In Vitro Propagation of Aconitum violaceum Jacq. ex Stapf through Seed Culture and Somatic Embryogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Chemicals
2.3. Explant Selection, Culture Conditions, and Establishment of In Vitro Cultures
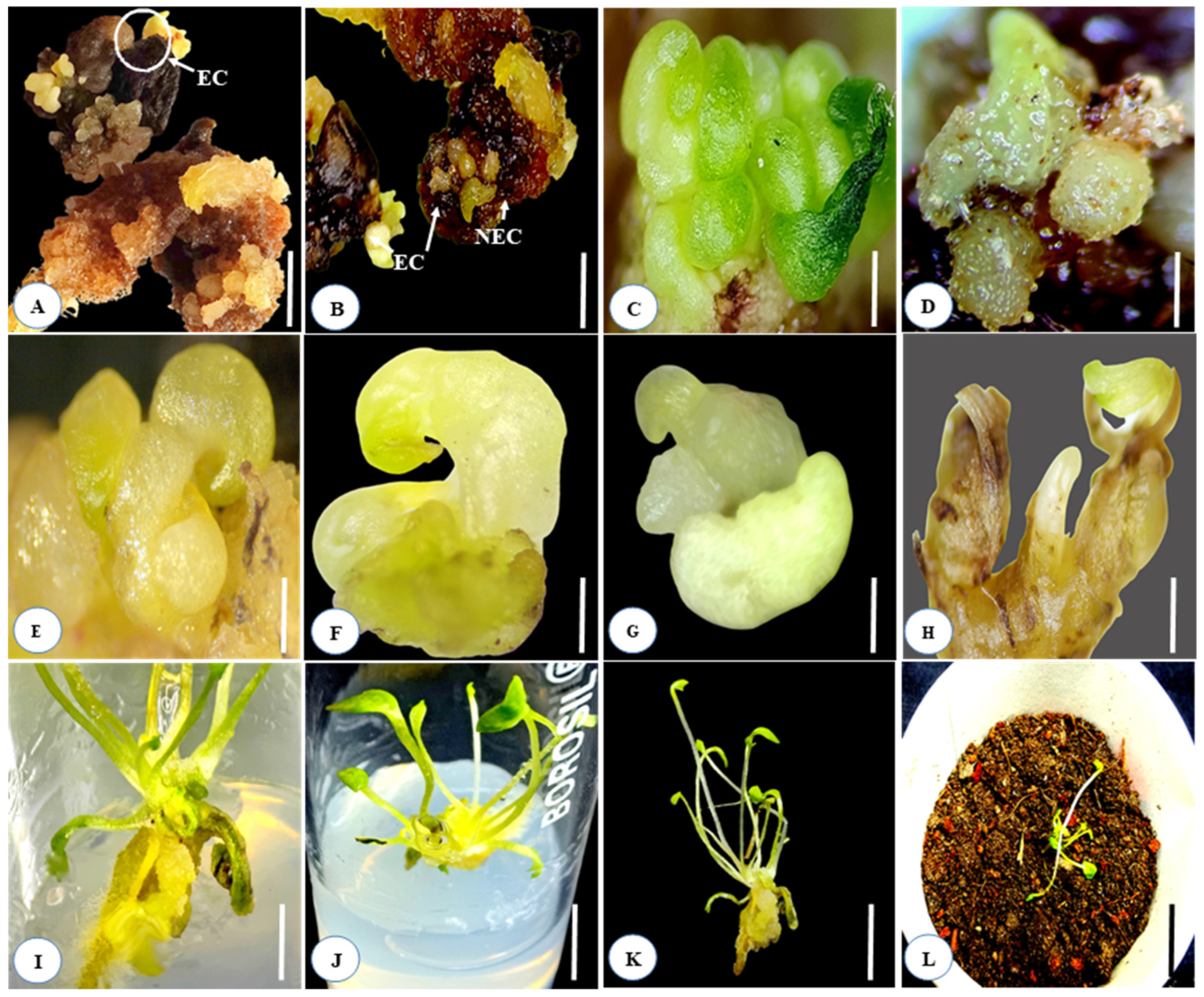
2.4. Embryogenic and Non-Embryogenic Callus Production
2.5. Effect of Light Requirement on Percentage of Somatic Embryo Formation
2.6. Whole Plant Regeneration from Somatic Embryos
2.7. In Vitro Rooting
2.8. Hardening and Acclimatization of In Vitro Plantlets
2.9. Experimental Design and Statistical Analysis
3. Results
3.1. Seed Germination
3.2. Effects of Kn, IAA, and 2, 4-D on Embryogenic and Non-Embryogenic Callus Formation
3.3. Effect of Light on Percentage of Somatic Embryo Formation
3.4. Complete Plant Development from Somatic Embryos
3.5. In Vitro Rooting
3.6. Hardening and Acclimatization of In Vitro Plantlets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ved, D.; Saha, D.; Ravikumar, K.; Haridasan, K. Aconitum violaceum . In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2015; p. e.T50126562A79581679. [Google Scholar] [CrossRef]
- Kala, C.P. Status and conservation of rare and endangered medicinal plants in the Indian Trans-Himalaya. Biol. Conserv. 2000, 93, 371–379. [Google Scholar] [CrossRef]
- Chaudhary, L.B.; Rao, R.R. Notes on the genus Aconitum L. (Ranunculaceae) in North West Himalaya (India). Feddes Repert. 1998, 109, 527–537. [Google Scholar] [CrossRef]
- Hadi, A.; Singh, S.; Nawchoo, I.A.; Rafiq, S.; Ali, S. Impacts of Habitat Variability on the Phenotypic Traits of Aconitum Violaceum Jacq. Ex Stapf. At different altitudes and environmental conditions in the Ladakh Himalaya, India. Plant Sci. Today 2022, 9. [Google Scholar] [CrossRef]
- CAMP. Threat assessment and management priorities of selected medicinal plants of Western Himalayan states, India. In Proceedings of the Conservation Assessment of Medicinal Plants Workshop, Shimla, Banglore, India, 22–26 May 2003. [Google Scholar]
- Anonymous. The wealth of India. Dictionary of Indian raw material and industrial products. In Raw Materials; National Institute of Science Communication and Information resources (NISCAIR): New Delhi, India, 1998; Volume I CSIR, p. 253. [Google Scholar]
- Sabir, S.; Arshad, M.; Hussain, M.; Sadaf, H.M.; Sohail; Imran, M.; Yasmeen, F.; Saboon; Chaudhari, S.K. A probe into biochemical potential of Aconitum violaceum: A medicinal plant from Himalaya. Asian Pac. J. Trop. Dis. 2016, 6, 502–504. [Google Scholar] [CrossRef]
- Hadi, A.; Singh, S.; Ali, S.; Mehdi, M. Traditional uses of medicinal plants by indigenous tribes of Ladakh union territory. Res. J. Agril. Sci. 2022, 13, 68–77. [Google Scholar]
- Pandey, M.R. Use of medicinal plants in traditional Tibetan therapy system in upper Mustang, Nepal. Our Nat. 2006, 4, 69–82. [Google Scholar] [CrossRef]
- Ameri, A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998, 56, 211–235. [Google Scholar] [CrossRef]
- Bhattarai, S.; Chaudhary, R.P.; Quave, C.L.; Taylor, R.S.L. The use of medicinal plants in the trans-Himalayan arid zone of Mustang district, Nepal. J. Ethnobiol. Ethnomed. 2010, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Lone, P.A.; Bhardwaj, A.K.; Shah, K.W.; Tabasum, S. Ethnobotanical survey of some threatened medicinal plants of Kashmir Himalaya, India. J. Med. Plant Res. 2014, 8, 1362–1373. [Google Scholar]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, S.; Khan, N.M.; Khan, H.; Khan, S. Antimicrobial and antioxidant role of the aerial parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021, 65, 84–93. [Google Scholar] [CrossRef]
- Yadav, S.; Verma, D.L. Acylated flavonol glycosides from the flowers of Aconitum violaceum Staph. Nat. Sci. 2010, 8, 239–243. [Google Scholar]
- Miana, G.A.; Ikram, M.; Khan, M.I.; Sultana, F. Alkaloids of Aconitum violaceum. Phytochemistry 1971, 10, 3320–3322. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Shrestha, K.P.; Bussmann, R.W. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J. Ethnobiol. Ethnomed. 2010, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprety, Y.; Asselin, H.; Boon, E.K.; Yadav, U.; Shrestha, K.K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed. 2010, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Dall’Acqua, S.; Shrestha, B.B.; Gewali, M.B.; Jha, P.K.; Carrara, M.; Innocenti, G. Diterpenoid alkaloids and phenol glycosides from Aconitum naviculare (Bruhl) Stapf. Nat. Prod. Commun. 2008, 3, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.M.; Yan, L.Y.; He, H.Y.; Wei, X.M. Norditerpenoid alkaloids from Aconitum spicatum Stapf. J. Integr. Plant Biol. 2006, 48, 364–369. [Google Scholar] [CrossRef]
- Mir, A.H.; Tyub, S.; Kamili, A.N. Ecology, distribution mapping and conservation implications of four critically endangered endemic plants of Kashmir Himalaya. Saudi J. Biol. Sci. 2020, 27, 2380–2389. [Google Scholar] [CrossRef]
- Rafiq, S.; Wagay, N.A.; Bhat, I.A.; Kaloo, Z.A.; Rashid, S.; Lin, F.; El-Abedin, T.K.Z.; Wani, S.H.; Mahmoud, E.A.; Almutairi, K.F.; et al. In vitro Propagation of Aconitum chasmanthum Stapf Ex Holmes: An Endemic and Critically Endangered Plant Species of the Western Himalaya. Horticulturae 2021, 7, 586. [Google Scholar] [CrossRef]
- Singh, M.; Chettri, A.; Pandey, A.; Sinha, S. In vitro propagation and phytochemical assessment of Aconitum ferox Wall: A threatened medicinal plant of Sikkim Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 313–321. [Google Scholar] [CrossRef]
- Giri, A.; Ahuja, P.S.; Ajay Kumar, P.V. Somatic embryogenesis and plant regeneration from callus cultures of Aconitum heterophyllum Wall. Plant Cell Tissue Organ Cult. 1993, 32, 213–218. [Google Scholar] [CrossRef]
- Deb, C.R.; Langhu, T. Development of in vitro propagation protocol of Aconitum nagarum Stapf. Plant Cell Biotechnol. Mol. Biol. 2017, 18, 324–332. [Google Scholar]
- Mou, Z.; Ye, F.; Shen, F.; Zhao, D. In vitro Germination and Micropropagation of Aconitum vilmorinianum: An Important Medicinal Plant in China. Python-Int. J. Exp. Bot. 2022, 1–18. [Google Scholar] [CrossRef]
- Hatano, K.; Shoyama, Y.; Nishioka, I. Somatic embryogenesis and plant regeneration from the anther of Aconitum carmichaeli Debx. Plant Cell Rep. 1987, 6, 446–448. [Google Scholar] [CrossRef]
- Xu, K.; Wang, W.; Yu, D.; Li, X.-L.; Chetn, J.M.; Feng, B.J.; Zhao, Y.W.; Cheng, M.J.; Liu, X.X.; Li, C.W. NAA at a high concentration promotes efficient plant regeneration via direct somatic embryogenesis and SE-mediated transformation system in Ranunculus sceleratus. Sci. Rep. 2020, 9, 18321. [Google Scholar] [CrossRef] [Green Version]
- Rawat, J.M.; Agnihotri, R.K.; Nautiyal, S.; Rawat, B.; Chandra, A. In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: A threatened medicinal herb. Acta Physiol. Plant. 2013, 35, 2589–2599. [Google Scholar] [CrossRef]
- Darrudi, R.; Hassandokht, M.R.; Nazeri, V. Effects of KNO3 and CaCl2 on seed germination of Rheum khorasanicum B. Baradaran & A. Jafari. J. Appl. Sci. Res. 2014, 10, 171–175. [Google Scholar]
- Nidhi, S.; Vikas, S.; Barkha, K.; Dobriyal, A.K.; Jadon, V.S. Advancement in research on Aconitum sp. (Ranunculaceae) under different area: A review. Biotechnology 2010, 9, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Sharma, S.; Sharma, S.S. Seed germination behaviour of some medicinal plants of Lahaul and Spiti cold desert (Himachal Pradesh): Implications for conservation and cultivation. Cur. Sci. 2006, 90, 1113–1118. [Google Scholar]
- Srivastava, N.; Sharma, V.; Kamal, B.; Jadon, V.S. Aconitum: Need for sustainable exploitation (with special reference to Uttarakhand). Int. J. Green Pharm. 2010, 4, 220–228. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Dong, K.H.; Yang, J.F. Efects of diferent treatment methods on seed germination of Iris lactea Pall. Anim. Husb. Feed. Sci. 2013, 34, 23. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Deep complex morphophysiological dormancy in seeds of the mesic woodland herb Delphinium tricorne (Ranunculaceae). Int. J. Plant Sci. 1994, 155, 738–743. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, C.C.; Baskin, J.M. Seeds of Thalictrum mirabile (Ranunculaceae) require cold stratification for loss of non- deep simple morphophysiological dormancy. Can. J. Bot. 1999, 77, 1769–1776. [Google Scholar] [CrossRef]
- Forbis, T.A.; Floyd, S.K.; DeQueiroz, A. The evolution of embryo size in angiosperms and other seed plants: Implications for the evolution of seed dormancy. Evolution 2002, 56, 2112–2125. [Google Scholar] [CrossRef]
- Paramanick, D.; Panday, R.; Shukla, S.S.; Sharma, V. Primary pharmacological and other important findings on the medicinal plant “Aconitum heterophyllum” (aruna). J. Pharmacopunct. 2017, 20, 89. [Google Scholar] [CrossRef] [Green Version]
- Maheswaran, G.; Williams, E.G. Direct somatic embryoid formation in immature embryos of Trifolium repens, T. pretense and Medicago sativa, and rapid clonal propagation of T. repens. Ann. Bot. 1984, 54, 201–211. [Google Scholar] [CrossRef]
- Joseph, R.; Yeoh, H.H.; Loh, C.S. Induced mutations in cassava using somatic embryos and the identification of mutant plants with altered starch yield and composition. Plant Cell Rep. 2004, 23, 91–98. [Google Scholar] [CrossRef]
- Chen, J.L.; Beversdorf, W.D.A. Combined use of microprojectile bombardment and DNA imbibition enhances transformation frequency of canola (Brassica napus L.). Theoret. Appl. Genet. 1994, 88, 187–192. [Google Scholar] [CrossRef]
- Vandelook, F.; Lenaerts, J.; Jozef, A.V.A. The role of temperature in post dispersal embryo growth and dormancy break in seed of Aconitum lycoctomum L. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 536–542. [Google Scholar] [CrossRef]
- Lan, T.H.; Hong, P.I.; Huang, C.C.; Chang, W.C.; Lin, C.S. High-frequency direct somatic embryogenesis from leaf tissues of Drimiopsis kirkii Baker (giant squill). Vitr. Cell. Dev. Biol. Plant 2009, 45, 44–47. [Google Scholar] [CrossRef]
- Jevremovic, S.; Jeknic, Z.; Subotic, A. Micropropagation of Iris sp. Methods Mol. Biol. 2013, 11013, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Boltenkov, E.V.; Zarembo, E.V. In vitro regeneration and callogenesis in tissue culture of foral organs of the genus Iris (Iridaceae). Biol. Bull. 2005, 32, 138. [Google Scholar] [CrossRef]
- Rhie, Y.H.; Lee, S.Y. Seed dormancy and germination of Epimedium koreanum Nakai. Sci. Hortic. 2020, 272, 109600. [Google Scholar] [CrossRef]
- Montalban, I.; Garcia-Mendiguren, O.; Goicoa, T.; Ugarte, M.; Moncalean, P. Cold storage of initial plant material afects positively somatic embryogenesis in Pinus radiata. New For. 2015, 46, 309–317. [Google Scholar] [CrossRef]
- Li, X.; Krasnyanski, S.; Korban, S.S. Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J. Plant Physiol. 2002, 159, 313–319. [Google Scholar] [CrossRef]




| MS Medium + PGRs (mg L−1) | MGT (days, Mean ± SEM) | Percentage Germination (Mean ± SEM) | Number of Shoot Buds Formed Per Seed (Mean ± SEM) | Shoot Length (cm, Mean ± SEM) | Remark | |||
|---|---|---|---|---|---|---|---|---|
| IAA | Kn | BAP | NAA | |||||
| - | - | - | - | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | No seed germination |
| 0.1 | - | - | - | 35.44 ± 0.18 de | 27.54 ± 0.18 d | 1.0 ± 0 b | 2.83 ± 0.03 cd | Healthy growth of seedlings |
| 0.5 | - | - | - | 34.11 ± 0.09 cd | 25.99 ± 0.25 d | 1.33 ± 0.33 b | 2.9 ± 0.05 c,d | Poor seed germination |
| 1.0 | - | - | - | 37.88 ± 0.12 ef | 24.44 ± 0.10 d | 1.0 ± 0 b | 2.56 ± 0.03 bc | Poor seed germination |
| 1.5 | - | - | - | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | No response |
| - | 0.1 | - | - | 31.33 ± 0.30 c | 36.22 ± 0.28 e | 1.33 ± 0.33 b | 3.8 ± 0.2 e | Healthy seed germination |
| - | 0.35 | - | - | 28.00 ± 0.38 b | 45.99 ± 0.33 f | 1.33 ± 0.33 b | 3.96 ± 0.20 ef | Healthy growth of seedlings |
| - | 0.5 | - | - | 27.22 ± 0.70 b | 77.32 ± 0.38 g | 2.0 ± 0 c | 4.3 ± 0.3 f | Healthy growth of seedlings |
| - | 1.0 | - | - | 26.88 ± 0.16 b | 75.33 ± 0.14 g | 2.0 ± 0 c | 4.7 ± 0.11 g | Healthy growth of seedlings |
| - | 1.5 | - | - | 31.88 ± 0.10 c | 6.64 ± 0.15 b | 1.0 ± 0 b | 2.26 ± 0.13 b | Poor seed germination |
| - | 2.0 | - | - | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | No response |
| - | - | 0.05 | - | 39.22 ± 0.45 f | 12.6 ± 0.13 c | 1.33 ± 033 b | 3.0 ± 0.05 d | Poor seed germination |
| - | - | 0.1 | - | 44.77 ± 0.15 g | 16.13 ± 0.35 c | 1.0 ± 0 b | 2.5 ± 0.11 b,c | Poor seed germination |
| - | - | - | 0.05 | 40.11 ± 0.44 f | 12.82 ± 0.04 c | 1.0 ± 0 b | 2.6 ± 0.05 bcd | Poor seed germination |
| MS Medium + PGRs (mg L−1) | Callus Initiation Time (Days, Mean ± SEM) | Callus Proliferation Rate | % Culture Response (Mean ± SEM) | Callus Attributes to Various Treatments | ||
|---|---|---|---|---|---|---|
| Kn | IAA | 2,4-D | ||||
| - | - | - | 0.0 ± 0 a | - | 0.0 ± 0 a | No callus formation |
| 0.1 | - | - | 46.66 ± 0.88 d | High | 35.53 ± 2.23 d | Embryogenic; friable, transparent or light greenish, proliferative |
| 0.1 | 0.3 | - | 51.66 ± 1.20 e | Moderate | 24.0 ± 2.0 b | Embryogenic; friable, creamy or light green, proliferative |
| 0.1 | 0.5 | - | 31.0 ± 0.57 b | High | 51.0 ± 1.0 e | Embryogenic; friable, healthy creamy or greenish, proliferative |
| 0.5 | 0.1 | - | 42.33 ± 1.20 cd | Low | 28.88 ± 2.93 bc | Compact, light brown |
| - | - | 0.1 | 0.0 ± 0 a | - | 0.0 ± 0.0 a | No callus formation |
| - | - | 0.5 | 41.66 ± 3.52 c | Moderate | 30.88 ± 2.44 cd | Compact, light brown |
| MS Medium + PGRs (mg L−1) | Initiation of Shoot (Days, Mean ± SEM) | Initiation of Root Days (Mean ± SEM) | % Culture Response (Mean ±SEM) | Root Length (cm) (Mean ± SEM) | Shoot Length (cm) (Mean ± SEM) | Number of Shoots (Mean ± SEM) | |
|---|---|---|---|---|---|---|---|
| Kn | IAA | ||||||
| - | - | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a | 0.0 ± 0 a |
| 0.1 | - | 26 ± 1.0 d | 34.66 ± 0.66 d | 56.00 ± 2.08 b | 4.33 ± 0.35 b | 8.16 ± 0.44 b | 5.33 ± 0.66 b |
| 0.1 | 0.3 | 21.33 ± 0.88 c | 28.33 ± 0.88 c | 56.33 ± 4.91 b | 4.16 ± 0.23 b | 9.0 ± 1.0 b | 5.66 ± 0.88 b |
| 0.1 | 0.5 | 16.66 ± 0.66 b | 25. 00 ± 1.15 b | 68.00 ± 1.52 c | 4.66 ± 0.20 b | 10.33 ± 0.6 c | 9.33 ± 1.76 c |
| Week | Plant Height (Mean ± SEM) | Number of Leaves (Mean ± SEM) | Number of Floral Buds (Mean ± SEM) |
|---|---|---|---|
| 1st | 2.33 ± 0.33 a | 2.66 ± 0.33 a | 0.0 ± 0 a |
| 2nd | 3.33 ± 0.66 a | 3.0 ± 0.57 a | 0.0 ± 0 a |
| 3rd | 6.0 ± 1.52 ab | 4.33 ± 0.33 a | 0.0 ± 0 a |
| 4th | 9.0 ± 1.15 b | 6.0 ± 0.57 b | 0.0 ± 0 a |
| 5th | 12.66 ± 0.88 c | 8.66 ± 0.33 c | 0.0 ± 0 a |
| 6th | 15.66 ± 0.88 c | 9.66 ± 0.66 c | 0.0 ± 0 a |
| 7th | 20.66 ± 1.45 d | 9.66 ± 0.66 c | 6.33 ± 0.66 b |
| 8th | 25.33 ± 1.76 e | 9.66 ± 0.66 c | 8.0 ± 0.57 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, A.; Singh, S.; Rafiq, S.; Nawchoo, I.A.; Wagay, N.A.; Mahmoud, E.A.; El-Ansary, D.O.; Sharma, H.; Casini, R.; Yessoufou, K.; et al. In Vitro Propagation of Aconitum violaceum Jacq. ex Stapf through Seed Culture and Somatic Embryogenesis. Horticulturae 2022, 8, 599. https://doi.org/10.3390/horticulturae8070599
Hadi A, Singh S, Rafiq S, Nawchoo IA, Wagay NA, Mahmoud EA, El-Ansary DO, Sharma H, Casini R, Yessoufou K, et al. In Vitro Propagation of Aconitum violaceum Jacq. ex Stapf through Seed Culture and Somatic Embryogenesis. Horticulturae. 2022; 8(7):599. https://doi.org/10.3390/horticulturae8070599
Chicago/Turabian StyleHadi, Abdul, Seema Singh, Shah Rafiq, Irshad A. Nawchoo, Nasir Aziz Wagay, Eman A. Mahmoud, Diaa O. El-Ansary, Hanoor Sharma, Ryan Casini, Kowiyou Yessoufou, and et al. 2022. "In Vitro Propagation of Aconitum violaceum Jacq. ex Stapf through Seed Culture and Somatic Embryogenesis" Horticulturae 8, no. 7: 599. https://doi.org/10.3390/horticulturae8070599








