Metabolically Tailored Selection of Ornamental Rose Cultivars through Polyamine Profiling, Osmolyte Quantification and Evaluation of Antioxidant Activities
Abstract
1. Introduction
- Proline and glycine betaine content.
- The amount of individual polyamines such as putrescine, spermidine and spermine.
- Antioxidant activities against ABTS and NO radicals as well as total condensed tannin amounts.
2. Materials and Methods
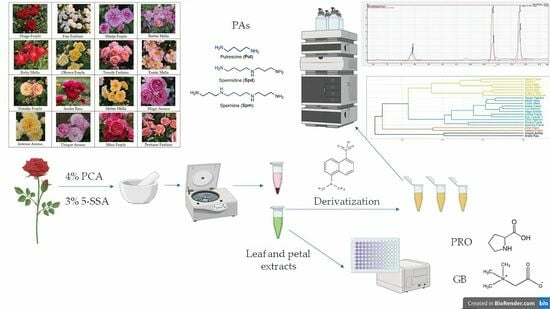
2.1. Plant Material and Sample Preparation
2.2. Phytochemical Characterization
2.2.1. HPLC Quantification of Polyamines (PAs)
2.2.2. Carbon and Nitrogen Elemental Analysis
2.2.3. Quantification of Free Proline (PRO)
2.2.4. Quantification of Glycine Betaine (GB) as a Predominant Quaternary Ammonium Compound
2.2.5. Quantification of Condensed Tannins (CTs)
2.3. Evaluation of Antioxidant Capacity
2.3.1. Extract Preparation
2.3.2. Assays of Antioxidant Defense Systems
2.4. Chemicals and Reagents
2.5. Statistical Analysis
3. Results
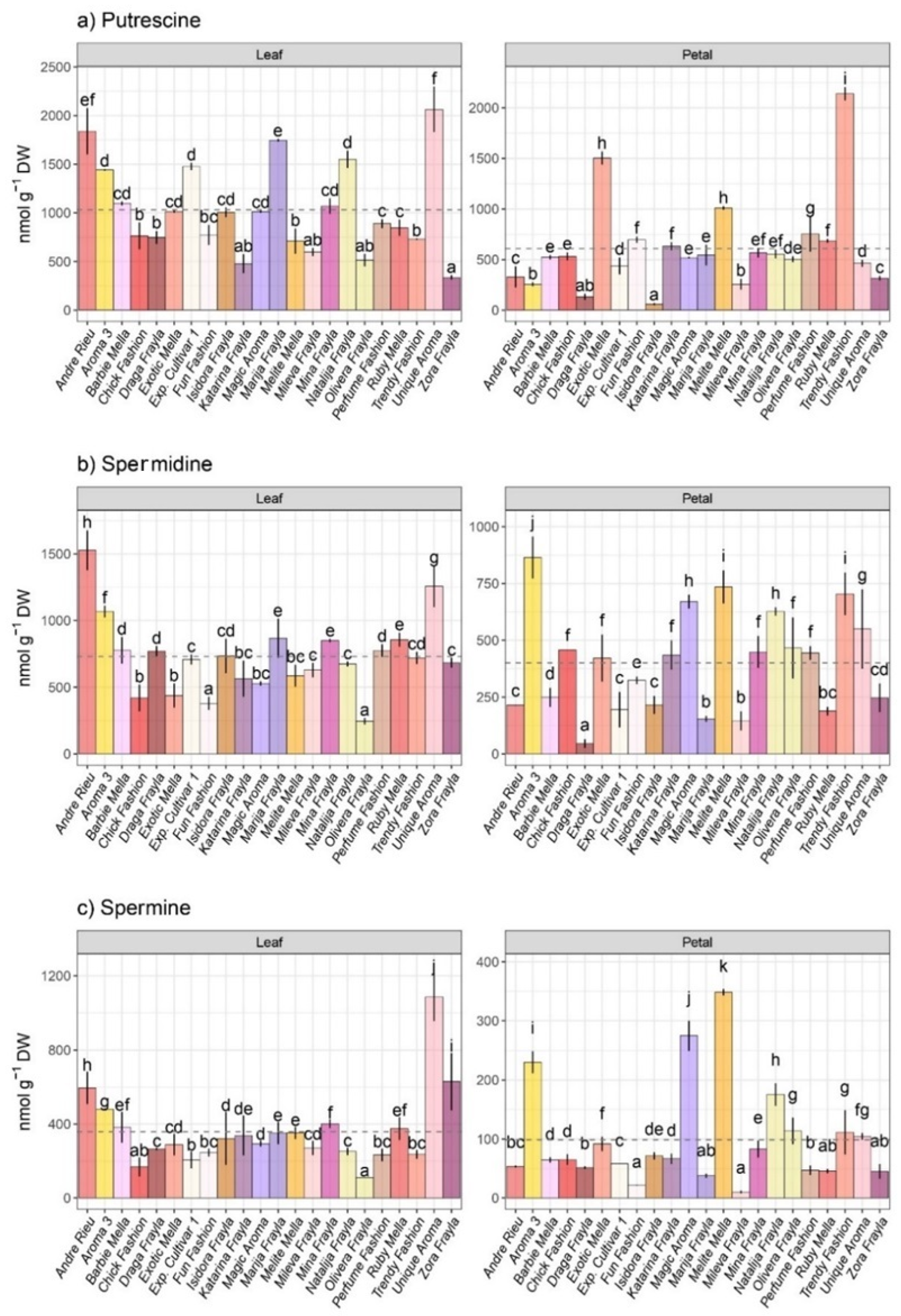
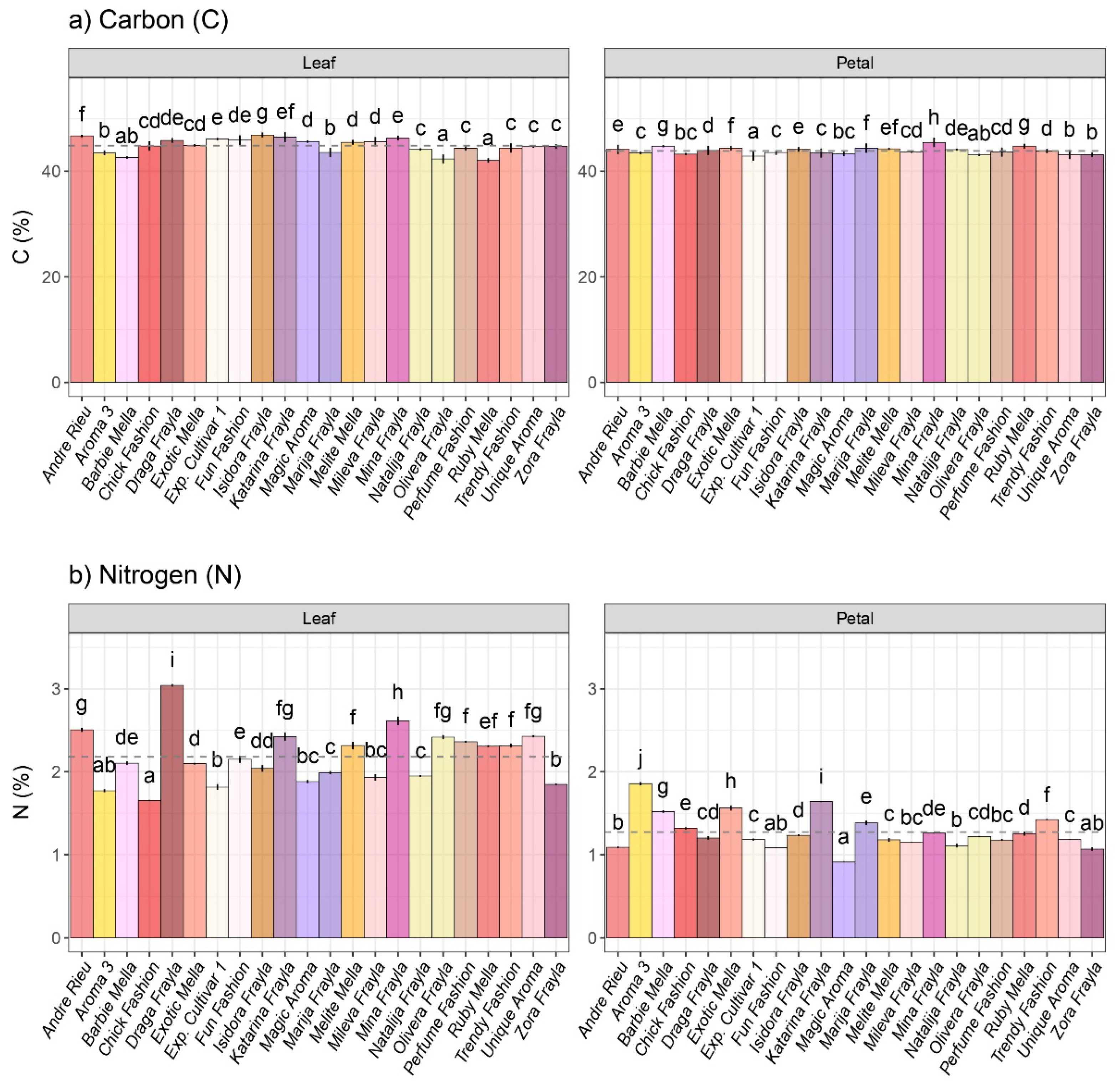
3.1. Osmolyte Variability among Inspected Rose Cultivars
3.2. Polyamine Profiling within Inspected Rose Cultivars
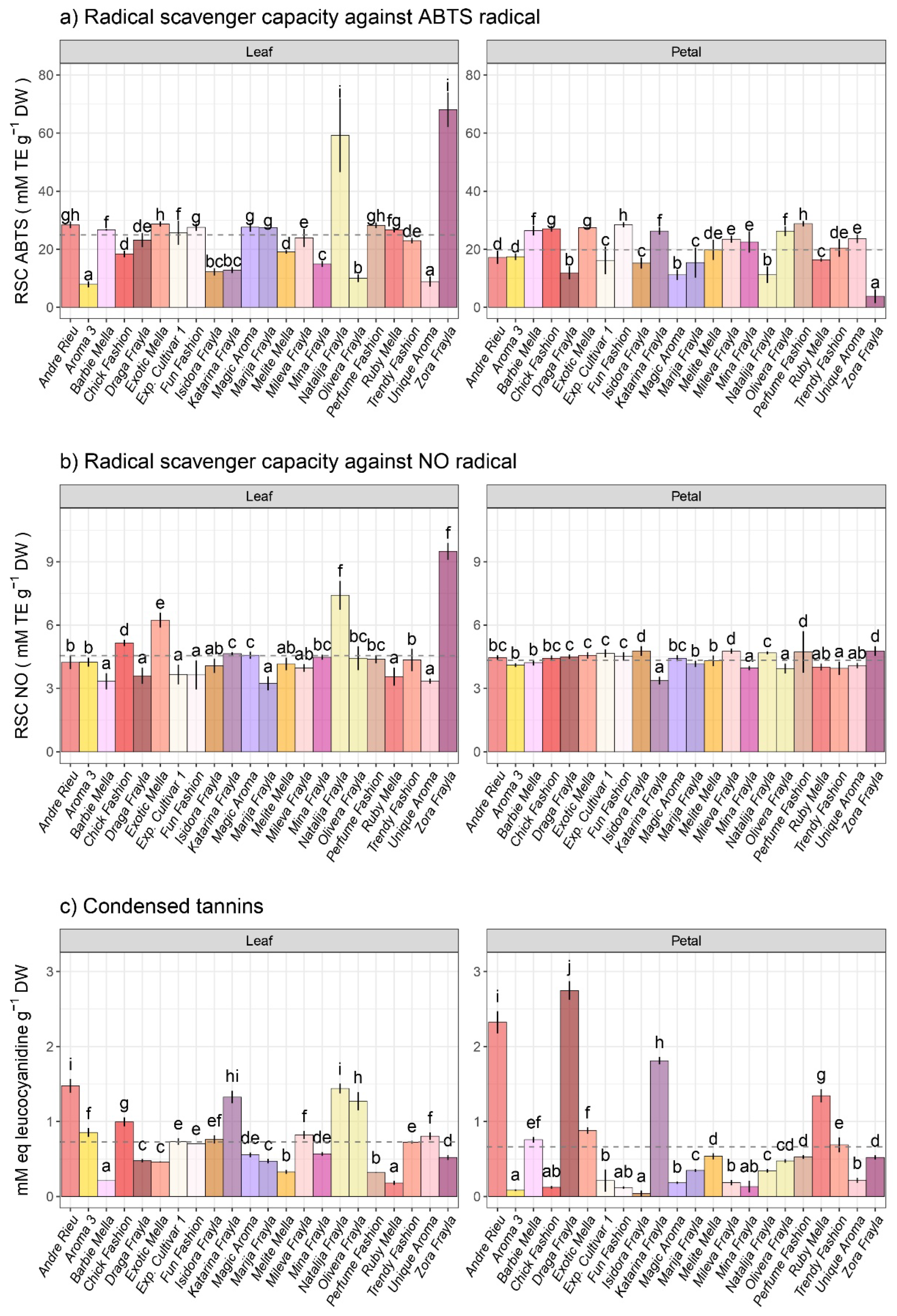
3.3. Variability in Antioxidant Capacities and Condensed Tannins among Examined Rose Cultivars
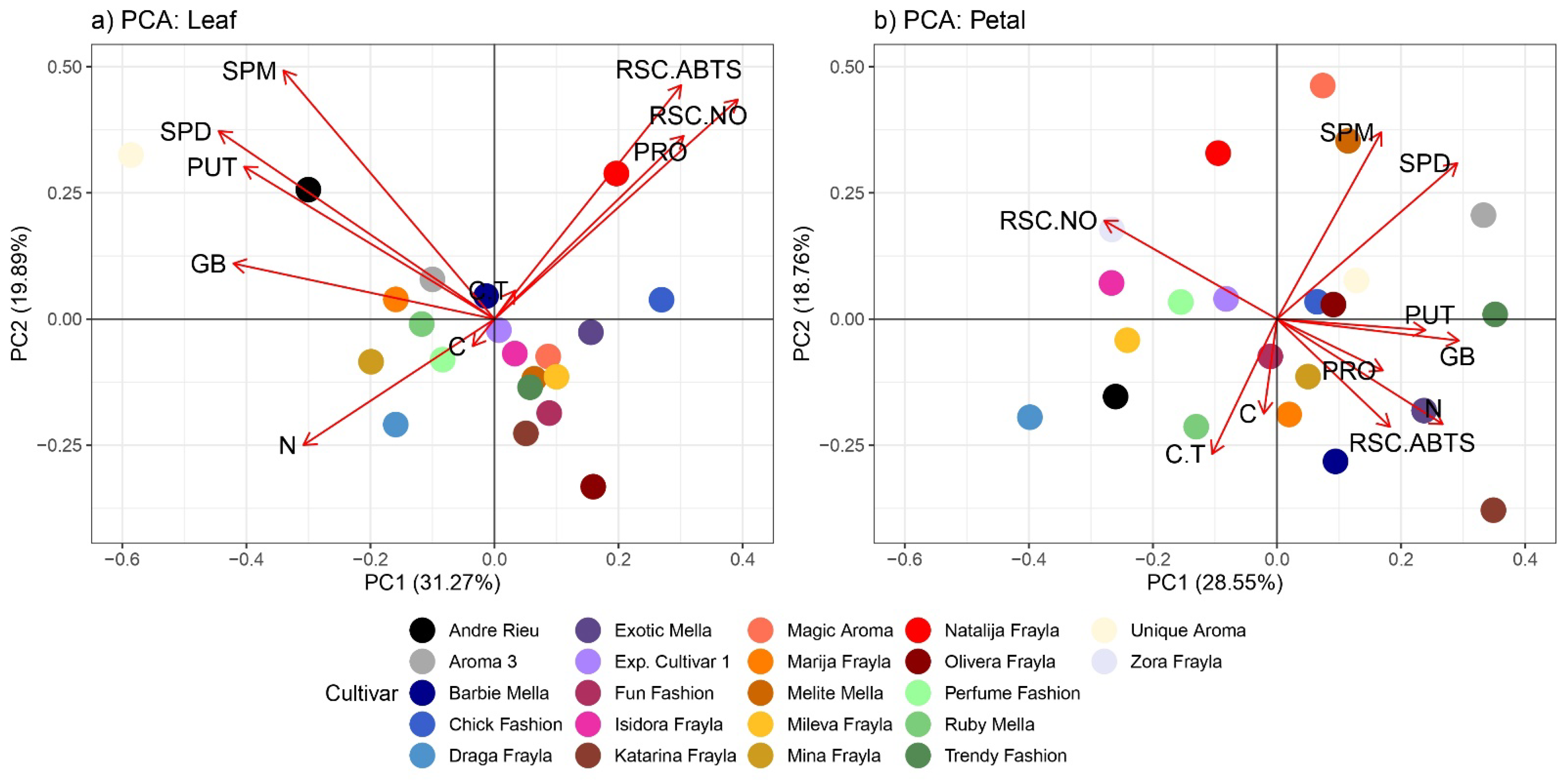
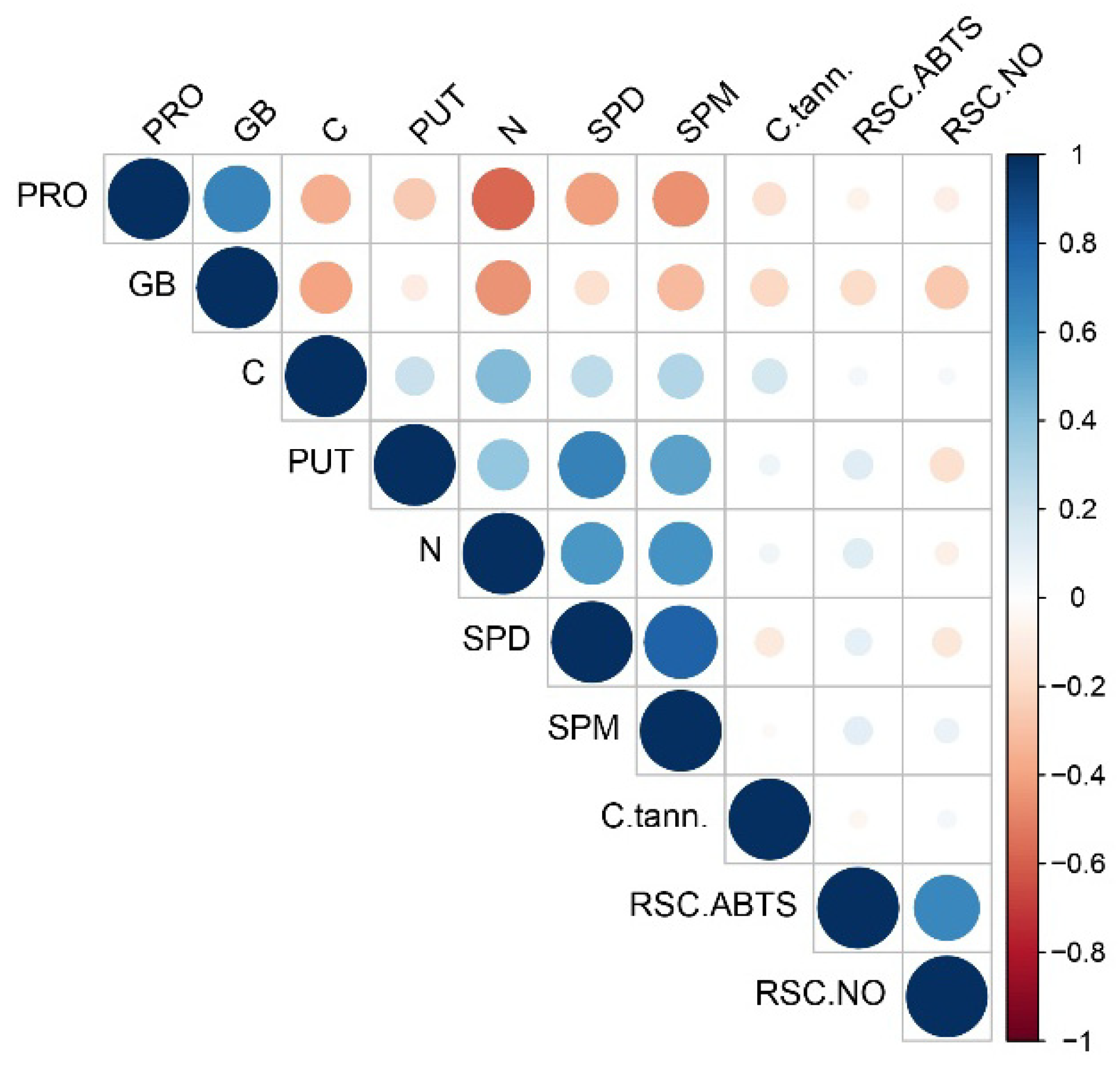
3.4. PCA Analysis, Hierarchical Clustering and Correlation Matrix
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, S. The true value of flowers and their effect on the Dutch economy. An interdisciplinary relationship between Art and Economics. Res. Econ. 2020, 74, 228–232. [Google Scholar] [CrossRef]
- Usman, M.; Ashfaq, M.; Taj, S.; Abid, M. An economic analysis of cut-rose flower in Punjab, Pakistan. JAPS 2014, 24, 651–655. [Google Scholar]
- Sharma, R.; Kumari, P.; Sahare, H.; Paul, S. Insight to the biotechnological interventions in flower crops for abiotic stress tolerance. Sci. Hortic. 2023, 318, 112102. [Google Scholar] [CrossRef]
- Debener, T.; Byrne, D.H. Disease resistance breeding in rose: Current status and potential of biotechnological tools. Plant Sci. 2014, 228, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Vukosavljev, M.; Zhang, J.; Esselink, G.D.; Westende, W.V.; Cox, P.; Visser, R.; Arens, P.; Smulders, M.J. Genetic diversity and differentiation in roses: A garden rose perspective. Sci. Hortic. 2013, 162, 320–332. [Google Scholar] [CrossRef]
- Sazzad, S.; Rajbongshi, A.; Shakil, R.; Akter, B.; Kaiser, M.S. RoseNet: Rose leave dataset for the development of an automation system to recognize the diseases of rose. Data Brief 2022, 44, 108497. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, C.; Wang, W.; Cao, Z.; Li, S.; Li, H.; Zhu, W.; Huang, Y.; Bao, M.; He, Y.; et al. Comparative transcriptome analysis of different heat stress responses between self-root grafting line and heterogeneous grafting line in rose. Hortic. Plant J. 2021, 7, 243–255. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.Z.; Cheng, C.; Jiang, X. Exogenous calcium enhances the physiological status and photosynthetic capacity of rose (Rosa hybrida L.) under drought stress. Hortic. Plant J. 2023; in press. [Google Scholar] [CrossRef]
- Carvalho, D.R.; Vasconcelos, M.W.; Lee, S.; Vreugdenhil, D.; Heuvelink, E.; Carvalho, S.M. Moderate salinity improves stomatal functioning in rose plants grown at high relative air humidity. Environ. Exp. Bot. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, F.; Chen, H.; Zhang, T.; Yan, J.; Hu, Y. Unraveling arbuscular mycorrhizal fungi-induced resistance of purple branch rose (Rosa rugosa ‘Zizhi’) to Lymantria dispar based on metabolomics. Biol. Control 2022, 172, 104971. [Google Scholar] [CrossRef]
- Khan, S.; Siraj, S.; Shahid, M.; Haque, M.M.; Islam, A. Osmolytes: Wonder molecules to combat protein misfolding against stress conditions. Int. J. Biol. Macromol. 2023, 234, 123662. [Google Scholar] [CrossRef]
- Fedotova, M.V. Compatible osmolytes-bioprotectants: Is there a common link between their hydration and their protective action under abiotic stresses? J. Mol. Liq. 2019, 292, 111339. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknic, Z.; Chen, T.H.H. Exogenous application of glycine betaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Biouki, R.Y.; Beyrami, H. Investigating the effects of glycine betaine on growth and flower yield of Damask Rose under salinity stress. J. Crop Improv. 2020, 22, 119–134. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Čapelja, E.; Vuksanović, V.; Stojnić, S.; Gavranović Markić, A.; Zlatković, M.; Milović, M.; Galović, V.; Orlović, S. Ectomycorrhizal fungi modulate pedunculate Oak’s heat stress responses through the alternation of polyamines, phenolics, and osmotica Content. Plants 2022, 11, 3360. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double agents in disease and plant immunity. Trends Plant. Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Rashan, A.I.; Altaee, R.T.; Salh, F.S.; Al-Abbasy, O.Y.; Al-Lehebe, N. The role of polyamines in plants: A review. Plant Sci. Today 2023, 10, 164–171. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 429326. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, X.; Yan, J.; Fan, L.; Rong, C.; Mo, C.; Zhang, M. Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’ apple (Malus domestica Borkh.). Sci. Rep. 2019, 9, 12777. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of roses (Rosa hybrida L. ‘Herbert Stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci. Rep. 2019, 9, 16025. [Google Scholar] [CrossRef]
- Ochir, S.; Park, B.; Nishizawa, M.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Simultaneous determination of hydrolysable tannins in the petals of Rosa rugosa and allied plants. J. Nat. Med. 2010, 64, 383–387. [Google Scholar] [CrossRef]
- Koopman, W.J.M.; Wissemann, V.; De Cock, K.; Van Huylen-Broeck, J.; De Riek, J.; Sabatino, G.J.H.; Visser, D.; Vosman, B.; Ritz, C.M.; Maes, B.; et al. AFLP markers as a tool to reconstruct complex relationships: A case study in Rosa (Rosaceae). Am. J. Bot. 2008, 95, 353–366. [Google Scholar] [CrossRef]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M.L. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef]
- Smulders, M.J.; Arens, P.; Bourke, P.M.; Debener, T.; Linde, M.; De Riek, J.; Leus, L.; Ruttink, T.; Baudino, S.; Hibrant Saint-Oyant, L.; et al. In the name of the rose: A roadmap for rose research in the genome era. Hortic. Res. 2019, 6, 65. [Google Scholar] [CrossRef]
- Scaramagli, S.; Franceschetti, M.; Michael, A.J.; Torrigiani, P.; Bagni, N. Polyamines and flowering: Spermidine biosynthesis in the different whorls of developing flowers of Nicotiana tabacum L. Plant Biosyst. 1999, 133, 229–237. [Google Scholar] [CrossRef]
- Biondi, S.; Antognoni, F.; Marincich, L.; Lianza, M.; Tejos, R.; Ruiz, K.B. The polyamine “multiverse” and stress mitigation in crops: A case study with seed priming in quinoa. Sci. Hortic. 2022, 304, 111292. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumaravel, S. Study on phenolic content, antioxidant activity and CHNS elemental analysis of Amorphophallus sylvaticus. Int. J. Agric. Life Sci. 2016, 2, 12–17. Available online: https://skyfox.co/ijals/index.php/als/article/view/9 (accessed on 8 March 2024).
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Rašeta, M.; Kebert, M.; Mišković, J.; Rakić, M.; Kostić, S.; Čapelja, E.; Karaman, M. Polyamines in edible and medicinal fungi from Serbia: A novel perspective on neuroprotective properties. J. Fungi 2024, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 15 March 2024).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological characters, phytochemical profile and biological activities of novel garden roses edible cultivars. Horticulturae 2023, 9, 1082. [Google Scholar] [CrossRef]
- Azizi, S.; Seyed Hajizadeh, H.; Aghaee, A.; Kaya, O. In vitro assessment of physiological traits and ROS detoxification pathways involved in tolerance of Damask rose genotypes under salt stress. Sci. Rep. 2023, 13, 17795. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Kostić, S.; Stojnić, S.; Čapelja, E.; Gavranović Markić, A.; Zorić, M.; Kesić, M.; Flors, V. A fine-tuning of the plant hormones, polyamines and osmolytes by ectomycorrhizal fungi enhances drought tolerance in pedunculate oak. Int. J. Mol. Sci. 2023, 24, 7510. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.; Tomar, R.S.; Sharma, R.A.; Singh, S.K. Proline: A key player to regulate biotic and abiotic stress in plants. In Towards Sustainable Natural Resources: Monitoring and Managing Ecosystem Biodiversity; Springer International Publishing: Cham, Switzerland, 2022; Volume 18, pp. 333–346. [Google Scholar] [CrossRef]
- Kumar, N.; Pal, M.; Singh, A.; SaiRam, R.K.; Srivastava, G.C. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L. ‘Grand Gala’). Sci. Hortic. 2010, 127, 79–85. [Google Scholar] [CrossRef]
- Zhang, L.; Becker, D. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 151855. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Wang, J. Increasing the activities of protective enzymes is an important strategy to improve resistance in cucumber to powdery mildew disease and melon aphid under different infection/infestation patterns. Front. Plant Sci. 2022, 13, 950538. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Zlatković, M.; Stojnic, S.; Čapelja, E.; Zorić, M.; Kiprovski, B.; Budakov, D.; Orlović, S. Ectomycorrhizal fungi modulate biochemical response against powdery mildew disease in Quercus robur L. Forests 2022, 13, 1491. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Montilla-Bascón, G.; Rubiales, D.; Prats, E. Changes in polyamine profile in host and non-host oat–powdery mildew interactions. Phytochem. Lett. 2014, 8, 207–212. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines and plant disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, W.; Kulma, A.; Namysł, K.; Preisner, M.; Szopa, J. Polyamine metabolism in flax in response to treatment with pathogenic and non–pathogenic Fusarium strains. Front. Plant Sci. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gai, C.; Shao, M.; Fang, L.; Li, X.; Song, Y.; Zeng, R.; Chen, D. Herbivory by striped stem borer triggers polyamine accumulation in host rice plants to promote its larval growth. Plants 2023, 12, 3249. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Gholami, M.; Baninasab, B. Changes in polyamines during bud dormancy in almond cultivars differing in their flowering date. Sci. Hortic. 2019, 258, 108788. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chaudhury, R.; Dutta, S.; Basak, M.; Dey, S.; Schäffner, A.R.; Das, M. Role of metabolites in flower development and discovery of compounds controlling flowering time. Plant Physiol. Biochem. 2022, 190, 109–118. [Google Scholar] [CrossRef]
- Tavallali, V.; Alhavi, N.; Gholami, H.; Abarghuei, F.M. Developmental and phytochemical changes in pot marigold (Calendula officinalis L.) using exogenous application of polyamines. Plant Physiol. Biochem. 2022, 183, 128–137. [Google Scholar] [CrossRef]
- Constabel, P.C.; Yoshida, K.; Walker, V. Diverse ecological roles of plant tannins: Plant defense and beyond. In Recent Advances in Polyphenol Research; Romani, A., Lattanzio, V., Quideau, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 115–142. [Google Scholar]
- Harding, S.A. Condensed tannins: Arbiters of abiotic stress tolerance? Tree Physiol. 2019, 39, 341–344. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]


 Mileva Frayla |  Zora Frayla |  Experimental cultivar |  Isidora Frayla |
 Draga Frayla |  Fun Fashion |  Marija Frayla |  Barbie Mella |
 Ruby Mella |  Olivera Frayla |  Trendy Fashion |  Exotic Mella |
 Natalija Frayla |  Andre Rieu |  Melite Mella |  Magic Aroma |
 Aroma 3 |  Unique Aroma |  Mina Frayla |  Perfume Fashion |
 Katarina Frayla |  Chick Fashion |  Jelena Frayla |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebert, M.; Rašeta, M.; Kostić, S.; Vuksanović, V.; Božanić Tanjga, B.; Ilić, O.; Orlović, S. Metabolically Tailored Selection of Ornamental Rose Cultivars through Polyamine Profiling, Osmolyte Quantification and Evaluation of Antioxidant Activities. Horticulturae 2024, 10, 401. https://doi.org/10.3390/horticulturae10040401
Kebert M, Rašeta M, Kostić S, Vuksanović V, Božanić Tanjga B, Ilić O, Orlović S. Metabolically Tailored Selection of Ornamental Rose Cultivars through Polyamine Profiling, Osmolyte Quantification and Evaluation of Antioxidant Activities. Horticulturae. 2024; 10(4):401. https://doi.org/10.3390/horticulturae10040401
Chicago/Turabian StyleKebert, Marko, Milena Rašeta, Saša Kostić, Vanja Vuksanović, Biljana Božanić Tanjga, Olivera Ilić, and Saša Orlović. 2024. "Metabolically Tailored Selection of Ornamental Rose Cultivars through Polyamine Profiling, Osmolyte Quantification and Evaluation of Antioxidant Activities" Horticulturae 10, no. 4: 401. https://doi.org/10.3390/horticulturae10040401
APA StyleKebert, M., Rašeta, M., Kostić, S., Vuksanović, V., Božanić Tanjga, B., Ilić, O., & Orlović, S. (2024). Metabolically Tailored Selection of Ornamental Rose Cultivars through Polyamine Profiling, Osmolyte Quantification and Evaluation of Antioxidant Activities. Horticulturae, 10(4), 401. https://doi.org/10.3390/horticulturae10040401