Poinsettia Growth and Development Response to Container Root Substrate with Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Substrate Treatments and Plant Materials
2.2. Fertigation Regimes
2.3. Measurements
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Growth Substrate Characteristics
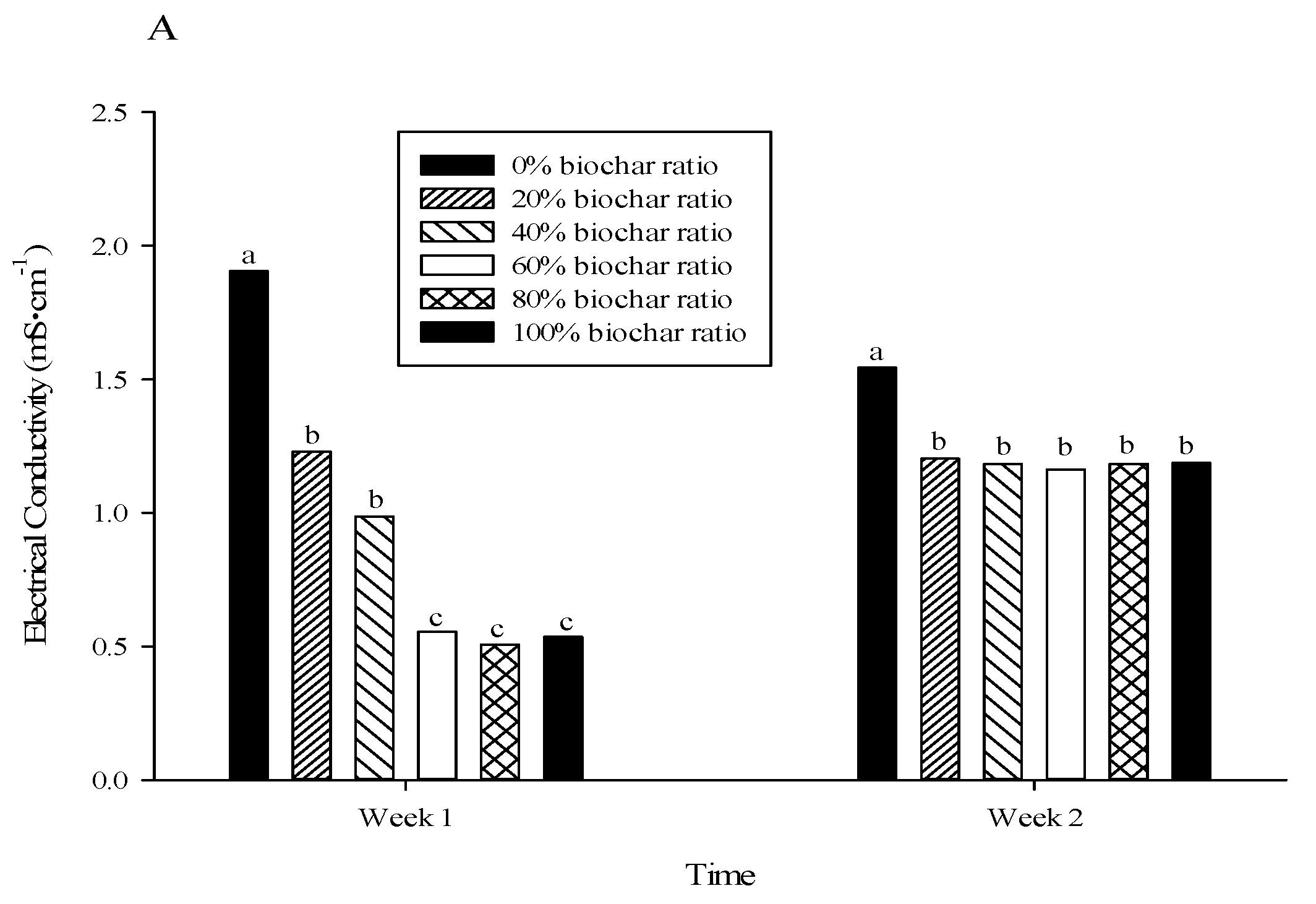
3.2. Root Substrate Electrical Conductivity
3.3. Plant Growth
3.4. Gas Exchange
3.5. Plant Quality
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Floriculture Crops 2015 Summary, 2016. National Agricultural Statistics Service, United States Department of Agriculture, April 2016. Available online: http://usda.mannlib.cornell.edu/usda/current/FlorCrop/FlorCrop-04-26-2016.pdf (accessed on 26 December 2017).
- Bilderback, T. Container Soils and Soilless Media; NCPM. No. 9; North Carolina Agricultural Extension Service: Raeigh, NC, USA, 1982. [Google Scholar]
- Hidalgo, P.R.; Harkess, R.L. Earthworm castings as a substrate for poinsettia production. HortScience 2002, 37, 304–308. [Google Scholar]
- Clarke, D. Wise use of peat in horticulture. Acta Hortic. 2008, 779, 161–164. [Google Scholar] [CrossRef]
- Apodaca, E.L. Mineral Commodity Summaries 2013; U.S. Geological Survey: Lakewood, CO, USA, 2013; pp. 114–116.
- Rivière, L.; Morel, P.J.M.; Charpentier, S. Growing media in French horticulture. Acta Hortic. 2008, 779, 33–38. [Google Scholar] [CrossRef]
- Robertson, R.A. Peat, horticulture and environment. Biodivers. Conserv. 1993, 2, 541–547. [Google Scholar] [CrossRef]
- Carlile, W.R. Growing media and environment lobby in UK 1997–2001. Acta Hortic. 2004, 644, 107–113. [Google Scholar] [CrossRef]
- Jackson, E.B.; Wright, R.D.; Barnes, M.C.; Hall, S. Pine tree substrate, nitrogen rate, article size, and peat amendment affect poinsettia growth and substrate physical properties. HortScience 2008, 43, 2155–2161. [Google Scholar]
- Gu, M.; Li, Q.; Steele, P.H.; Niu, G.; Yu, F. Growth of ‘Fireworks’ gomphrena grown in substrates amended with biochar. J. Food Agric. Environ. 2013, 11, 819–821. [Google Scholar]
- Wang, Y.; Blessington, T.M. Growth and interior performance of poinsettia in media containing composted cotton burrs. HortScience 1990, 25, 407–408. [Google Scholar]
- Ku, S.M.C.; Bouwkamp, J.C.; Gouin, F.R. Effects of compost source and timing of fertigation initiation on growth of potted poinsettia. Compost Sci. Util. 1998, 6, 57–66. [Google Scholar] [CrossRef]
- Papafotiou, M.; Phsyhalou, M.; Kargas, G.; Chatzipavlidis, I.; Chronopoulos, J. Olive-mill wastes compost as growing medium component for production of poinsettia. Sci. Hortic. 2004, 102, 167–175. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Kisiki, N.H.; Yusuf, H.M.; Ghani, W.A.W.A.K. Gasification of biochar from empty fruit bunch in a fluidized bed reactor. Energies 2010, 3, 1344–1352. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Filiberto, D.M.; Gaunt, J.L. Practicality of biochar additions to enhance soil and crop productivity. Agriculture 2013, 3, 715–725. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Jirka, S.; Tomlinson, T. 2013 State of the Biochar Industy, Asurvey of Commercial Activity in the Biochar Field; International Biochar Initiative (IBI): Philadelphia, PA, USA, 2014; 61p, Available online: http://www.biochar-international.org/sites/default/files/State_of_the_Biochar_Industry_2013.pdf (accessed on 26 December 2017).
- Altland, J.E.; Locke, J.C. Biochar affects macronutrient leaching from a soilless substrate. HortScience 2012, 47, 1136–1140. [Google Scholar]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Cavins, T.J.; Gibson, J.L.; Whipker, B.E.; Fonteno, W.C. pH and EC Meters-Tool for Substrate Analysis; North Carolina State University: Raleigh, NC, USA, 2000; Available online: https://www.ces.ncsu.edu/depts/hort/floriculture/Florex/PH%20EC%20Meter%20Comparison.pdf (accessed on 26 December 2017).
- Ecke, P.; Faust, J.E.; Higgins, A.; Williams, J. The Ecke Poinsettia Manual; Ball Publishing: West Chicago, IL, USA, 2004. [Google Scholar]
- Ecke, P., Jr.; Matkin, O.A.; Hartley, D.E. Poinsettia Manual, 3rd ed.; Paul Ecke Poinsettias: Encinitas, CA, USA, 1990. [Google Scholar]
- LeBude, A.V.; Bilderback, T.E. Pour-through Extraction Procedure: A Nutrient Management Tool for Nursery Crops. North Carolina Cooperative Extension 1–8. August 2009. Available online: https://content.ces.ncsu.edu/the-pour-through-extraction-procedure-a-nutrient-management-tool-for-nursery-crops (accessed on 26 December 2017).
- Wright, R.D.; Grueber, K.L.; Leda, C. Medium nutrient extraction pour-through and saturated with medium extract procedures for poinsettia. HortScience 1990, 25, 658–660. [Google Scholar]
- Fonteno, W.C.; Cassel, D.K.; Larson, R.A. Physical properties of three container media and their effect on poinsettia height. J. Am. Soc. Hortic. Sci. 1981, 106, 736–741. [Google Scholar]
- Yeager, T.H.; Fare, D.C.; Lea-Cox, J.; Ruter, J.; Bilderback, T.E.; Gilliam, C.H.; Niemiera, A.X.; Warren, S.L.; Whitwell, T.E.; Wright, R.D.; et al. Best Management Practices: Guide for Producing Container-Grown Plants, 2nd ed.; Sourn Nurserymen’s Assoc.: Marietta, GA, USA, 2007. [Google Scholar]
- Dole, M.J.; Wilkins, H.F. Floriculture Principles and Species, 2nd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Steiner, C.; Harttung, T. Biochar as growing media additive and peat substitute. Solid Earth Discuss. 2014, 6, 1023–1035. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. Can biochar reduce soil greenhouse gas emissions from a Miscanthus bioenergy crop? Glob. Chang. Biol. Bioenergy 2014, 6, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of Biochar on Manure Carbon Stabilization and Greenhouse Gas Emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Ku, S.M.C.; Hershey, R.D. Leachate electrical conductivity and growth of potted poinsettia with leaching fractions of 0 to 0.4 J. Am. Soc. Hortic. Sci. 1991, 116, 802–806. [Google Scholar]
- Yelanich, M.V.; Biernbaum, J.A. Root-medium nutrient concentration and growth of poinsettia at three fertilizer concentrations and four leaching fractions. HortScience 1993, 118, 771–776. [Google Scholar]
- Gaborcik, N. Relationship between contents of chlorophyll (a + b) (SPAD values) and nitrogen of some temperate grasses. Photosynthetica 2003, 41, 285–287. [Google Scholar] [CrossRef]
- Li, Y.C.; Alva, A.K.; Calvert, D.V.; Zhang, M. A rapid nondestructive technique to predict leaf nitrogen status of grapefruit tree with various nitrogen fertilization practices. HortTechnology 1998, 8, 81–86. [Google Scholar]
- Neilsen, D.; Hogue, E.J.; Neilsen, G.H. Using SPAD-502 values to assess nitrogen status of apple trees. HortScience 1995, 30, 508–512. [Google Scholar]
- Sibley, J.L.; Eakes, D.J.; Gilliam, C.H.; Keever, G.J.; Dozier, W.A.; Himelrick, D.G. Foliar SPAD-502 meter values, nitrogen levels, and extractable chlorophyll for red maple selections. HortScience 1996, 31, 468–470. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Kammann, C.; Ratering, S.; Eckhard, C.; Müller, C. Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J. Environ. Qual. 2012, 41, 1052–1066. [Google Scholar] [CrossRef] [PubMed]


| Biochar Percentage | TP y (% vol) | CC x (% vol) | AS w (% vol) | BD v (g·cm−3) | BD u at CC (g·cm−3) |
|---|---|---|---|---|---|
| 0% | 84.2 ab z | 62.8 a | 21.5 e | 0.10 f | 0.73 a |
| 20% | 86.5 a | 61.5 a | 24.9 d | 0.11 e | 0.72 a |
| 40% | 79.8 bcd | 55.8 b | 24.0 d | 0.12 d | 0.68 b |
| 60% | 75.3 d | 46.3 c | 29.0 c | 0.14 c | 0.60 d |
| 80% | 78.5 cd | 47.2 c | 31.3 b | 0.16 b | 0.63 c |
| 100% | 82.6 abc | 46.9 c | 35.7 a | 0.18 a | 0.65 c |
| Suitable Range t | 50–85 | 45–65 | 10–30 | 0.19–0.70 | 0.64–0.96 |
| Treatment | Growth Index (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 3 | Week 5 | Week 7 | Week 9 | Week 11 | Week 13 | Week 15 | |
| Biochar | ||||||||
| 0% | 12.3 a z | 20.8 a | 25.4 a | 29.0 a | 32.7 ab | 37.7 b | 40.8 b | 44.1 ab |
| 20% | 12.5 a | 20.1 ab | 24.7 a | 30.0 a | 33.7 a | 39.5 a | 42.6 a | 45.2 a |
| 40% | 12.3 a | 20.3 a | 24.9 a | 29.1 a | 33.0 ab | 37.5 b | 40.8 b | 44.2 ab |
| 60% | 12.5 a | 19.5 b | 24.5 a | 28.6 a | 32.2 b | 36.9 b | 40.6 b | 43.7 ab |
| 80% | 12.0 a | 18.7 c | 24.2 a | 28.4 a | 30.7 c | 35.7 c | 39.5 b | 42.7 b |
| 100% | 11.8 a | 17.0 d | 22.3 b | 24.6 b | 26.4 d | 32.5 d | 35.1 c | 37.5 c |
| Fertigation | ||||||||
| F1 | 12.1 a | 19.4 a | 24.8 a | 29.1 a | 31.6 a | 35.6 b | 37.6 b | 40.9 b |
| F2 | 12.3 a | 19.2 a | 23.6 a | 28.6 ab | 31.7 a | 36.8 ab | 40.4 a | 43.1 a |
| F3 | 12.2 a | 19.7 a | 24.7 a | 27.9 bc | 31.4 a | 37.2 a | 40.8 a | 44.0 a |
| F4 | 12.4 a | 19.1 a | 24.2 a | 27.1 c | 31.0 a | 36.9 ab | 40.8 a | 43.7 a |
| Significance | ||||||||
| Biochar | NS y | *** | *** | *** | *** | *** | *** | *** |
| Fertigation | NS | NS | NS | *** | NS | *** | *** | *** |
| Biochar × Fertigation | NS | NS | NS | NS | NS | NS | NS | NS |
| Dry Weight (g) | |||
|---|---|---|---|
| Treatment | Total DW | Green Leaf DW | Stem DW |
| Biochar | |||
| 0% | 39.8 b z | 15.1 ab | 10.6 a |
| 20% | 43.1 a | 16.1 a | 10.9 a |
| 40% | 38.1 bc | 14.7 b | 10.7 a |
| 60% | 35.9 c | 14.2 b | 9.7 a |
| 80% | 32.0 d | 12.4 c | 8.7 b |
| 100% | 24.1 e | 9.3 d | 6.7 c |
| Fertigation | |||
| F1 | 32.5 b | 12.2 b | 9.5 a |
| F2 | 36.3 a | 13.9 a | 10.0 a |
| F3 | 36.6 a | 14.1 a | 9.4 a |
| F4 | 38.0 a | 14.9 a | 9.5 a |
| Treatment | Photosynthetic Rate (μmol CO2 m−2·s−1) | Stomatal Conductance (mol H2O m−2·s−1) | Transpiration Rate (mmol H2O m−2·s−1) |
|---|---|---|---|
| Biochar | |||
| 0% | 10.83 a z | 0.41 a | 4.47 a |
| 40% | 8.13 b | 0.41 a | 4.55 a |
| 100% | 8.11 b | 0.42 a | 4.58 a |
| Fertigation | |||
| F1 | 7.09 b | 0.36 b | 4.23 b |
| F2 | 9.47 a | 0.42 ab | 4.63 ab |
| F3 | 10.51 a | 0.45 a | 4.74 a |
| Significance | |||
| Biochar | ** y | NS | NS |
| Fertigation | *** | * | * |
| Biochar × Fertigation | NS | NS | NS |
| Biochar | Fertigation Regime | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Total Number of Leaves | ||||
| 0% | 235.7 a z | 219.2 a | 225.3 a | 211.5 a |
| 20% | 214.6 ab | 217.8 a | 232.8 a | 221.8 a |
| 40% | 222.5 ab | 210.4 a | 202.7 ab | 231.0 a |
| 60% | 188.4 b | 213.3 a | 224.9 a | 224.0 a |
| 80% | 194.2 b | 190.0 ab | 209.3 ab | 188.3 a |
| 100% | 155.0 c | 161.4 b | 187.0 b | 118.8 b |
| Total Number of Red Bracts | ||||
| 0% | 143.9 a | 128.3 a | 137.2 ab | 136.1 a |
| 20% | 127.7 ab | 130.8 a | 143.9 a | 137.5 a |
| 40% | 128.7 ab | 122.1 a | 121.9 ab | 142.1 a |
| 60% | 106.9 bc | 123.0 a | 134.0 ab | 136.4 a |
| 80% | 113.8 bc | 107.0 ab | 120.9 ab | 115.4 a |
| 100% | 96.8 c | 96.9 b | 115.7 b | 66.5 b |
| Dry Weight of Red Bract (g) | ||||
| 0% | 13.8 a z A y | 13.7 ab A | 13.8 ab A | 13.9 ab A |
| 20% | 12.2 a B | 15.0 a A | 15.1 a A | 15.4 a A |
| 40% | 10.3 b C | 12.4 bc B | 12.7 bc B | 14.4 ab A |
| 60% | 9.9 b B | 12.1 c A | 12.7 bc A | 12.7 b A |
| 80% | 9.6 b B | 11.0 c A | 11.9 c A | 11.0 c A |
| 100% | 7.1 c BC | 8.4 d AB | 9.7 d A | 5.9 d C |
| Treatment | SPAD Values | Average Number of Red Bracts | |||||
|---|---|---|---|---|---|---|---|
| Week 10 | Week 12 | Week 14 | Week 11 | Week 12 | Week 13 | Week 14 | |
| Biochar | |||||||
| 0% | 41.7 a z | 45.1 a | 53.1 a | 1.5 ab | 5.2 b | 8.9 a | 10.1 ab |
| 20% | 41.5 a | 45.6 a | 53.7 a | 1.8 a | 5.8 a | 9.1 a | 10.5 a |
| 40% | 40.1 a | 44.1 a | 54.1 a | 1.3 ab | 4.9 b | 8.4 a | 9.8 ab |
| 60% | 40.3 a | 45.4 a | 54.4 a | 1.2 ab | 4.8 b | 8.4 a | 10.0 ab |
| 80% | 40.1 a | 46.2 a | 54.2 a | 1.2 ab | 4.8 b | 8.3 a | 9.7 b |
| 100% | 39.9 a | 45.4 a | 54.9 a | 1.1 b | 4.8 b | 8.4 a | 9.5 b |
| Fertigation | |||||||
| F1 | 39.0 b | 43.5 b | 53.0 b | 1.0 b | 4.6 c | 8.1 b | 9.3 b |
| F2 | 40.4 ab | 45.1 a | 54.1 ab | 1.2 ab | 4.8 bc | 8.4 ab | 9.7 b |
| F3 | 41.5 a | 46.4 a | 55.0 a | 1.5 a | 5.2 ab | 8.9 a | 10.2 a |
| F4 | 41.5 a | 46.2 a | 54.1 ab | 1.6 a | 5.5 a | 8.9 a | 10.3 a |
| Significance | |||||||
| Biochar | NS y | NS | NS | ** | *** | ** | ** |
| Fertigation | ** | *** | ** | ** | *** | *** | *** |
| Biochar × Fertigation | NS | NS | NS | NS | NS | NS | NS |
| Biochar | Fertigation | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Final Shoot Rating | ||||
| 0% | 4.4 a z A y | 4.1 a AB | 4.5 a A | 3.9 a B |
| 20% | 4.6 a A | 4.0 a A | 4.2 a A | 4.2 a A |
| 40% | 4.9 a A | 4.1 a B | 4.1 a B | 4.4 a B |
| 60% | 4.4 a A | 4.2 a A | 4.2 a A | 3.6 a A |
| 80% | 4.5 a A | 4.3 a A | 4.1 a A | 3.3 a B |
| 100% | 4.5 a A | 3.3 a A | 4.3 a A | 1.8 b B |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Niu, G.; Starman, T.; Volder, A.; Gu, M. Poinsettia Growth and Development Response to Container Root Substrate with Biochar. Horticulturae 2018, 4, 1. https://doi.org/10.3390/horticulturae4010001
Guo Y, Niu G, Starman T, Volder A, Gu M. Poinsettia Growth and Development Response to Container Root Substrate with Biochar. Horticulturae. 2018; 4(1):1. https://doi.org/10.3390/horticulturae4010001
Chicago/Turabian StyleGuo, Yanjun, Genhua Niu, Terri Starman, Astrid Volder, and Mengmeng Gu. 2018. "Poinsettia Growth and Development Response to Container Root Substrate with Biochar" Horticulturae 4, no. 1: 1. https://doi.org/10.3390/horticulturae4010001





