Effect of Paclobutrazol Application on Plant Photosynthetic Performance and Leaf Greenness of Herbaceous Peony
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Photosynthetic and Chlorophyll Fluorescence Parameters
2.3. Chlorophyll Content
2.4. Morphological Parameters
2.5. Light and Electron Microscopy Analysis
2.6. Microstructures Observation
2.7 Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, C.Z.; Sun, Y.; Zhao, D.Q.; Tao, J. Relationship between Mechanical Strength and Morphological Index of Inflorescence Stem of Herbaceous Peony (Paeonia lactiflora Pall.). Acta Agric. Zhejiangensis 2015, 27, 182–188. (In Chinese) [Google Scholar]
- Ogata, T.; Hasukawa, H.; Shiozaki, S.; Horiuchi, S.; Kawase, K.; Iwagaki, I.; Okuda, H. Seasonal Changes in Endogenous Gibberellin Contents in Satsuma Mandarin during Flower Differentiation and the Influence of Paclobutrazol on Gibberellin Synthesis. J. Jpn. Soc. Hortic. Sci. 1996, 65, 245–253. [Google Scholar] [CrossRef][Green Version]
- Kondhare, K.R.; Hedden, P.; Kettlewell, P.S.; Farrell, A.D.; Monaghan, J.M. Use of the hormone-biosynthesis inhibitors fluridone and paclobutrazol to determine the effects of altered abscisic acid and gibberellin levels on pre-maturity α-amylase formation in wheat grains. J. Cereal Sci. 2014, 60, 210–216. [Google Scholar] [CrossRef]
- Pal, S.; Zhao, J.S.; Khan, A.; Yadav, N.S.; Batushansky, A.; Barak, S.; Rewald, B.; Fait, A.; Lazarovitch, N.; Rachmilevitch, S. Paclobutrazol Induces Tolerance in Tomato to Deficit Irrigation through Diversified Effects on Plant Morphology, Physiology and Metabolism. Sci. Rep. 2016, 6, 39321. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, B.S.A.; Sinniah, U.R.; Ismail, M.R.; Swamy, M.K. Optimization of Paclobutrazol Concentration and Application Time for Increased Lodging Resistance and Yield in Field-Grown Rice. Philipp. Agric. Sci. 2016, 99, 221–228. [Google Scholar]
- Srivastav, M.; Kishor, A.; Dahuja, A.; Sharma, R.R. Effect of Paclobutrazol and Salinity on Ion Leakage, Proline Content and Activities of Antioxidant Enzymes in Mango (Mangifera indica L.). Sci. Hortic. 2010, 125, 785–788. [Google Scholar] [CrossRef]
- Manivarman, P.; Jaleel, C.A.; Kishorekumar, A.; Sankar, B.; Somasundaram, R.; Panneerselvam, R. Protection of Vigna unguiculata (L.) Walp. Plants from Salt Stress by Paclobutrazol. Colloid Surf. B. 2008, 61, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wang, Y.Y.; Sun, L.Q.; Wei, T.T.; Gu, X.H.; Gao, F.; Li, X.D. Effects of Paclobutrazol on the Yield, Quality, and Related Enzyme Activities of Different Quality Type Peanut Cultivars. Chin. J. Appl. Ecol. 2013, 24, 2850–2856. (In Chinese) [Google Scholar]
- Zhao, J.; Zhang, W.; Qiu, Q.; Li, Z.P.; Zhang, M.H.; Yan, X.Y.; Du, D.H. Effects of PP333 Spraying at Different Stages on Soybean Agronomic and Physiological Characters. Soybean Sci. 2011, 30, 211–214. (In Chinese) [Google Scholar]
- Zhou, W.J.; Xi, H.F. Effects of Mixtalol and Paclobutrazol on Photosynthesis and Yield of Rape (Brassica napus). J. Plant Growth Regul. 1993, 12, 157–161. [Google Scholar] [CrossRef]
- Tekalign, T.; Hammes, P.S. Response of Potato Grown under Non-inductive Condition to Paclobutrazol: Shoot Growth, Chlorophyll Content, Net Photosynthesis, Sssimilate Partitioning, Tuber Yield, Quality, and Dormancy. Plant Growth Regul. 2004, 43, 227–236. [Google Scholar] [CrossRef]
- Feng, L.J.; Yuan, Z.H.; Yi, Y.L.; Zhao, X.Q. Effects of Paclobutrazol on Photosynthetic Characteristics and Ultrastructure of Dahurian Leaves. Acta Pratacult. Sin. 2014, 23, 114–121. (In Chinese) [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wu, F.B.; Wang, X.D.; Zhang, G.P. Heterosis in CMS Hybrids of Cotton for Photosynthetic and Chlorophyll Fluorescence Parameters. Euphytica 2005, 144, 353–361. [Google Scholar] [CrossRef]
- Berova, M.; Zlatko, Z. Physiological Response and Yield of Paclobutrazol Treated Tomato Plants (Lycopersicon esculentum Mill.). Plant Growth Regul. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Nivedithadevi, D.; Somasundaram, R.; Pannerselvam, R. Effect of Abscisic Acid, Paclobutrazol and Salicylic Acid on the Growth and Pigment Variation in Solanum Trilobatum (l). Int. J. Drug Dev. Res. 2015, 4, 236–246. [Google Scholar]
- Fletcher, F.A.; Gilley, A.; Sankhla, N. Triazoles as Plant Growth Regulator and Stress Protectants. Hortic. Rev. 2000, 24, 55–138. [Google Scholar]
- Nouriyani, H.; Majidi, E.; Seyyednejad, S.M.; Siadat, S.A.; Naderi, A. Evaluation of Interaction of Paclobutrazol and Nitrogen on Correlation between Yield and Photosynthetic Pigments Contents in Two Wheat Cultivars (Triticum aestivum L.). Res. Crop. 2012, 13, 446–452. [Google Scholar]
- Kishorekumar, A.; Jaleel, C.A.; Manivannan, P. Differential Effects of Hexaconazole and Paclobutrazol on the Foliage Characteristics of Chinese Potato (Solenostemon rotundifolius Poir., J.K. Morton). Acta Biol. Szeged. 2006, 50, 127–129. [Google Scholar]
- Fletcher, R.A.; Kallidumbil, V.; Steele, P. An Improved Bioassay for Cytokinins Using Cucumber Cotyledons. Plant Physiol. 1982, 69, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.K.; Ganesh, R.; Mathur, S.R. Paclobutrazol Delayed Dark-induced Senescence of Mung Bean Leaves. Biologia 2007, 62, 185–188. [Google Scholar] [CrossRef]
- Costa, J.; Bosch, M.; Blanco, A. Growth and Cropping of Blanquilla Pear Trees Treated with Paclobutrazol. J. Hortic. Sci. 1995, 70, 433–443. [Google Scholar] [CrossRef]
- Yu, G.R. A Study on Modeling Stomatal Conductance of Maize (Zea mays L.) Leaves. Tech. Bull. Fac. Hort. Chiba Univ. 1999, 53, 145–239. [Google Scholar]
- Berova, M.; Zlatev, V. Physiological response of paclobutrazol-treated triticale plants to water stress. Biol. Plant. 2003, 46, 133–136. [Google Scholar] [CrossRef]
- Ma, B.L.; Morrison, M.J.; Voldeng, D.H. Leaf greenness and photosynthetic rates in soybean. Crop Sci. 1995, 35, 1411–1414. [Google Scholar] [CrossRef]
- Turner, F.T.; Jund, M.E. Assessing the Nitrogen Requirements of Rice Crops with a Chlorophyll Meter. Aust. J. Exp. Agric. 1994, 34, 1001–1005. [Google Scholar] [CrossRef]
- Messmer, R.; Fracheboud, Y.; Banziger, M.; Stamp, P.; Ribaut, J.M. Drought Stress and Tropical Maize: QTL-by-environment Interactions and Stability of QTLs across Environments for Yield Components and Secondary Traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Giunta, F.; Motzo, R.; Deidda, M. SPAD Readings and Associated Leaf Traits in Durum Wheat, Barley and Triticale Cultivars. Euphytica 2002, 125, 197–205. [Google Scholar] [CrossRef]
- Kim, D.S.; Kin, J.K.; Kim, S.D. Varietal Difference in Relationship between SPAD Value and Chlorophyll and Nitrogon Concentration in Rice Leaf. Korean J. Crop Sci. 2002, 47, 263–267. [Google Scholar]
- Fu, X.Y.; Zhou, L.Y.; Huang, J.B.; Mo, W.P.; Zhang, J.Y.; Li, J.G.; Wang, H.C.; Huang, X.M. Relating Photosynthetic Performance to Leaf Greenness in Litchi: A Comparison among Genotypes. Sci. Hortic. 2013, 152, 16–25. [Google Scholar] [CrossRef]
- Jalikop, S.H. Inheritance of Foliage Characteristics in (Annona cherimola Mill. x A. squamosa L.) x A. reticulata L. Hybrids. Euphytica 2010, 171, 175–180. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative Photosynthesis of Sun and Shade Plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Jiang, C.D.; Wang, X.; Gao, H.Y.; Wang, x.; Gao, H.Y.; Shi, L.; Chow, W.S. Systemic Regulation of Leaf Anatomical Structure, Photosynthetic Performance, and High-Light Tolerance in Sorghum. Plant Physiol. 2011, 155, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Pantin, F.; Simonneau, T.; Rolland, G.; Dauzat, M.; Muller, B. Control of Leaf Expansion: A Developmental Switch from Metabolics to Hydraulics. Plant Physiol. 2011, 156, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Bondada, B.R.; Syvertsen, J.P. Leaf Chlorophyll, Net Gas Exchange and Chloroplast Ultrastructure in Citrus Leaves of Different Nitrogen Status. Tree Physiol. 2003, 23, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Cao, J.R.; Qin, H.Y.; Zhao, Y.; Chen, L.; Ai, J.; Liu, T. The Pigment Content and Structure of the Color Leaves of Kiwi (Actinidia kolomikta) Affect the Leaf Color. Plant Physiol. J. 2016, 52, 1921–1926. (In Chinese) [Google Scholar]
- Banon, S.; Ochoa, J.; Gonzalez, A. Manipulation of Oleander Growth, Development and Foliage Colour by Paclobutrazol and Ethephon. Gartenbauwissenschaft 2001, 66, 123–132. [Google Scholar]
- Anderson, J.M.; Goodchild, D.J.; Boardman, N.K. Composition of the Photosystems and Chloroplast Structure in Extreme Shade Plants. Biochim. Biophys. Acta 1973, 325, 573–585. [Google Scholar] [CrossRef]
- Wan, L.Q.; Shi, Y.H.; Li, X.L.; He, F.; Jia, Y.X. Alterations in leaf cellular ultra-structure of three varieties of Lolium perenne subjected to high temperature and soil drought stress. Acta Pratacult. Sin. 2009, 18, 25–31. (In Chinese) [Google Scholar]
- Hang, J.S.; Wang, L.R.; Yu, X.S. The Ultrastructure of Chloroplasts of Virusinfected Barley Leaves. J. Integr. Plant Biol. 1986, 28, 444–445. (In Chinese) [Google Scholar]
- Wang, X.J.; Yuan, X.F.; Lou, C.R.; Dong, H.; He, Z.G. Effects of Different Nitrogen and Potassium Levels on Tomato Growth and Leaf Ultrastructure. Soil Fert. Sci. China 2014, 3, 44–48. (In Chinese) [Google Scholar]
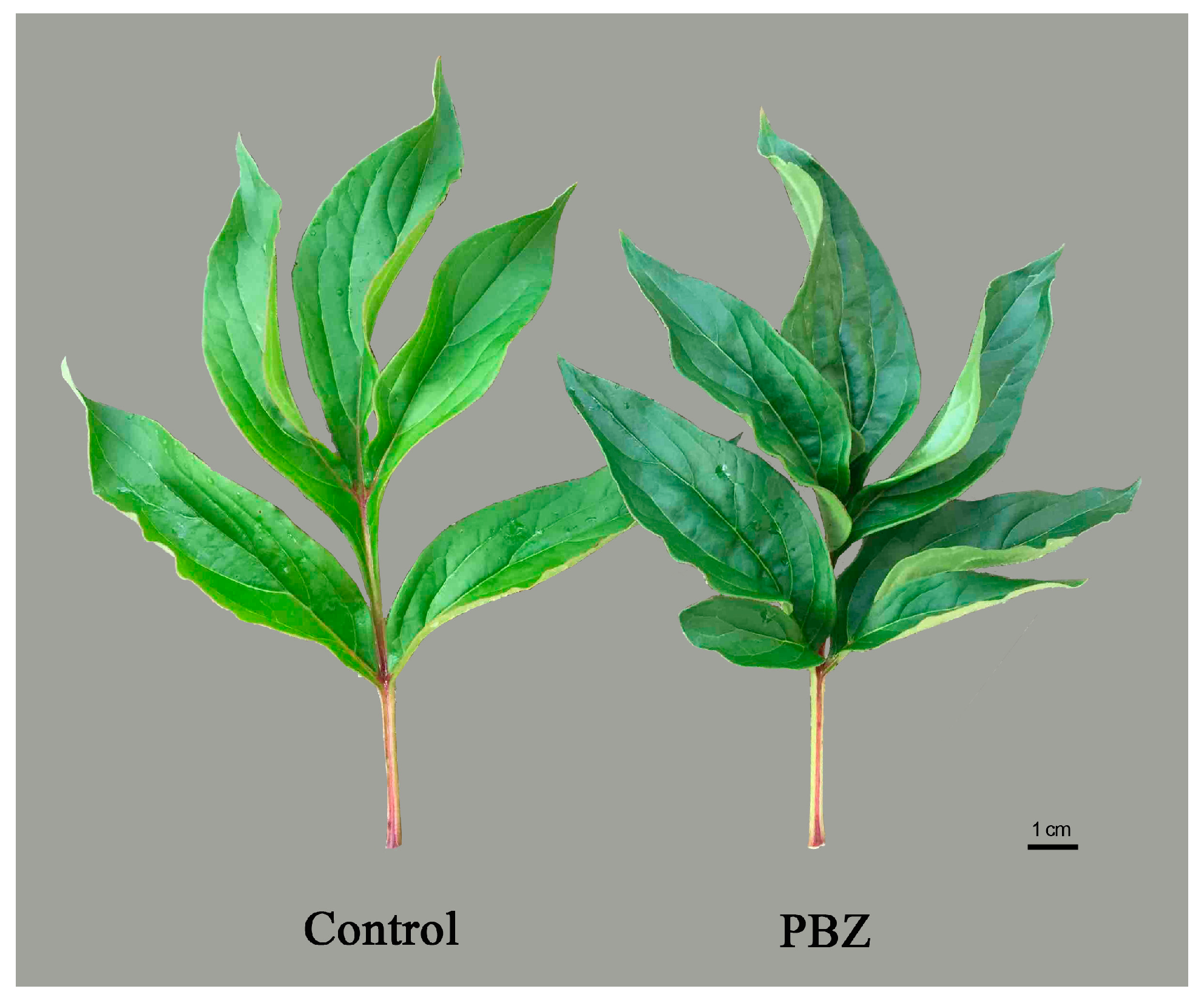

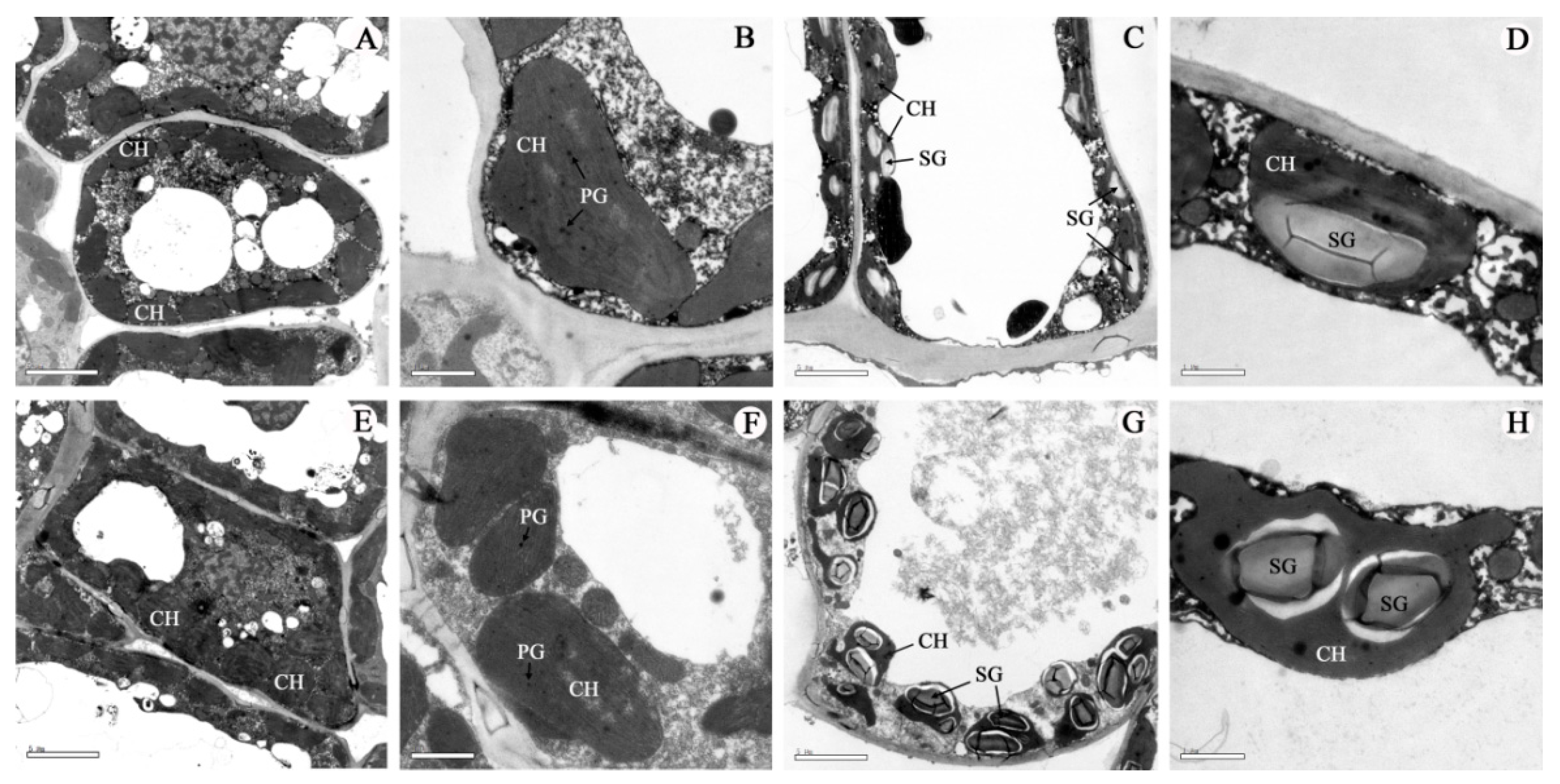
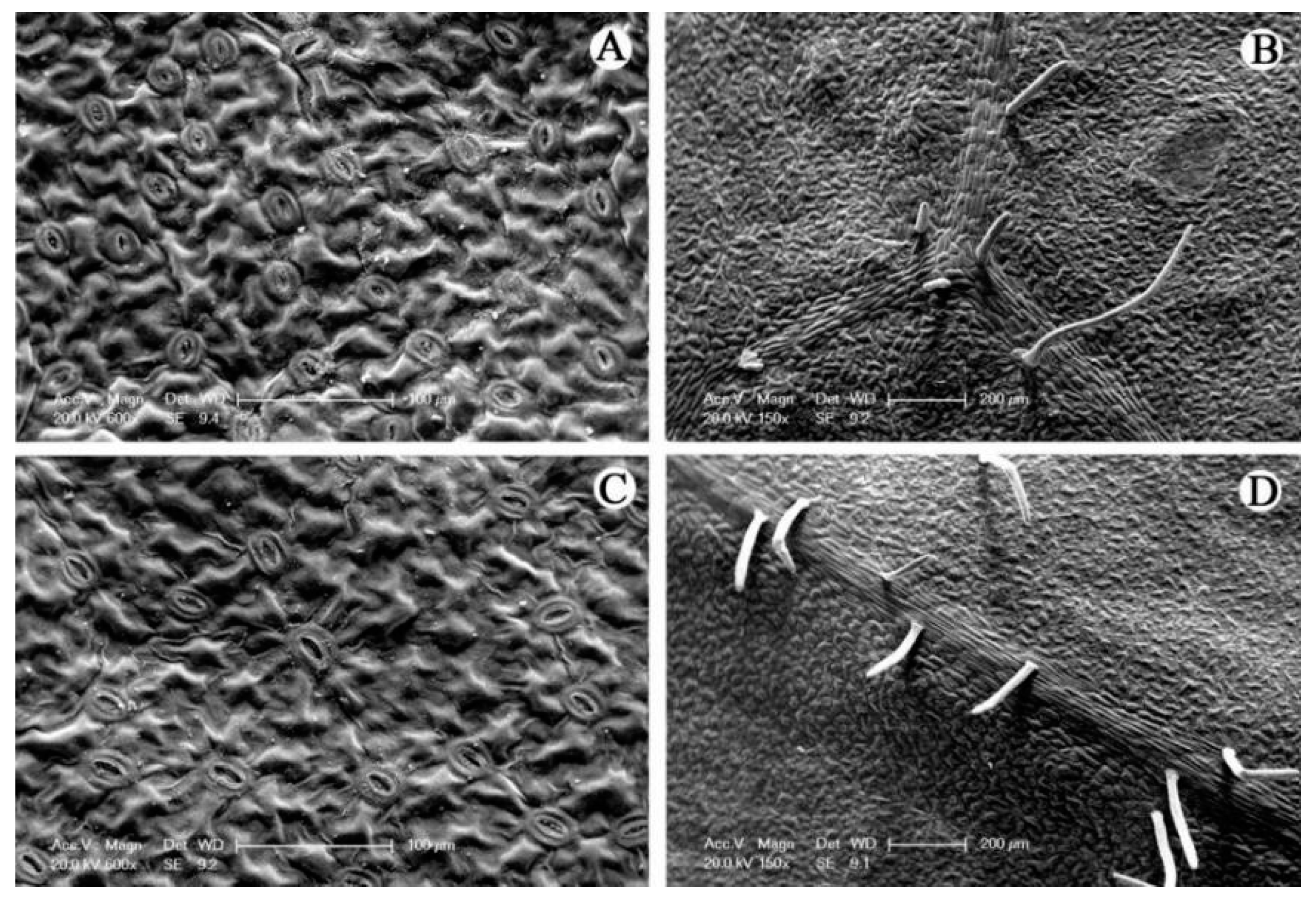
| PH z (cm) | USW (mm) | MSW (mm) | LSW (mm) | |
|---|---|---|---|---|
| Control | 102.3 ± 8.51 y a | 3.41 ± 0.44 a | 6.82 ± 0.43 a | 9.16 ± 0.82 a |
| PBZ | 86.22 ± 3.43 b | 2.99 ± 0.47 b | 6.11 ± 0.49 b | 9.25 ± 0.71 a |
| Leaf Trait | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage z | Tmt y | Chla x | Chlb | Chl | SPAD | L * | a * | b * | c * | h° | |||
| S1 | Control | 6.94 ± 0.05 w e | 2.11 ± 0.07 f | 9.03 ± 0.12 h | 37.10 ± 2.51 h | 45.08 ± 1.55 a | −12.40 ± 0.82 h | 20.90 ± 1.67 a | 24.20 ± 0.94 a | 114.50 ± 1.15 g | |||
| PBZ | 8.09 ± 0.31 c | 2.43 ± 0.11 f | 10.51 ± 0.25 g | 50.30 ± 4.13 d | 39.57 ± 1.43 e | −9.20 ± 0.50 b | 13.20 ± 0.80 e | 16.90 ± 0.94 e | 123.70 ± 1.40 c | ||||
| S2 | Control | 7.99 ± 0.08 c | 2.76 ± 0.05 e | 10.74 ± 0.03 e | 40.65 ± 3.22 g | 44.65 ± 2.72 b | −10.04 ± 2.09 e | 20.52 ± 4.70 c | 22.97 ± 4.57 c | 116.66 ± 6.08 f | |||
| PBZ | 8.59 ± 0.22 b | 2.71 ± 0.04 e | 11.29 ± 0.26 d | 54.80 ± 1.73 c | 38.31 ± 1.98 f | −8.74 ± 1.42 a | 12.86 ± 2.39 g | 15.60 ± 2.43 g | 124.44 ± 4.91 b | ||||
| S3 | Control | 7.99 ± 0.23 c | 2.61 ± 0.10 d | 10.59 ± 0.07 f | 42.70 ± 2.46 f | 42.67 ± 2.82 c | −11.76 ± 1.43 g | 20.83 ± 6.07 b | 24.03 ± 5.70 b | 120.64 ± 5.99 d | |||
| PBZ | 9.81 ± 0.44 a | 3.85 ± 0.18 b | 13.65 ± 0.33 a | 55.90 ± 5.38 b | 37.58 ± 1.20 g | −9.72 ± 0.70 d | 12.95 ± 2.48 f | 16.25 ± 2.23 f | 127.41 ± 4.71 a | ||||
| S4 | Control | 7.46 ± 0.07 d | 4.30 ± 0.20 a | 11.74 ± 0.17 c | 45.50 ± 3.19 e | 42.48± 2.52 d | −10.34 ± 1.41 f | 19.26 ± 5.50 d | 22.01 ± 5.14 d | 119.29 ± 6.01 e | |||
| PBZ | 8.53 ± 0.19 b | 3.52 ± 0.13 c | 12.04 ± 0.32 b | 58.40 ± 3.66 a | 37.09 ± 1.69 h | −9.29 ± 0.96 c | 12.14 ± 1.64 h | 15.32 ± 1.69 h | 127.57 ± 3.16 a | ||||
| Leaf Trait z | Chla | Chlb | Chl | SPAD | L * | a * | b * | c * | h° |
|---|---|---|---|---|---|---|---|---|---|
| Chlb | 1 | 0.815 * y | 0.459 | −0.387 | 0.327 | −0.246 | −0.279 | 0.423 | |
| Chl | 1 | 0.757* | −0.714 * | 0.575 | −0.591 | −0.615 | 0.770 * | ||
| SPAD | 1 | −0.989 ** | 0.790 * | −0.949 ** | −0.959 ** | 0.971 ** |
| Stage z | Tmt y | Chlorophyll Fluorescence Parameter | ||||
|---|---|---|---|---|---|---|
| Fv/Fm | qP | qN | ΦPSII | NPQ | ||
| S1 | CK | 0.770 ± 0.009 w b,c | 0.883 ± 0.054 c,d | 0.094 ± 0.004 c,d | 0.673 ± 0.048 c,d | 0.084 ± 0.004 c,d |
| PBZ | 0.790 ± 0.008 a,b | 0.906 ± 0.008 b,c | 0.085 ± 0.013 d,e | 0.705 ± 0.011 a,b,c | 0.077 ± 0.011 d,e | |
| S2 | CK | 0.759 ± 0.037 c | 0.873 ± 0.047 d | 0.116 ± 0.008 a | 0.666 ± 0.056 d | 0.107 ± 0.008 a |
| PBZ | 0.793 ± 0.003 a,b | 0.915 ± 0.008 a,b | 0.106 ± 0.010 b-d | 0.714 ± 0.008 a,b | 0.090 ± 0.022 b−d | |
| S3 | CK | 0.783 ± 0.009 a-c | 0.906 ± 0.023 b,c | 0.095 ± 0.012 c,d | 0.696 ± 0.035 b−d | 0.086 ± 0.011 c,d |
| PBZ | 0.805 ± 0.004 a | 0.934 ± 0.006 a,b | 0.075 ± 0.013 e | 0.735 ± 0.007 a | 0.067 ± 0.012 e | |
| S4 | CK | 0.791 ± 0.011 a,b | 0.925 ± 0.010 a,b | 0.112 ± 0.013 a,b | 0.719 ± 0.024 a,b | 0.102 ± 0.011 a,b |
| PBZ | 0.806 ± 0.006 a | 0.941 ± 0.003 a | 0.106 ± 0.007 a-c | 0.738 ± 0.006 a | 0.099 ± 0.008 a-c | |
| Trait z | Fv/Fm | qP | qN | ΦPSII | NPQ | Chl |
|---|---|---|---|---|---|---|
| Fv/Fm | 1 | |||||
| qP | 0.972 **,y | 1 | ||||
| qN | −0.386 | −0.225 | 1 | |||
| ΦPSII | 0.985 ** | 0.991 ** | −0.271 | 1 | ||
| NPQ | −0.387 | −0.209 | 0.980 ** | −0.263 | 1 | |
| Chl | 0.739 * | 0.783 * | −0.227 | 0.811 * | −0.201 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Tang, Y.; Wei, M.; Zhao, D. Effect of Paclobutrazol Application on Plant Photosynthetic Performance and Leaf Greenness of Herbaceous Peony. Horticulturae 2018, 4, 5. https://doi.org/10.3390/horticulturae4010005
Xia X, Tang Y, Wei M, Zhao D. Effect of Paclobutrazol Application on Plant Photosynthetic Performance and Leaf Greenness of Herbaceous Peony. Horticulturae. 2018; 4(1):5. https://doi.org/10.3390/horticulturae4010005
Chicago/Turabian StyleXia, Xing, Yuhan Tang, Mengran Wei, and Daqiu Zhao. 2018. "Effect of Paclobutrazol Application on Plant Photosynthetic Performance and Leaf Greenness of Herbaceous Peony" Horticulturae 4, no. 1: 5. https://doi.org/10.3390/horticulturae4010005
APA StyleXia, X., Tang, Y., Wei, M., & Zhao, D. (2018). Effect of Paclobutrazol Application on Plant Photosynthetic Performance and Leaf Greenness of Herbaceous Peony. Horticulturae, 4(1), 5. https://doi.org/10.3390/horticulturae4010005





