Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rose Cultivation and Abiotic Stress Treatment
2.2. Bioinformatic Analysis of RcTINY2
2.3. Determination of Transcriptional Activation and Subcellular Localization
2.4. Extraction and Quantification of RNA
2.5. Detection of Abiotic Stress Response in Arabidopsis
2.6. Examination of the Effects of RcTINY2 Silencing
2.7. Statistical Analyses
3. Results
3.1. Phylogenetic Relationship and Sequence Analysis of RcTINY2
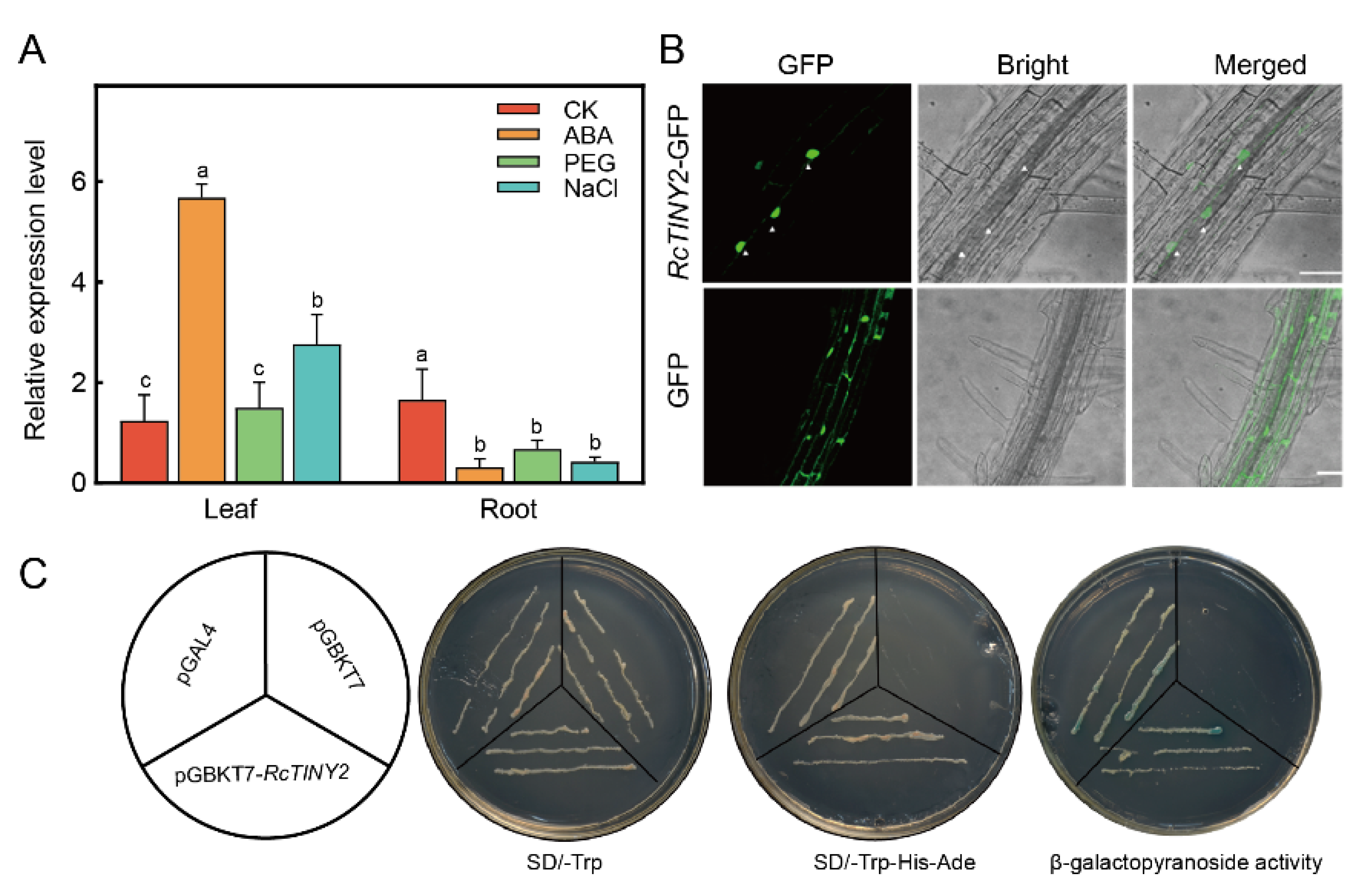
3.2. Expression Characteristics, Subcellular Localization and Transcription Activation Analysis
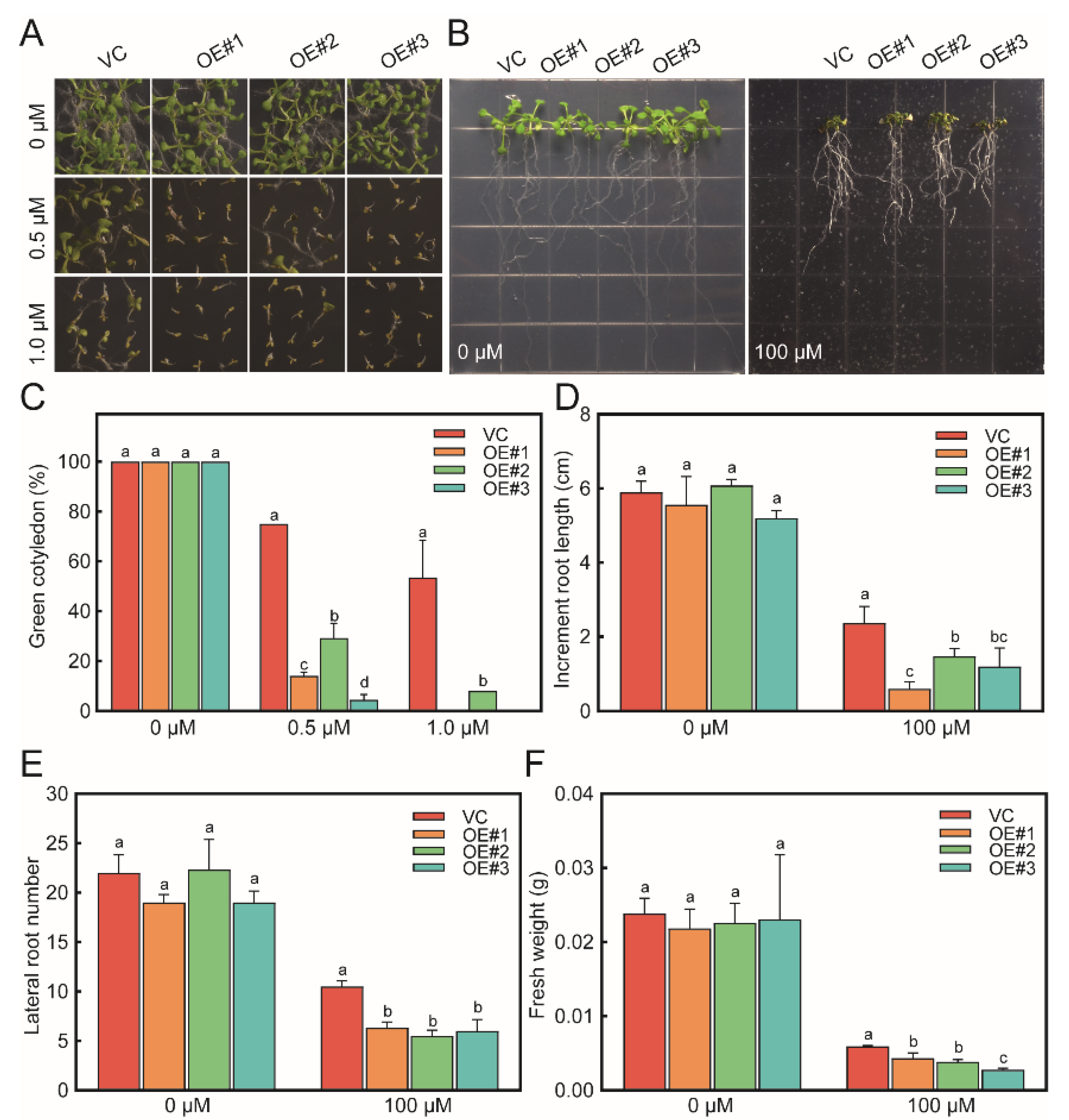
3.3. Sensitivity of RcTINY2-OE Plants to ABA Exposure
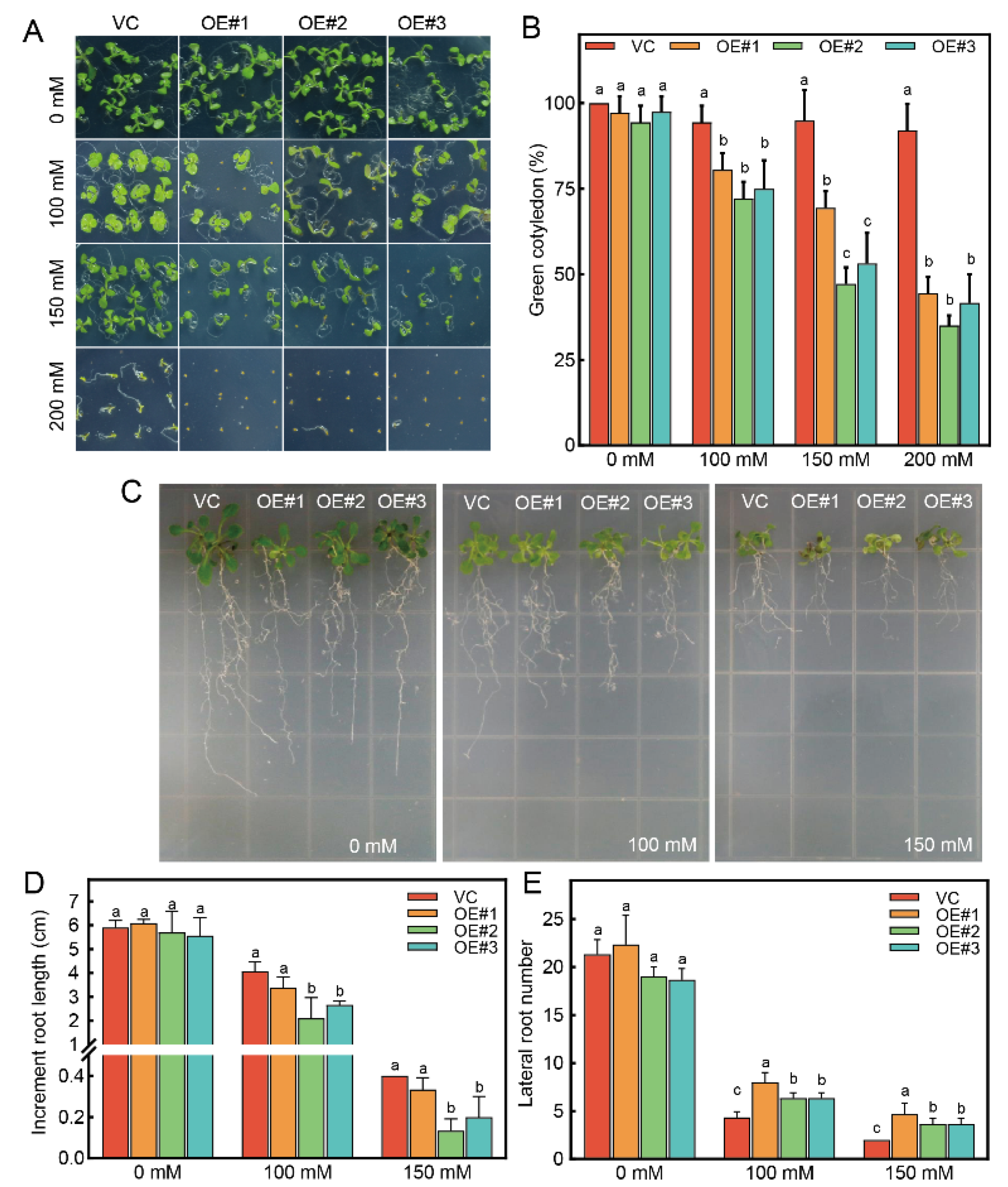
3.4. Sensitivity of RcTINY2-OE Plants to Salinity Stress
3.5. Sensitivity of RcTINY2-OE Plants to Water Stress and Accumulation of ROS
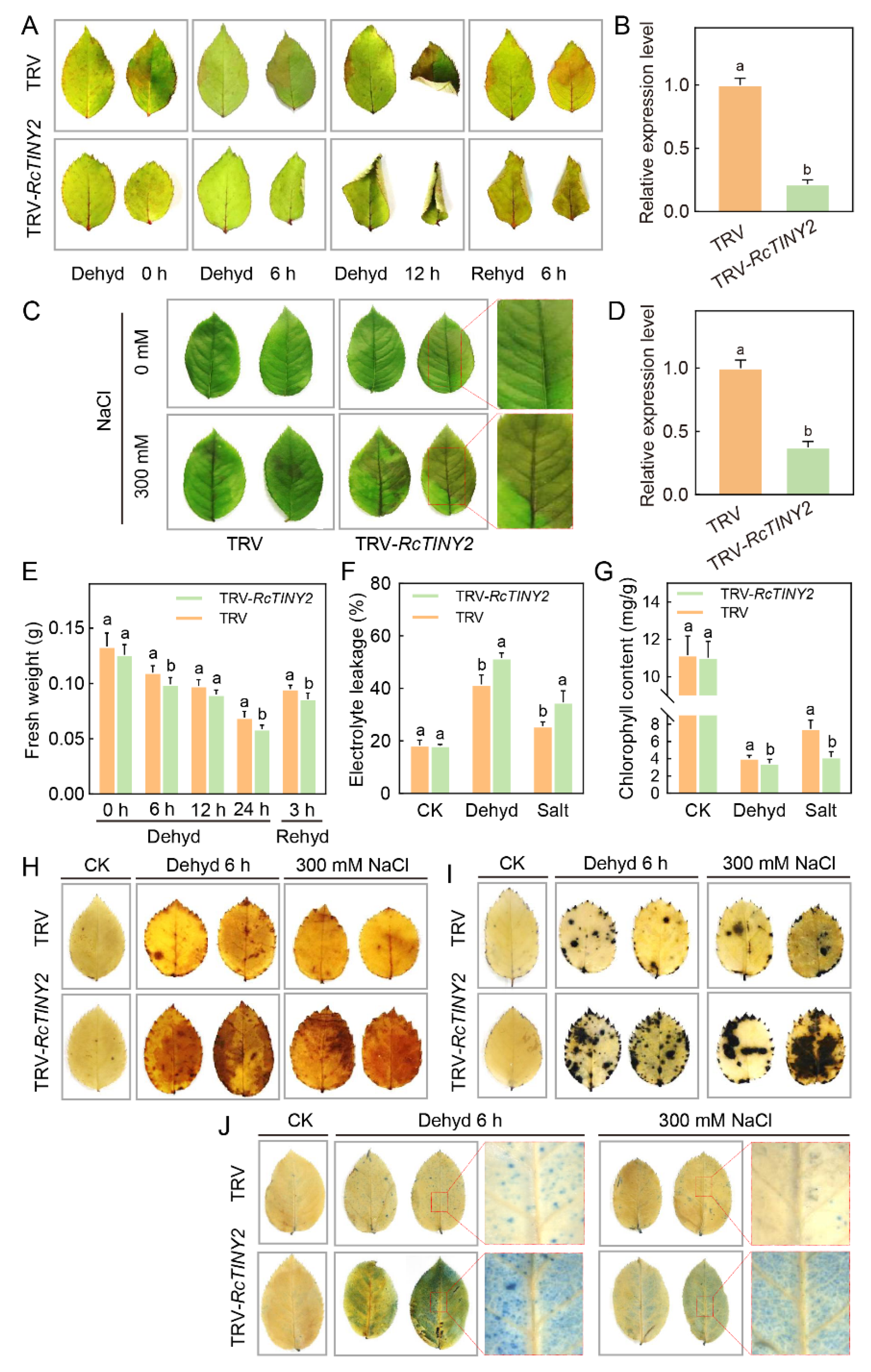
3.6. Silencing of RcTINY2 Increases Salt and Drought Sensitivity in Rose Plants
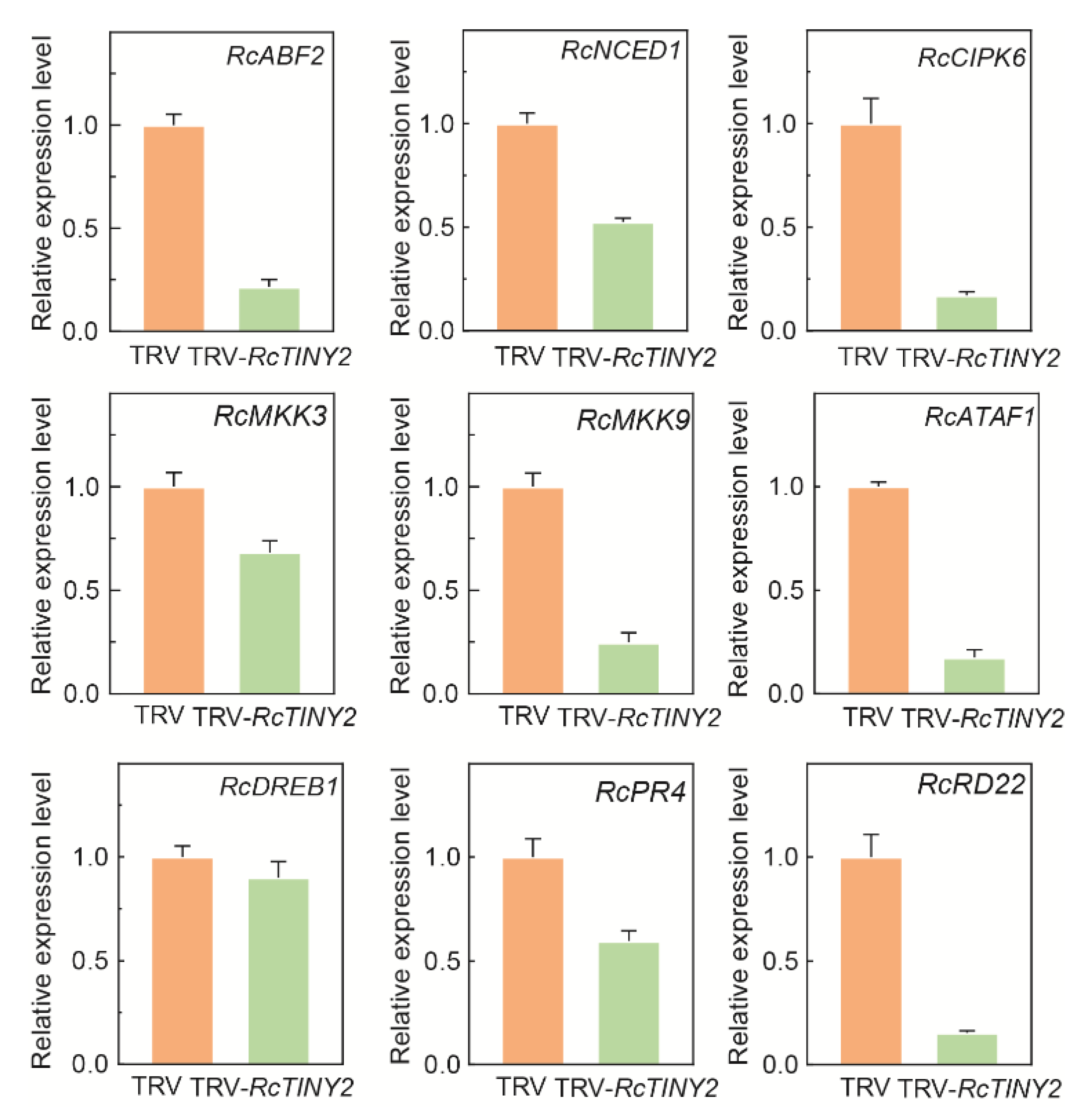
3.7. Effect of RcTINY2 on Stress-Responsive and ABA-Related Gene Expression in Rose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shomali, A.; Aliniaeifard, S. Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 231–258. [Google Scholar]
- Hang, Q.; Chen, J.; Li, L.; Zhao, M.; Zhang, M.; Wang, Y. Research progress on plant AP2/ERF transcription factor family. Biotechnol. Bull. 2018, 34, 7–13. [Google Scholar]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, R.; Duan, L.; Wang, J. The APETALA2/ethylene-responsive factor transcription factor OsDERF2 negatively modulates drought stress in rice by repressing abscisic acid responsive genes. J. Agric. Sci. 2017, 155, 966–977. [Google Scholar] [CrossRef]
- Li, W.; Geng, Z.; Zhang, C.; Wang, K.; Jiang, X. Whole-genome characterization of Rosa chinensis AP2/ERF transcription factors and analysis of negative regulator RcDREB2B in Arabidopsis. BMC Genom. 2021, 22, 90. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abea, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Bioph. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, X.; Yin, W. Expression profiling and functional characterization of a DREB2-type gene from Populus euphratica. Biochem. Biophys. Res. Commun. 2009, 378, 483–487. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Gao, X.; Xu, M.; Guo, Y. DEAR4, a member of DREB/CBF family, positively regulates leaf senescence and response to multiple stressors in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 367. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.-Y.; Cheng, X.-G.; Xu, Z.-S.; Li, L.-C.; Ye, X.-G.; Xia, L.-Q.; Ma, Y.-Z. GmDREB2, a soybean dre-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Bioph. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Xu, J.; Wang, Y.; Zhu, J.; Zhang, Y. The apple columnar gene candidate MdCoL and the AP2/ERF factor MdDREB2 positively regulate aba biosynthesis by activating the expression of MdNCED6/9. Tree Physiol. 2020, 41, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, K.; Yang, Y.; Wu, J.; Chen, F.; Yu, D. Molecular cloning, expression profiling and trans-activation property studies of a DREB2-like gene from chrysanthemum (Dendranthema vestitum). J. Plant Res. 2008, 121, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, W.; He, S.; Zhang, J.; Liu, Q.; Chen, S. An EREPB/AP2-type protein in Triticum aestivum was a dre-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J. Cloning and functional analysis of the novel gene GhDBP3 encoding a DRE-binding transcription factor from Gossypium hirsutum. BBA-Gene Struct. Expr. 2006, 1759, 263–269. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Bio. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, C.; Lü, P.; Jiang, G.; Liu, X.; Dai, F.; Gao, J. RhNAC3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol. J. 2014, 12, 38–48. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, D.T. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.; Peng, T.; Huang, X.; Fan, Q.; Liu, J. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 7, 914–922. [Google Scholar] [CrossRef]
- Su, L.; Zhao, X.; Geng, L.; Fu, L.; Lu, Y.; Liu, Q.; Jiang, X. Analysis of the thaumatin-like genes of Rosa chinensis and functional analysis of the role of RcTLP6 in salt stress tolerance. Planta 2021, 254, 118. [Google Scholar] [CrossRef]
- Li, H.S. Experimental principles and techniques of plant physiology and biochemistry. High. Educ. Press 2000, 2000, 105–108. [Google Scholar]
- Wang, T.; Zheng, T.; Nan, M. Isolation and expression analysis of Rh-DREB1s gene in cut roses (Rosa hybrida) under ethylene treatment and water deficit stress. Acta Hortic. Sin. 2009, 36, 65–72. [Google Scholar]
- Fu, L.; Zhang, Z.; Wang, H.; Zhao, X.; Su, L.; Geng, L.; Lu, Y.; Tong, B.; Liu, Q.; Jiang, X. Genome-wide analysis of BURP genes and identification of a BURP-V gene RcBURP4 in Rosa chienesis. Plant Cell Rep. 2021, 41, 395–413. [Google Scholar] [CrossRef]
- Geng, L.; Su, L.; Fu, L.; Lin, S.; Zhang, J.; Liu, Q.; Jiang, X. Genome-wide analysis of the rose (Rosa chinensis) NAC family and characterization of RcNAC091. Plant Mol. Biol. 2022, 108, 605–619. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Shen, X. Regulatory network established by transcription factors transmits drought stress signals in plant. Stress Biol. 2022, 2, 26. [Google Scholar] [CrossRef]
- Wei, G.; Pan, Y.; Lei, J.; Zhu, Y.X. Molecular cloning, phylogenetic analysis, expressional profiling and in vitro studies of TINY2 from Arabidopsis thaliana. J. Biochem. Mol. Biol. 2005, 38, 440–446. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhu, J.J.; Wei, C.J.; Guo, Q.; Bian, C.K.; Xiang, Z.K.; Zhao, A.C. Genome-wide identification and characterization of the DREB transcription factor gene family in mulberry. Biol. Plant 2015, 59, 253–265. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.-D.; Chi, X.-J.; Wu, C.; Li, Y.-Z.; Song, L.-L.; Liu, X.-M.; Wang, Y.-F.; Wang, F.-W.; Zhang, C.; et al. Dwarf apple MbDREB1 enhances plant tolerance to low temperature, drought, and salt stress via both ABA-dependent and ABA-independent pathways. Planta 2011, 233, 219–229. [Google Scholar] [CrossRef]
- Herath, V. Small family, big impact: In silico analysis of DREB2 transcription factor family in rice. Computat. Biol. Chem. 2016, 65, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Morran, S.; Eini, O.; Pyvovarenko, T.; Parent, B.; Singh, R.; Ismagul, A.; Eliby, S.; Shirley, N.; Langridge, P.; Lopato, S. Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol. J. 2011, 9, 230–249. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Muhovski, Y.; Clippe, A.; Žižková, E.; I. Dobrev, P.; Motyka, V.; Lutts, S. SlDREB2, a tomato dehydration-responsive element-binding 2 transcription factor, mediates salt stress tolerance in tomato and Arabidopsis. Plant Cell Environ. 2015, 39, 62–79. [Google Scholar] [CrossRef]
- Sun, S.; Yu, J.P.; Chen, F.; Zhao, T.J.; Fang, X.H.; Li, Y.Q.; Sui, S.F. TINY, a dehydration-responsive element (DRE)-binding protein-like transcription factor connecting the DRE- and ethylene-responsive element-mediated signaling pathways in Arabidopsis. J. Biol. Chem. 2008, 283, 6261–6271. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, C.G.; Wu, X.; Hu, R.X.; Yu, G.; Zhang, X.H.; Liu, J.L.; Pan, H.Y. ZmDBF3, a novel transcription factor from maize (Zea mays L.), is involved in multiple abiotic stress tolerance. Plant Mol. Biol. Rep. 2016, 34, 353–364. [Google Scholar] [CrossRef]
- Bouaziz, D.; Jbir, R.; Charfeddine, S.; Saidi, M.N.; Gargouri, R. The StDREB1 transcription factor is involved in oxidative stress response and enhances tolerance to salt stress. Plant Cell Tiss. Org. 2015, 121, 237–248. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, K.; Zhang, J.; Li, X.; Xu, K.; Zhang, Y.; Qi, J.; Yu, D.; Wang, J.; Li, C. JcDREB2, a physic nut AP2/ERF gene, alters plant growth and salinity stress responses in transgenic rice. Front. Plant Sci. 2017, 8, e0131599. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Li, J.; Liu, Q.; Li, Y.Q.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004, 45, 1042–1052. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Dong, C.; Li, X.; Du, J.C.; Qian, M.; Sun, X.D.; Yang, Y.P. A novel Ap2/ERF transcription factor from Stipa purpurea leads to enhanced drought tolerance in Arabidopsis thaliana. Plant Cell Rep. 2016, 35, 2227–2239. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki., K.; Yamaguchi-Shinozaki., K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signaling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022. [Google Scholar] [CrossRef]
- Li, R.; Zhang, G.; Wei, G. Functions and mechanisms of the CBL-CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci. 2009, 19, 667–676. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant mitogen-activated protein kinase cascades in environmental stresses. Int. J. Mol. Sci. 2021, 3, 1543. [Google Scholar] [CrossRef]
- Wang, M.; Yue, H.; Feng, K.; Deng, P.; Song, W.; Nie, X. Genome-wide identification, phylogeny and expressional profiles of mitogen activated protein kinase kinase kinase (MAPKKK) gene family in bread wheat (Triticum aestivum L.). BMC Genom. 2016, 17, 668. [Google Scholar] [CrossRef]
- Kim, S.-H.; Woo, D.-H.; Kim, J.-M.; Lee, S.-Y.; Chung, W.S.; Moon, Y.-H. Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem. Bioph. Res. Commun. 2011, 412, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Raymond, O.; Gouzy, J.; Just, J.; Badouin, H.; Verdenaud, M.; Lemainque, A.; Vergne, P.; Moja, S.; Choisne, N.; Pont, C. The Rosa genome provides new insights into the domestication of modern roses. Nat. Genet. 2018, 50, 772–777. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, L.; Su, L.; Wang, Y.; Geng, Z.; Lin, S.; Zhang, Y.; Yu, S.; Fu, L.; Liu, Q.; Cheng, C.; et al. Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose. Horticulturae 2022, 8, 747. https://doi.org/10.3390/horticulturae8080747
Geng L, Su L, Wang Y, Geng Z, Lin S, Zhang Y, Yu S, Fu L, Liu Q, Cheng C, et al. Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose. Horticulturae. 2022; 8(8):747. https://doi.org/10.3390/horticulturae8080747
Chicago/Turabian StyleGeng, Lifang, Lin Su, Yuan Wang, Ziwen Geng, Shang Lin, Yichang Zhang, Shuang Yu, Lufeng Fu, Qinghua Liu, Chenxia Cheng, and et al. 2022. "Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose" Horticulturae 8, no. 8: 747. https://doi.org/10.3390/horticulturae8080747




