LEDs Combined with CHO Sources and CCC Priming PLB Regeneration of Phalaenopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. CHO Sources and LED Lights
2.3. CCC Concentrations
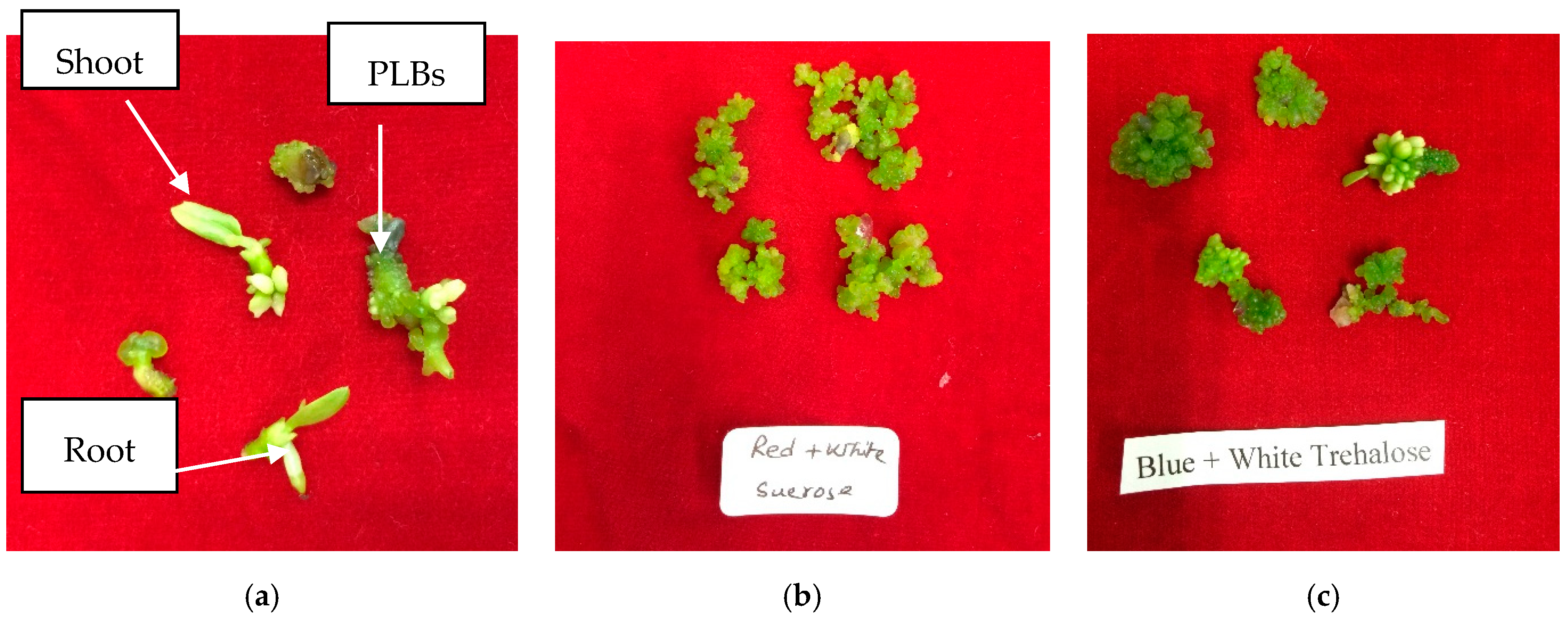
2.4. PLB Culture, Data Collection, and Data Analysis
- ■
- Average number = Number of cultured explants with new PLBs/Total number of cultured explants
- ■
- Percentage of PLB (%) = (Number of cultured explants with new PLBs/Total number of cultured explants) × 100
3. Results
3.1. CHO Sources and LED Lights
3.2. CCC Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arditti, J.; Ernst, R. Micropropagation of Orchids; Wiley: New York, NY, USA, 1993; pp. 1–682. [Google Scholar]
- Sheelavanthmath, S.S.; Murthy, H.N.; Hema, B.P.; Hahn, E.J.; Paek, K.Y. High frequency of protocorm like bodies (PLBs) induction and plant regeneration from protocorm and leaf sections of Aerides Crispum. Sci. Hortic. 2005, 106, 395–401. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, S. Micropropagation of Phalaenopsis through the culture of lateral buds from young flower stalks. Lindleyana 1992, 7, 208–215. [Google Scholar]
- Tanaka, M.; Senda, Y.; Hasegawa, A. Plantlet formation in root-tip culture in Phalaenopsis. Am. Orchid Soc. Bull. 1976, 45, 1022–1024. [Google Scholar]
- Park, S.Y.; Murthy, H.N.; Paek, K.Y. Rapid propagation of Phalaenopsis from floral stalk-derived leaves. In Vitro Cell. Dev. Biol. Plant 2002, 38, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Tokuhara, K.; Mii, M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds in Phalaenopsis (Orchidaceae). In Vitro Cell. Dev. Biol. Plant 2001, 37, 457–461. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. In Vitro Cell. Dev. Biol. Plant 2003, 39, 635–639. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, C.; Chang, W.C. A reliable protocol for plant regeneration from callus culture of Phalaenopsis. In Vitro Cell. Dev. Biol. Plant 2000, 36, 420–423. [Google Scholar] [CrossRef]
- Park, S.Y.; Yeung, E.C.; Chakrabarty, D.C.; Paek, K.Y. An efficient direct induction of protocorm-like bodies from leaf sub epidermal cells of Doritaenopsis hybrid using thin-section culture. Plant Cell Rep. 2002, 21, 46–51. [Google Scholar] [CrossRef]
- Bustam, S.; Sinniah, U.R.; Swamy, M.K. Simple and efficient in vitro method of storing Dendrobium sw Shavin White protocorm like bodies (PLBs). Bangladesh J. Bot. 2017, 46, 439–449. [Google Scholar]
- Murdad, R.; Latip, M.A.; Aziz, Z.A.; Ripin, R. Effects of carbon source and potato homogenate on in vitro growth and development of Sabah’s endangered orchid: Phalaenopsis gigantea. AsPac J. Mol. Biol. Biotechnol. 2010, 18, 199–202. [Google Scholar]
- Al-Khateeb, A.A. Regulation of in vitro bud formation of date palm (Phoenix dactylifera L.) cv. Khanezi by different carbon sources. Bioresour. Technol. 2008, 99, 6550–6555. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.A.; Lin, J.J.; Wu, R.Y. The effects of using trehalose as a carbon source on the proliferation of Phalaenopsis and Doritaenopsis protocorm-like-bodies. Plant Cell Tissue Org. Cult. 2006, 86, 125–129. [Google Scholar] [CrossRef]
- Akter, S.; Nasiruddin, K.M.; Khaldun, A.B.M. Organogenesis of Dendrobium orchid using traditional media and organic extracts. J. Agric. Rural Dev. 2007, 5, 30–35. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 1st ed.; Benjamin-Cumings Publishing Co.: New York, NY, USA, 1991. [Google Scholar]
- Agarwal, A.; Gupta, S.D. Impact of light-emitting diodes (LEDs) and its potential on plant growth and development in controlled environment plant production system. Curr. Biotechnol. 2016, 5, 28–43. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Pérez-Sato, J.A.; Cruz-Cruz, C.A.; Martínez-Estrada, E. Light-emitting diodes: Progress in plant micropropagation. In Chlorophyll; Jacob-Lopes, E., Ed.; InTech: London, UK, 2017; pp. 747–1216. [Google Scholar]
- Shukla, M.R.; Singh, A.S.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods 2017, 13, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Both, A.J.; Bourget, C.M.; Burr, J.F.; Kubota, C.; Lopez, R.G.; Morrow, R.C.; Runkle, E.S. LEDs: The future of greenhouse lighting. Chron. Hortic. 2012, 52, 6–10. [Google Scholar]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Matioc-Precup, M.M.; Cachiţă-Cosma, D. The germination and growth of Brassica oleracea L. var. capitata f. rubra plantlets under the influence of colored light of different provenance. Studia Univ. Vasile Goldis Arad Ser. Stiintele Vietii 2012, 22, 193–202. [Google Scholar]
- Saebo, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Org. Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Senger, H. The effect of blue light on plants and microorganisms. Phytochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, A.S. Green light: A signal to slow down or stop. J. Expt. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.J.; Haque, S.M.; Shimasaki, K. Organogenesis of Cymbidium finlaysonianum under different sources of lights. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 2095–2101. [Google Scholar]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Alam, M.M. Effects of different light quality on growth and development of protocorm-like bodies (PLBs) in Dendrobium kingianum cultured in vitro. Bangladesh Res. Public J. 2014, 10, 223–227. [Google Scholar]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Alam, M.M. Effect of 6-Benzylaminopurine (BA) and Hyaluronic Acid (HA) under white light emitting diode (LED) on organogenesis in protocorm-like bodies (PLBs) of Dendrobium kingianum. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 605–609. [Google Scholar]
- Kamal, M.M.; Shimasaki, K.; Akhter, N. Effect of light emitting diode (LED) and N-Acetyleglucoseamine (NAG) on organogenesis in protocorm-like-bodies (PLBs) of Cymbidium hybrid cultured In Vitro. Plant Tissue Cult. Biotech. 2014, 24, 273–277. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. The response of protocorm-like bodies of nine hybrid Cymbidium cultivars to light-emitting diodes. Environ. Exp. Biol. 2014, 12, 155–159. [Google Scholar]
- Wang, Z.; Li, G.; He, S.; Teixeira da Silva, J.A.; Tanaka, M. Effect of cold cathode fluorescent lamps on growth of Gerbera jamesonii plantlets in vitro. Sci. Hortic. 2011, 130, 482–484. [Google Scholar] [CrossRef]
- Sultana, K.S.; Mustafa, K.H.; Mehedi, K.H.; Sultana, S.; Mehraj, H.; Shimasaki, K.; Habiba, S.U. Effect of Hyaluronic Acid (HA) on organogenesis in protocorm-like-bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1721–1724. [Google Scholar]
- Sultana, K.S.; Mustafa, K.H.; Mehedi, K.H.; Sultana, S.; Mehraj, H.; Shimasaki, K.; Habiba, S.U. Effect of two elicitors on organogenesis in protocorm-like bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. World Appl. Sci. J. 2015, 33, 1528–1532. [Google Scholar]
- Silva Batista, D.; Felipe, S.; Heitor, S.; Dulcineia Silva, T.; Motta de Castro, K.; Mamedes-Rodrigues, T.C.; Amaral Miranda, N.; Ríos-Ríos, A.M.; Vidal Faria, D.; Alexandre Fortini, E.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Ruter, J.M. Growth and landscape establishment of Pyracantha and Juniperus after application of paclobutrazol. HortScience 1994, 29, 1318–1320. [Google Scholar] [CrossRef]
- Kozak, D.; Grodek, J. The consequent effect of growth retardants on the growth and development of Tibouchina urvilleana Cogn. shoots in vitro. Acta Sci. Pol. Hortorum Cultus 2005, 4, 123–128. [Google Scholar]
- Sharma, N.; Kaur, N.; Gupta, A.K. Effects of gibberellic acid and chlorocholine chloride on tuberization and growth of potato (Solanumtuberosum L.). J. Sci. Food Agric. 1998, 78, 466–470. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, N.; Gupta, A.K. Effect of chlorocholine chloride sprays on the carbohydrate composition and activities of sucrose metabolising enzymes in potato (Solanum tuberosum L.). Plant Growth Regul. 1998, 26, 97–103. [Google Scholar] [CrossRef]
- Berova, M.; Zlatev, Z. Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.). Plant Growth Regul. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Liu, F.; Xiao, L. Chlorocholine chloride application effects on photosynthetic capacity and photoassimilates partitioning in potato (Solanum tuberosum L.). Sci. Hortic. 2009, 119, 113–116. [Google Scholar] [CrossRef]
- Wang, H.Q.; Xiao, L.T. Effects of chlorocholine chloride on phytohormones and photosynthetic characteristics in potato (Solanum tuberosum L.). J. Plant Growth Regul. 2009, 28, 21–27. [Google Scholar] [CrossRef]
- Mares, D.J.; Marschner, H.; Krauss, A. Effect of gibberellic acid on growth and carbohydrate metabolism of developing tubers of potato (Solanum tuberosum L.). Physiol. Plant. 1981, 52, 267–274. [Google Scholar] [CrossRef]
- Shimasaki, K.; Uemoto, S. Micropropagation of a terrestrial Cymbidium species using rhizomes developed from seeds and pseudobulbs. Plant Cell Tissue Org. Cult. 1990, 22, 237–244. [Google Scholar] [CrossRef]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.E.; Coskun, Y. In vitro culture response of Phalaenopsis orchid by different carbon sources. J. Biotechnol. 2015, 208, S107. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Morales-Ramos, V. Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr. J. Biotechnol. 2016, 15, 272–277. [Google Scholar] [CrossRef]
- Heo, J.W.; Shin, K.S.; Kim, S.K.; Paek, K.Y. Light quality affects in vitro growth of grape ‘Teleki 5BB7’. J. Plant Biol. 2006, 49, 276–280. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Org. Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Dueck, T.A.; Trouwborst, G.; Hogewoning, S.; Meinen, E. Can a high red: Far red ratio replace temperature induced inflorescence development in Phalaenopsis? Environ. Exp. Bot. 2016, 121, 139–144. [Google Scholar] [CrossRef]
- Tanaka, M.; Watanabe, T.; Giang, D.T.; Tanaka, M.; Takamura, T.; Watanabe, H. Morphogenesis in the PLB segments of Phalaenopsis cultured under LED irradiation system (Abstract only). J. Jpn. Soc. Hortic. Sci. 2001, 70 (Suppl. 1), 306. [Google Scholar]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Org. Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Topchiy, N.M.; Sytnik, S.K.; Syvash, O.O.; Zolotareva, O.K. The effect of additional red irradiation on the photosynthetic apparatus of Pisumsativum. Photosynthetica 2005, 43, 451–456. [Google Scholar] [CrossRef]
- Li, H.M.; Tang, C.M.; Xu, Z.G.; Liu, X.Y.; Han, X.L. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262–273. [Google Scholar] [CrossRef]
- Li, H.M.; Xu, Z.G.; Tang, C.M. Effect of light emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Org. Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Kurilcik, A.; Miklusyte-Canova, R.; Dapkuniene, S.; Zilinskaite, S.; Kurilcik, G.; Tamulaitis, G.; Duchovskis, P.; Zukauskas, A. In vitro culture of chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Li, H.M.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Anuchai, J.; Hsieh, C.H. Effect of change in light quality on physiological transformation of in vitro Phalaenopsis ‘Fortune Saltzman’ seedlings during the growth period. Hortic. J. 2017, 86, 395–402. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A. Influence of LED lighting on in vitro plant regeneration and associated cellular redox balance. In Light Emitting Diodes for Agriculture; Dutta Gupta, S., Ed.; Springer: Singapore, 2017. [Google Scholar]
- Zheng, R.; Wu, Y.; Xia, Y. Chlorocholine chloride and paclobutrazol treatments promote carbohydrate accumulation in bulbs of Lilium Oriental hybrids ‘Sorbonne’. J. Zhejiang Univ. Sci. B 2012, 13, 136–144. [Google Scholar] [CrossRef]
- Tezuka, T.; Takahara, C.; Yamamoto, Y. Aspects regarding the action of CCC in hollyhock plants. J. Exp. Bot. 1989, 40, 689–692. [Google Scholar] [CrossRef]
- Grossmann, K. Plant growth retardants as tools in physiological research. Physiol. Plant. 1990, 78, 640–648. [Google Scholar] [CrossRef]
- Hussain, I.; Chaudhry, Z.; Muhammad, A.; Aasghar, R.; Naqvi, S.M.S.; Rashid, H. Effect of chlorocholine chloride, sucrose and BAP on in vitro tuberization in potato (Solanum tuberosum L. cv. Cardinal). Pak. J. Bot. 2006, 38, 275–282. [Google Scholar]
- Ray, A.; Bhattacharya, S. An improved micropropagation of Ecliptaalba by in vitro priming with chlorocholine chloride. Plant Cell Tissue Org. Cult. 2007, 92, 315–319. [Google Scholar] [CrossRef]
- Zakaria, M.; Hossain, M.M.; KhalequeMian, M.A.; Hossain, T.; Uddin, M.Z. In vitro tuberization of potato influenced by benzyl adenine and chloro choline chloride. Bangladesh J. Agric. Res. 2008, 33, 419–425. [Google Scholar] [CrossRef]
- Coleman, W.K.; Donnelly, D.J.; Coleman, S.E. Potato microtubers as research tools: A review. Am. J. Potato Res. 2001, 78, 47–55. [Google Scholar] [CrossRef]
- El-Sawy, A.; Bekheet, S.; Aly, U.I. Morphological and molecular characterization of potato microtubers production on coumarin inducing medium. J. Agric. Biol. 2007, 9, 675–680. [Google Scholar]
- Gopal, J.; Chamail, A.; Sarkar, D. In vitro production of microtubers for conservation of potato germplasm: Effect of genotype, abscisic acid, and sucrose. In Vitro Cell. Dev. Biol. Plant 2004, 40, 485–490. [Google Scholar] [CrossRef]
- Fletcher, A.; Gilley, A.; Sankhla, N.; Davies, T. Triazoles as plant growth regulators and stress protectants. Hortic. Rev. 1999, 24, 55–138. [Google Scholar] [CrossRef]
- Mansuroglu, S.; Karaguzel, O.; Ortacesme, V.; Sayan, M. Effect of paclobutrazol on flowering, leaf and flower colour of Consolida orientalis. Pak. J. Bot. 2009, 41, 2323–2332. [Google Scholar]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chikara, J.; Chaudhary, D.; Prakash, A.; Boricha, G.; Zala, A. Paclobutrazol arrests vegetative growth and unveils unexpressed yield potential of Jatropha curcas. J. Plant Growth Regul. 2010, 29, 307–315. [Google Scholar] [CrossRef]
- Tsegaw, T.; Hammes, P. Growth responses of potato (Solanum tuberosum) grown in a hot tropical lowland to applied paclobutrazol: 2. Tuber attributes. New Z. J. Crop Hortic. Sci. 2005, 33, 43–51. [Google Scholar] [CrossRef]




| Light z | Mean Number of PLBs | Fresh Weight (g) | ||||
|---|---|---|---|---|---|---|
| Sucrose | Trehalose | Maltose | Sucrose | Trehalose | Maltose | |
| Control | 37.73 ± 4.40 y ab | 28.13 ± 4.87 abcd | 1.60 ± 0.53 b | 0.098 ± 0.057 a | 0.059 ± 0.033 ab | 0.043 ± 0.023 ab |
| R | 26.20 ± 3.38 bc | 15.87 ± 2.89 bcde | 2.73 ± 0.97 b | 0.062 ± 0.042 a | 0.059 ± 0.032 ab | 0.033 ± 0.019 b |
| G | 21.93 ± 3.76 bcd | 8.87 ± 1.55 de | 6.00 ± 1.35 b | 0.066 ± 0.045 a | 0.041 ± 0.022 ab | 0.033 ± 0.018 b |
| B | 20.47 ± 3.92 bcd | 27.67 ± 3.21 abcd | 2.27 ± 0.76 b | 0.043 ± 0.024 a | 0.137 ± 0.074 ab | 0.017 ± 0.009 b |
| W | 24.80 ± 4.49 bcd | 22.80 ± 2.92 abcde | 3.60 ± 0.67 b | 0.071 ± 0.039 a | 0.079 ± 0.042 ab | 0.028 ± 0.017 b |
| RG | 11.07 ± 3.97 cd | 19.33 ± 3.44 abcde | 4.47 ± 1.04 b | 0.029 ± 0.022 a | 0.063 ± 0.034 ab | 0.043 ± 0.023 ab |
| RB | 11.60 ± 3.11 cd | 15.33 ± 5.55 bcde | 1.47 ± 0.45 b | 0.026 ± 0.020 a | 0.091 ± 0.065 ab | 0.024 ± 0.013 b |
| RW | 54.13 ± 8.85 a | 12.13 ± 2.82 cde | 14.47 ± 3.39 a | 0.109 ± 0.063 a | 0.027 ± 0.020 b | 0.066 ± 0.041 ab |
| GB | 19.93 ± 4.83 bcd | 22.73 ± 3.67 abcde | 2.27 ± 1.03 b | 0.061 ± 0.035 a | 0.117 ± 0.062 ab | 0.020 ± 0.011 b |
| GW | 26.40 ± 3.60 bc | 8.73 ± 2.19 de | 4.80 ± 1.11 b | 0.080 ± 00.047 a | 0.047 ± 0.026 ab | 0.051 ± 0.027 ab |
| BW | 10.67 ± 4.11 cd | 36.33 ± 5.08 a | 3.80 ± 1.76 b | 0.020 ± 0.020 a | 0.129 ± 0.071 ab | 0.045 ± 0.024 ab |
| RGB | 5.47 ± 1.98 d | 5.00 ± 1.70 e | 8.40 ± 1.85 a | 0.015 ± 0.015 a | 0.066 ± 0.048 ab | 0.079 ± 0.047 ab |
| RBW | 13.53 ± 2.88 cd | 35.13 ± 4.36 ab | 13.73 ± 2.09 a | 0.034 ± 0.024 a | 0.167 ± 0.098 a | 0.112 ± 0.068 a |
| RGW | 19.07 ± 2.60 bcd | 29.40 ± 4.45 abc | 1.47 ± 0.35 b | 0.030 ± 0.021 a | 0.090 ± 0.048 ab | 0.022 ± 0.013 b |
| GBW | 12.20 ± 2.79 cd | 32.00 ± 7.77 ab | 4.73 ± 1.27 b | 0.056 ± 0.038 a | 0.088 ± 0.063 ab | 0.030 ± 0.017 b |
| Light z | Number of Shoots | Mean Shoot Length | ||||
|---|---|---|---|---|---|---|
| Sucrose | Trehalose | Maltose | Sucrose | Trehalose | Maltose | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| R | 0 | 0.38 ± 0.14 | 0 | 0 | 0.26 ± 0.10 | 0 |
| G | 0 | 0.38 ± 0.14 | 0 | 0 | 0.04 ± 0.02 | 0 |
| B | 0 | 0.75 ± 0.29 | 0 | 0 | 0.11 ± 0.05 | 0 |
| W | 0.13 ± 0.07 | 0.25 ± 0.09 | 0 | 0.05 ± 0.05 | 0.08 ± 0.03 | 0 |
| RG | 0 | 0.13 ± 0.07 | 0.13 ± 0.13 | 0 | 0.08 ± 0.04 | 0.03 ± 0.03 |
| RB | 0 | 0.25 ± 0.13 | 0 | 0 | 0.05 ± 0.03 | 0 |
| RW | 0 | 0.13 ± 0.07 | 0 | 0 | 0.03 ± 0.01 | 0 |
| GB | 0 | 0.13 ± 0.07 | 0 | 0 | 0.05 ± 0.03 | 0 |
| GW | 0 | 0 | 0 | 0 | 0 | 0 |
| BW | 0 | 0.38 ± 0.14 | 0 | 0 | 0.12 ± 0.04 | 0 |
| RGB | 0 | 0 | 0 | 0 | 0 | 0 |
| RBW | 0 | 0 | 0 | 0 | 0 | 0 |
| RGW | 0.25 ± 0.13 | 0 | 0 | 0.03 ± 0.03 | 0 | 0 |
| GBW | 0 | 0 | 0 | 0 | 0 | 0 |
| CCC (mgL−1) | Number of PLBs | PLB Formation (%) | Fresh Weight (g) |
|---|---|---|---|
| 0 | 12.53 ± 1.71 z a | 93.33 | 0.175 ± 0.028 a |
| 0.01 | 15.67 ± 1.01 a | 100.00 | 0.211 ± 0.018 a |
| 0.1 | 13.73 ± 1.62 a | 93.33 | 0.191 ± 0.022 a |
| 1 | 11.07 ± 2.08 ab | 80.00 | 0.182 ± 0.027 a |
| 10 | 4.40 ± 1.74 b | 33.33 | 0.049 ± 0.019 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs Combined with CHO Sources and CCC Priming PLB Regeneration of Phalaenopsis. Horticulturae 2019, 5, 34. https://doi.org/10.3390/horticulturae5020034
Mehraj H, Alam MM, Habiba SU, Mehbub H. LEDs Combined with CHO Sources and CCC Priming PLB Regeneration of Phalaenopsis. Horticulturae. 2019; 5(2):34. https://doi.org/10.3390/horticulturae5020034
Chicago/Turabian StyleMehraj, Hasan, Md. Meskatul Alam, Sultana Umma Habiba, and Hasan Mehbub. 2019. "LEDs Combined with CHO Sources and CCC Priming PLB Regeneration of Phalaenopsis" Horticulturae 5, no. 2: 34. https://doi.org/10.3390/horticulturae5020034






