, J.F. A Magnesium Transporter OsMGT1 Plays a Critical Role in Salt Tolerance in Rice. Plant Physiol. 2017, 174, 1837–1849. [Google Scholar] [CrossRef]
- Saquet, A.A.; Almeida, D. Sensory and instrumental assessments during ripening of ‘Rocha’ pear: The role of temperature and the inhibition of ethylene action on fruit quality. Technol. Hortic. 2023, 3, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; Linghu, T.; Zhao, Z.; Wang, A.; Zhai, R.; Yang, C.; Xu, L.; Wang, Z. Overexpression of a pear B-class MADS-box gene in tomato causes male sterility. Fruit. Res. 2023, 3, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Tang, C.; Lv, S.; Zhang, S.; Wu, J.; Wang, P. PbRbohH/J mediates ROS generation to regulate the growth of pollen tube in pear. Plant Physiol. Biochem. 2024, 207, 108342. [Google Scholar] [CrossRef]
- Li, X.; Qi, L.; Zang, N.; Zhao, L.; Sun, Y.; Huang, X.; Wang, H.; Yin, Z.; Wang, A. Integrated metabolome and transcriptome analysis of the regulatory network of volatile ester formation during fruit ripening in pear. Plant Physiol. Biochem. 2022, 185, 80–90. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Xue, C.; Xu, L.; Sun, H.; Qin, M.-F.; Zhang, S.; Wu, J. Distinct transcriptome profiles reveal gene expression patterns during fruit development and maturation in five main cultivated species of pear (Pyrus L.). Sci. Rep. 2016, 6, 28130. [Google Scholar] [CrossRef]
- Ahmad, S.; Jeridi, M.; Siddiqui, S.; Ali, S.; Shah, A.Z. Genome-wide identification, characterization, and expression analysis of the Chalcone Synthase gene family in Oryza sativa under Abiotic Stresses. Plant Stress. 2023, 9, 100201. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 2018, 289660. [Google Scholar]
- Ahmad, S.; Ali, S.; Shah, A.Z.; Khan, A.; Faria, S. Chalcone synthase (CHS) family genes regulate the growth and response of cucumber (Cucumis sativus L.) to Botrytis cinerea and abiotic stresses. Plant Stress. 2023, 8, 100159. [Google Scholar] [CrossRef]
- Tan, Y.; Xiao, L.; Zhao, J.; Zhang, J.; Ahmad, S.; Xu, D.; Xu, G.; Ge, L. Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum. Int. J. Mol. Sci. 2023, 24, 8587. [Google Scholar] [CrossRef]
- Ahmad, S.; Chen, Y.; Shah, A.Z.; Wang, H.; Xi, C.; Zhu, H.; Ge, L. The Homeodomain-Leucine Zipper Genes Family Regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioengineering 2022, 9, 398. [Google Scholar] [CrossRef]
- Ullah, U.; Shalmani, A.; Ilyas, M.; Raza, A.; Ahmad, S.; Shah, A.Z.; Khan, F.U.; AzizUd, D.; Bibi, A.; Rehman, S.U.; et al. BZR proteins: Identification, evolutionary and expression analysis under various exogenous growth regulators in plants. Mol. Biol. Rep. 2022, 49, 12039–12053. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhu, H.; Chen, Y.; Xi, C.; Shah, A.Z.; Ge, L. Comprehensive Bioinformatics and Expression Analysis of the TLP Gene Family Revealed Its Role in Regulating the Response of Oryza sativa to Nilaparvata lugens, Laodelphax striatellus, and Jinggangmycin. Agronomy 2022, 12, 1297. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Karikari, B.; Sharif, R.; Yadav, V.; Mubarik, M.S.; Habib, M.; Zhuang, Y.; Zhang, C.; Chen, H.; et al. miRNAs for crop improvement. Plant Physiol. Biochem. 2023, 201, 107857. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Ji, H.; Xu, Z.; Zhou, Y.; Yang, S. The specific W-boxes of GAPC5 promoter bound by TaWRKY are involved in drought stress response in wheat. Plant Sci. Int. J. Exp. Plant Biol. 2020, 296, 110460. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological Essence of Magnesium in Plants and Its Widespread Deficiency in the Farming System of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Tian, X.-Y.; He, D.-D.; Bai, S.; Zeng, W.-Z.; Wang, Z.; Wang, M.; Wu, L.-Q.; Chen, Z.-C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil. 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Xu, R.; Ali, A.; Li, Y.; Zhang, X.; Sharif, R.; Feng, X.; Ding, B. Transcriptome-Wide Analysis Revealed the Influential Role of PbrMTP (Metal Tolerance Protein) in the Growth and Fruit Development of Chinese White Pear. J. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Shalmani, A.; Ullah, U.; Muhammad, I.; Zhang, D.; Sharif, R.; Jia, P.; Saleem, N.; Gul, N.; Rakhmanova, A.; Tahir, M.M.; et al. The TAZ domain-containing proteins play important role in the heavy metals stress biology in plants. Environ. Res. 2021, 197, 111030. [Google Scholar] [CrossRef]
- Pan, J.; Tu, J.; Sharif, R.; Qi, X.; Xu, X.; Chen, X. Study of JASMONATE ZIM-Domain gene family to waterlogging stress in Cucumis sativus L. Veg. Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Wang, J.; Cao, Z.; Zhang, H.; Chen, P.; Yuhong, L. Genome wide identification, characterization and expression analysis of HD-ZIP gene family in Cucumis sativus L. under biotic and various abiotic stresses. Int. J. Biol. Macromol. 2020, 158, 502–520. [Google Scholar] [CrossRef]
- Jia, P.; Sharif, R.; Li, Y.; Sun, T.; Li, S.; Zhang, X.; Dong, Q.; Luan, H.; Guo, S.; Ren, X.; et al. The BELL1-like homeobox gene MdBLH14 from apple controls flowering and plant height via repression of MdGA20ox3. Int. J. Biol. Macromol. 2023, 242, 124790. [Google Scholar] [CrossRef]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP Gene Family: Potential Roles in Improving Plant Growth and Regulating Stress-Responsive Mechanisms in Plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Zhao, X.; Cao, H.; Wang, L.; Liu, S.; Wang, C.; Liu, M.; Wang, L.; Liu, Z. Superstar microRNA, miR156, involved in plant biological processes and stress response: A review. Sci. Hortic. 2023, 316, 112010. [Google Scholar] [CrossRef]
- Fan, X.; Li, H.; Guo, Y.; Sun, H.; Wang, S.; Qi, Q.; Jiang, X.; Wang, Y.; Xu, X.; Qiu, C.; et al. Integrated multi-omics analysis uncovers roles of mdm-miR164b-MdORE1 in strigolactone-mediated inhibition of adventitious root formation in apple. Plant Cell Env. 2022, 45, 3582–3603. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Liu, J.; Dou, F.; Wang, H.; Song, Y.; Ren, Y.; He, J.; Wang, L.; Zhang, C.; et al. Identification of grape miRNA revealed Vvi-miR164b involved in auxin induced root development. Sci. Hortic. 2022, 295, 110804. [Google Scholar] [CrossRef]
- Jin, L.F.; Yarra, R.; Yin, X.X.; Liu, Y.Z.; Cao, H.X. Identification and function prediction of iron-deficiency-responsive microRNAs in citrus leaves. 3 Biotech 2021, 11, 121. [Google Scholar] [CrossRef]
- Sorin, C.; Declerck, M.; Christ, A.; Blein, T.; Ma, L.; Lelandais-Brière, C.; Njo, M.F.; Beeckman, T.; Crespi, M.; Hartmann, C. A miR169 isoform regulates specific NF-YA targets and root architecture in Arabidopsis. New Phytol. 2014, 202, 1197–1211. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic. Res. 2022, 9, uhab024. [Google Scholar] [CrossRef]
- Su, L.; Rahat, S.; Ren, N.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Wang, M.; Chen, X.; Qi, X. Cytokinin and auxin modulate cucumber parthenocarpy fruit development. Sci. Hortic. 2021, 282, 110026. [Google Scholar] [CrossRef]
- Su, L.; Wang, M.; Wang, Y.; Sharif, R.; Ren, N.; Qian, C.; Xu, J.; Chen, X.; Qi, X. Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber. Horticulturae 2021, 7, 128. [Google Scholar] [CrossRef]
- Jia, P.; Wang, Y.; Sharif, R.; Ren, X.; Qi, G. MdIPT1, an adenylate isopentenyltransferase coding gene from Malus domestica, is involved in branching and flowering regulation. Plant Sci. 2023, 333, 111730. [Google Scholar] [CrossRef]
- Prasad, R. Cytokinin and Its Key Role to Enrich the Plant Nutrients and Growth Under Adverse Conditions-An Update. Front. Genet. 2022, 13, 883924. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Jiao, J.; Li, J.; Chang, J.; Li, J.; Chen, X.; Li, Z.; Song, Z.; Xie, D.; Zhang, B. Magnesium Effects on Carbohydrate Characters in Leaves, Phloem Sap and Mesocarp in Wax Gourd (Benincasa hispida (Thunb.) Cogn.). Agronomy 2023, 13, 455. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]

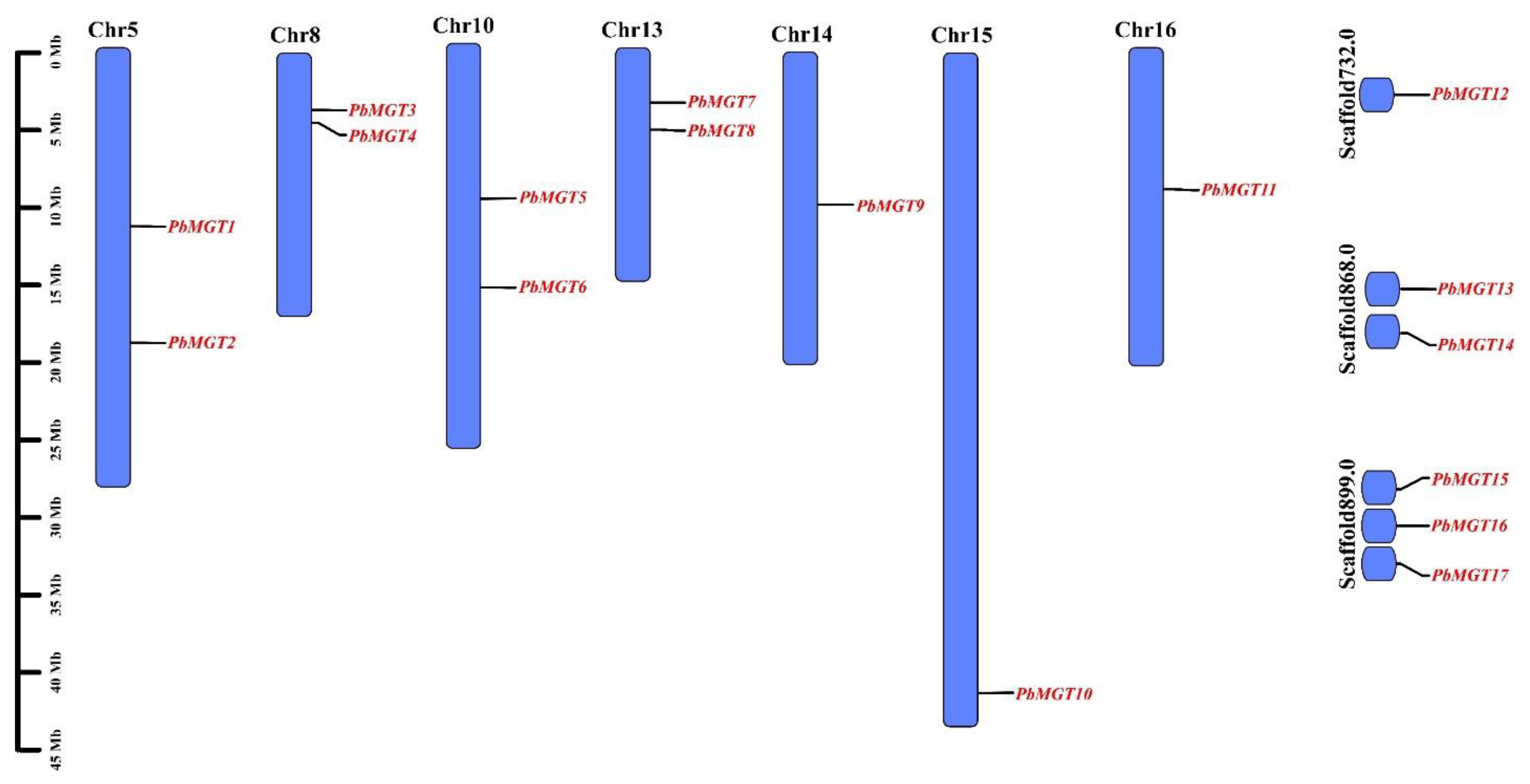
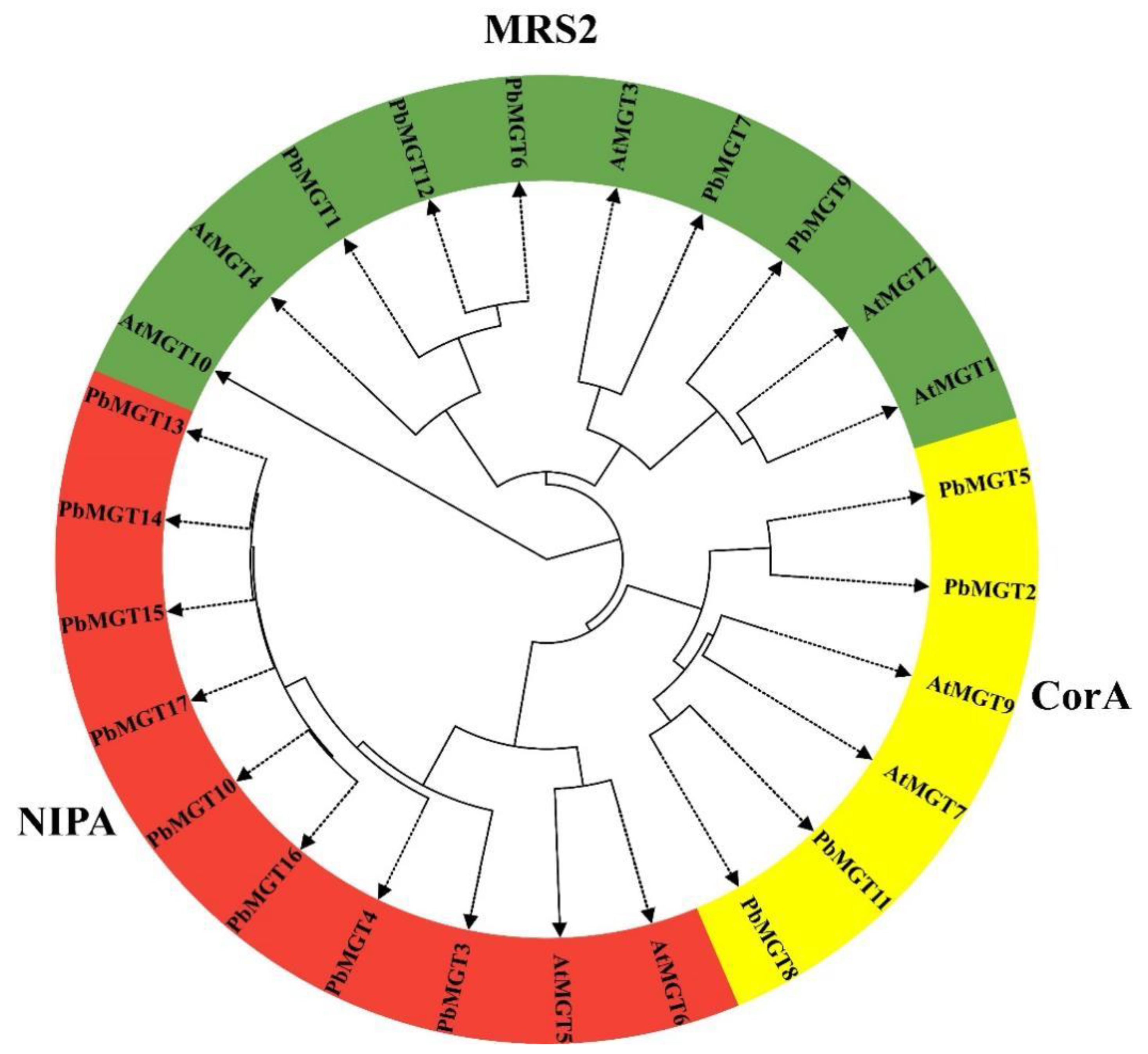
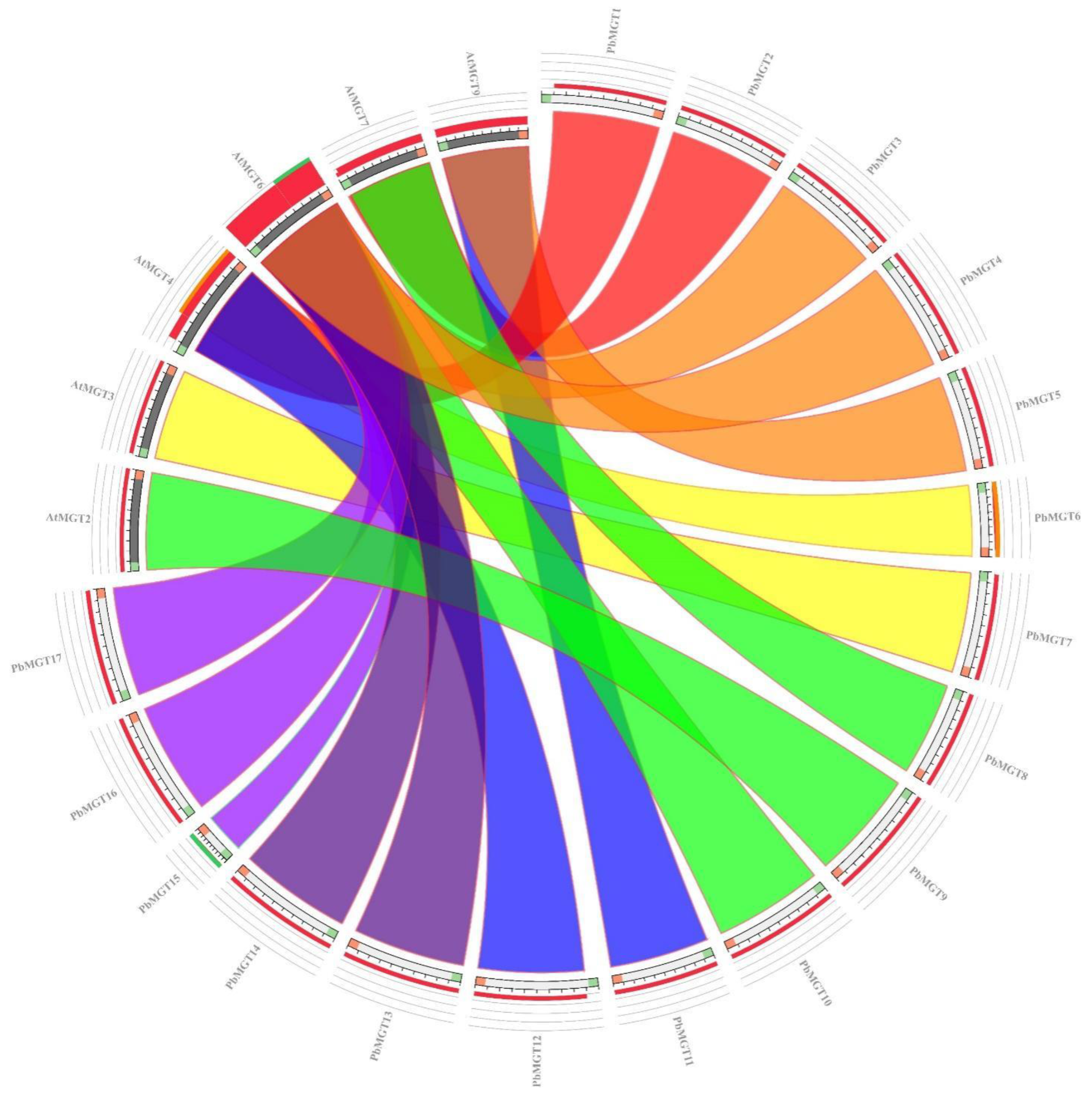

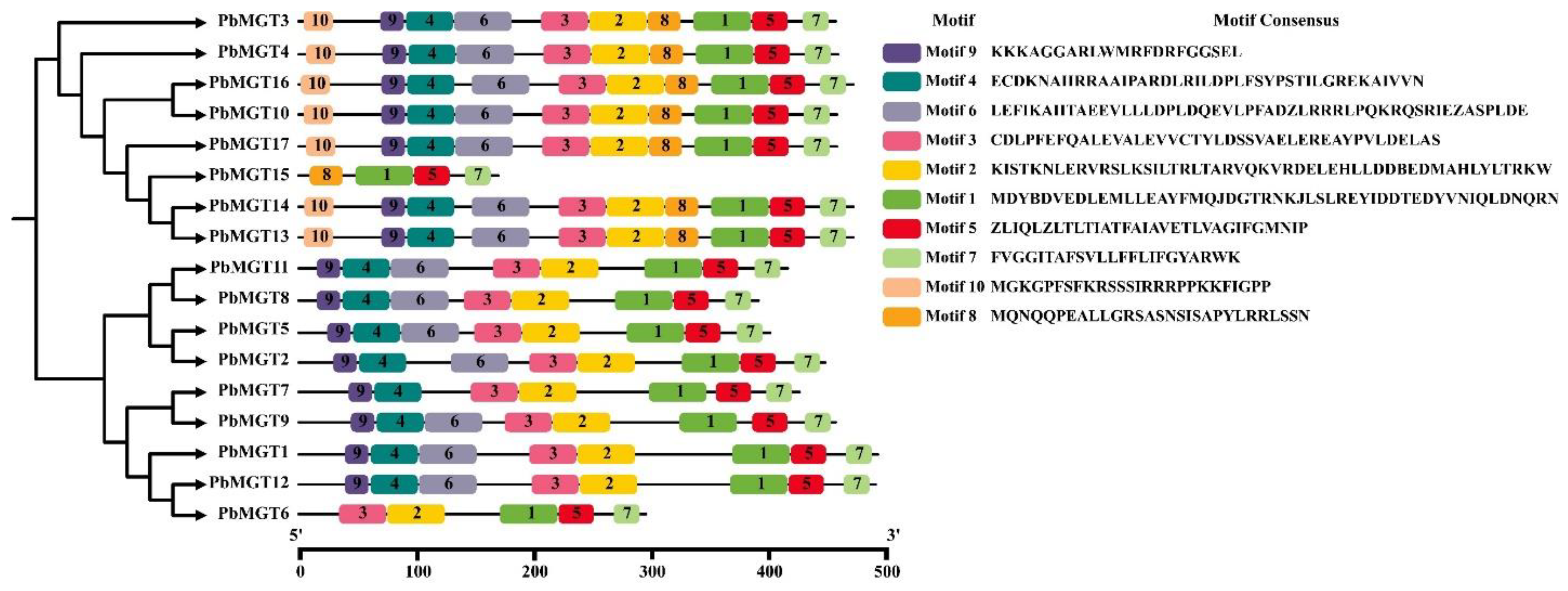



| Locus ID | Gene | Chr. | Start | End | No. AA | MW (kDa) | PI | SL |
|---|---|---|---|---|---|---|---|---|
| Pbr035629.1 | PbMGT1 | 5 | 11530499 | 11533234 | 499 | 55.14 | 4.95 | Nucleus |
| Pbr025298.1 | PbMGT2 | 5 | 19052560 | 19055228 | 454 | 50.32 | 5.22 | Nucleus |
| Pbr026553.1 | PbMGT3 | 8 | 4019326 | 4022927 | 463 | 52.08 | 8.38 | Chloroplast |
| Pbr026552.1 | PbMGT4 | 8 | 4025306 | 4029889 | 465 | 51.96 | 5.77 | Chloroplast |
| Pbr009062.1 | PbMGT5 | 10 | 10014987 | 10017700 | 407 | 45.22 | 4.71 | Nucleus |
| Pbr018771.1 | PbMGT6 | 10 | 15755036 | 15756903 | 301 | 33.22 | 4.55 | Nucleus |
| Pbr029967.3 | PbMGT7 | 13 | 4534375 | 4536674 | 432 | 48.69 | 5.35 | Nucleus |
| Pbr014721.1 | PbMGT8 | 13 | 5286444 | 5292607 | 397 | 44.21 | 4.85 | Chloroplast |
| Pbr018319.1 | PbMGT9 | 14 | 9847472 | 9851439 | 463 | 52.35 | 5.05 | Nucleus |
| Pbr003194.1 | PbMGT10 | 15 | 41303362 | 41306863 | 464 | 52.12 | 8.77 | Chloroplast |
| Pbr001806.1 | PbMGT11 | 16 | 9145142 | 9149449 | 422 | Undefined | Undefined | Nucleus |
| Pbr036745.1 | PbMGT12 | Scaffold732.0 | 29832 | 38286 | 497 | 54.97 | 4.97 | Nucleus |
| Pbr039912.1 | PbMGT13 | Scaffold868.0 | 54166 | 57561 | 478 | 53.48 | 7.31 | Chloroplast |
| Pbr039915.1 | PbMGT14 | Scaffold868.0 | 96276 | 99671 | 478 | 53.48 | 7.31 | Chloroplast |
| Pbr040583.1 | PbMGT15 | Scaffold899.0 | 81130 | 82089 | 175 | 19.97 | 4.99 | Cell membrane |
| Pbr040585.1 | PbMGT16 | Scaffold899.0 | 100391 | 103310 | 478 | 53.70 | 8.03 | Chloroplast |
| Pbr040588.1 | PbMGT17 | Scaffold899.0 | 140233 | 143150 | 464 | 52.06 | 7.32 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Ding, B.; Khan, K.; Lin, Y.; Ali, A.; Li, L. Transcriptomic Database Analysis of Magnesium Transporter (MGT) Gene Family in Pear (Pyrus bretschneideri) Revealed Its Role in Reproductive Stage Development. Horticulturae 2024, 10, 333. https://doi.org/10.3390/horticulturae10040333
Ma Y, Ding B, Khan K, Lin Y, Ali A, Li L. Transcriptomic Database Analysis of Magnesium Transporter (MGT) Gene Family in Pear (Pyrus bretschneideri) Revealed Its Role in Reproductive Stage Development. Horticulturae. 2024; 10(4):333. https://doi.org/10.3390/horticulturae10040333
Chicago/Turabian StyleMa, Yuchen, Baopeng Ding, Khushboo Khan, Yujing Lin, Ahmad Ali, and Liulin Li. 2024. "Transcriptomic Database Analysis of Magnesium Transporter (MGT) Gene Family in Pear (Pyrus bretschneideri) Revealed Its Role in Reproductive Stage Development" Horticulturae 10, no. 4: 333. https://doi.org/10.3390/horticulturae10040333





