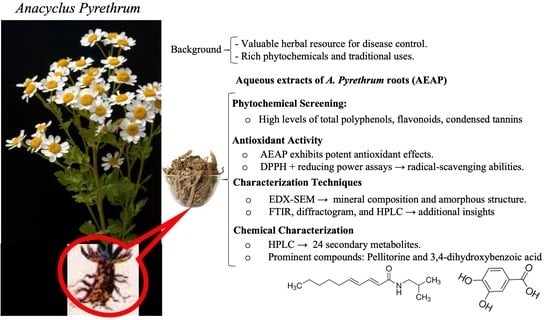
Phytochemistry, Antioxidant Potential, and Antibacterial Activities of Anacyclus pyrethrum: Promising Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Samples and Extraction
2.1.1. Plant Samples
2.1.2. Plant Extraction
2.2. Phytochemical Study
2.2.1. Determination of Total Phenolic Content (TPC)
2.2.2. Determination of Total Flavonoid Content (TFC)
2.2.3. Quantification of Condensed Tannins
2.3. Antioxidant Activity
2.3.1. Determination of DPPH Radical Scavenging Activity
2.3.2. Determination of Ferric Reducing Ability Power (FRAP) Activity
2.4. Antibacterial Activity
2.4.1. Microbial Strains and Culture Conditions
2.4.2. Agar Well Diffusion Inhibition Zones Test
2.4.3. MIC and MBC/MFC Determination
2.5. Chemical Characterization
2.5.1. High-Performance Liquid Chromatography with Photodiode Array Detection-Tandem Mass Spectrometer (HPLC–PDA–MS/MS)
2.5.2. Fourier Transform Infrared (FTIR)
2.5.3. X-ray Diffraction (XRD)
2.5.4. Scanning Electron Microscope (SEM)
2.5.5. Energy-Dispersive X-ray Spectroscopy (EDX)
2.6. Statistical Analyses
3. Results
3.1. Phytochemical Screening
3.2. Antioxidant Activity
3.3. Antibacterial Activity
3.4. Characterization of the Extract
3.4.1. HPLC Analysis
3.4.2. EDX–SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi, H.; Fadili, K.; Cherrat, A.; Amalich, S.; Zekri, N.; Zerkani, H.; Zair, T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- The Global Biodiversity Information Facility. Anacyclus pyrethrum (L.). Available online: https://www.gbif.org/fr/species/3148578 (accessed on 20 October 2023).
- Usmani, A.; Khushtar, M.; Arif, M.; Siddiqui, M.A.; Sing, S.P.; Mujahid, M. Pharmacognostic and phytopharmacology study of Anacyclus pyrethrum: An insight. J. Appl. Pharm. Sci. 2016, 6, 144–150. [Google Scholar] [CrossRef]
- Fennane, M.; bn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Pratique du Maroc. Trav. Inst. Sci. Sér. Bot. 1999, 1, 36. [Google Scholar]
- Elazzouzi, H.; Soro, A.; Elhilali, F.; Bentayeb, A.; El Belghiti, M.A.; Zair, T. Phytochemical study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in vitro study of antibacterial activity of pyrethrum. Adv. Nat. Appl. Sci. 2014, 8, 131–141. [Google Scholar]
- Boonen, J.; Sharma, V.; Dixit, V.K.; Burvenich, C.; Spiegeleer, B.D. LC-MS N-alkylamide Profiling of an Ethanolic Anacyclus pyrethrum Root Extract. Planta Med. 2012, 80, 1787–1795. [Google Scholar] [CrossRef]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-inflammatory, Antinociceptive, and Antiox-idant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Badhe, S.R.; Badhe, R.V.; Ghaisas, M.M.; Chopade, V.V.; Deshpande, A.D. Evaluations of antidepressant activity of Anacyclus pyrethrum root extract. Int. J. Green Pharm. IJGP. 2010, 4, 2. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Kalim, M.D.; Bhattacharyya, D.; Banerjee, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Immunomodulatory activity of petroleum ether extract of Anacyclus pyrethrum. Pharm Biol. 2010, 48, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Manouze, H.; Bouchatta, O.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anticonvulsive and neuroprotective effects of aqueous and methanolic extracts of Anacyclus pyrethrum root in kainic acid-induced-status epilepticus in mice. Epilepsy Res. 2019, 158, 106225. [Google Scholar] [CrossRef]
- Khare, C.P. Anacyclus pyrethrum DC. In Indian Medicinal Plants: An Illustrated Dictionary; Khare, C.P., Ed.; Springer: New York, NY, USA, 2007; p. 1. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Kumar, V.K.; Lalitha, K.G. Pharmacognostical Studies on the Root of Anacyclus pyrethrum DC; NISCAIR-CSIR: Delhi, India, 2012. [Google Scholar]
- Manzanilla, V.; Teixidor-Toneu, I.; Martin, G.J.; Hollingsworth, P.M.; de Boer, H.J.; Kool, A. Using target capture to address con-servation challenges: Population-level tracking of a globally-traded herbal medicine. Mol. Ecol. Resour. 2022, 22, 212–224. [Google Scholar] [CrossRef]
- Gulland, J.M.; Hopton, G.U., II. —Pellitorine, the pungent principle of Anacyclus pyrethrum. J. Chem. Soc. Resumed. 1930, 15, 6–11. [Google Scholar] [CrossRef]
- Ee, G.C.L.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F.J. Pellitorine, a Potential Anti-Cancer Lead Compound against HL60 and MCT-7 Cell Lines and Microbial Transformation of Piperine from Piper Nigrum. Molecules 2010, 15, 2398–2404. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Zeng, T.; Zhou, L.; Li, J.; Hu, H.; Wang, C. Overexpression of TcCHS Increases Pyrethrin Content When Using a Genotype-Independent Transformation System in Pyrethrum (Tanacetum cinerariifolium). Plants 2022, 11, 1575. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of folinciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity of Phaseolus vulgaris L. Dry Beans. J. Agric Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Aitbaba, A.; Sokar, Z.; Chait, A. Analgesic and anti-inflammatory effects of hydroalcoholic extract of Astragalus ibrahimianus. Bangladesh J. Pharmacol. 2023, 18, 41–48. [Google Scholar]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Alayed, H.S.; Devanesan, S.; AlSalhi, M.S.; Alkindi, M.G.; Alghamdi, O.G.; Alqhtani, N.R. Investigation of Antibacterial Activity of Carob-Mediated Calcium Hydroxide Nanoparticles against Different Aerobic and Anaerobic Bacteria. Appl. Sci. 2022, 12, 12624. [Google Scholar] [CrossRef]
- Ma, W. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. CLSI NCCLS 2006, 26, M7-A7. [Google Scholar]
- Al-Dulimi, A.G.; Al-Saffar, A.Z.; Sulaiman, G.M.; Khalil, K.A.; Khashan, K.S.; Al-Shmgani, H.S.; Ahmed, E.M. Immobilization of L-asparaginase on Gold Nanoparticles for Novel Drug Delivery Approach as Anti-Cancer Agent Against Human Breast Carcinoma Cells. J. Mater Res. Technol. 2020, 25, 15394–15411. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Bari, A. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, A.; Amalich, S.; Regragui, M.; Bouzoubae, A.; Elamrani, M.; Mahjoubi, M.; Zair, T. Polyphenols content and evaluation of antioxidant activity of Anacyclus pyrethrum (L.) lag. from timahdite a moroccan middle atlas region. Int. J. Adv. Res. 2017, 5, 569–577. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Nagendrappa, G. An appreciation of free radical chemistry 3. Free radicals in diseases and health. Resonance 2005, 10, 65–74. [Google Scholar] [CrossRef]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimarães, J.T. Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sreeja, P.S.; Yang, X. The Antioxidant Properties of Mushroom Polysaccharides can Potentially Mitigate Oxidative Stress, Beta-Cell Dysfunction and Insulin Resistance. Front Pharmacol 2022, 13, 874474. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environ-mental stress in plants. Front Environ Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Front. Environ. Sci. 2020, 30, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras Farmacogn. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Kaliaperumal, J.; Dulari, S.; Pandurangan, D.; Rathore, M.; Mittal, A.; Alam, M. Neuroprotective Activity of Protocatechuic Acid (3,4-Dihydroxybenzoic Acid) to Treat Parkinson’s Disease. J. Evol. Med. Dent Sci. 2020, 9, 2466–2471. [Google Scholar] [CrossRef]
- Nguyen, D.-M.; Seo, D.-J.; Kim, K.-Y.; Park, R.-D.; Kim, D.-H.; Han, Y.-S.; Kim, T.-H.; Jung, W.-J. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59–60, 52–59. [Google Scholar] [CrossRef]
- Guglielmi, F.; Luceri, C.; Giovannelli, L.; Dolara, P.; Lodovici, M. Effect of 4-coumaric and 3,4-dihydroxybenzoic acid on oxidative DNA damage in rat colonic mucosa. Br. J. Nutr. 2003, 89, 581–587. [Google Scholar] [CrossRef]
- Ji, R.; Quan, Q.; Guo, X.; Zhang, J.; Song, Y.; Zhu, M.; Tan, P.; Han, J.; Liu, Y. Simultaneous determination of five N-alkylamides in the root of Anacyclus pyrethrum by HPLC and profiling of components in its methanolic root extract by UPLC/Q-TOF-MS. Rev. Bras. Farmacogn. 2019, 29, 152–161. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Imtara, H.; El Moussaoui, A.; Khalis, H.; Es-Safi, I.; Al Kamaly, O.; Bari, A. Reproductive Biology of the Two Varieties of Anacyclus pyrethrum L.—Anacyclus pyrethrum var. pyrethrum (L.) Link and Anacyclus pyrethrum var. depressus (Ball.) Maire—An Endemic Endangered Species. Plants 2022, 11, 2299. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Kerboua, K.A.; Benosmane, L.; Namoune, S.; Ouled-Diaf, K.; Ghaliaoui, N.; Bendjeddou, D. Anti-inflammatory and anti-oxidant activity of the hot water-soluble polysaccharides fromzAnacyclus pyrethrum (L.). Lag. roots. J. Ethnopharmacol. 2021, 281, 114491. [Google Scholar] [CrossRef] [PubMed]
- Doudach, L.; Meddah, B.; Alnamer, R.; Chibani, F.; Cherrah, Y. In vitro antibacterial activity of the methanolic and aqueous ex-tracts of Anacyclus pyrethrum used in Moroccan traditional medicine. Int. J. Pharm. Pharm. Sci. 2012, 4, 4. [Google Scholar]
- Elazzouzi, H.; Zekri, N.; Zair, T.; El Belghiti, M.A. Volatiles profiling and antioxidant activity of Moroccan Artemisia ifranensis J. Didier and Anacyclus pyrethrum Link essential oils. Egypt J. Chem. 2020, 63, 3937–3947. [Google Scholar] [CrossRef]
- Daoudi, A.; Mohamed, B.; Jamal, I.; Laila, N. Antibacterial activity of aqueous extracts of Anacyclus pyrethrum (L) link and Corrigiola telephiifolia Pourr. from the middle atlas Region-Morocco. Eur. Sci. J. 2017, 13, 116. [Google Scholar]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Bari, A. Anacyclus pyrethrum (L): Chemical Com-position, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef]
- Bezza, K.; Gabbas, Z.E.; Laadraoui, J.; Laaradia, M.A.; Oufquir, S.; Chait, A. Ameliorative potential of Anacyclus pyrethrum extract in generalized seizures in rat: Possible cholinergic mediated mechanism. Bangladesh J. Pharmacol. 2019, 14, 188–195. [Google Scholar] [CrossRef]
- Baslam, A.; Aitbaba, A.; Hanchi, A.L.; Tazart, Z.; Aboufatima, R.; Soraa, N.; Chait, A. Modulation of Gut Microbiome in Ec-stasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. Int. J. Mol. Sci. 2023, 24, 9086. [Google Scholar] [CrossRef]



| Total Polyphenol Content mg GAE/g DM | Flavonoids mg CE/gDM | Tanins mg CE/g DM | |
|---|---|---|---|
| Crude extracts (mg/g) | 21.98 ± 0.05 | 8.1 ± 0.16 | 5.49 ± 0.08 |
| Antioxidant Assay | Plant Extract (IC50 = mg/mL) | Standard Antioxidant (IC50 = mg/mL) | |
|---|---|---|---|
| Quercetin | BHT | ||
| DPPH | 1.6 ± 0.04 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| FRAP | 1.4 ± 0.04 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Bacteria Group | Bacterial Strains | Reference | Diameter Inhibition Zone (mm) | |
|---|---|---|---|---|
| AEAP | Gentamicin | |||
| Gram-positive bacteria | Staphylococcus aureus | ATCC 25923 | 17.65 ± 1.14 | 23 |
| Enterococcus faecium | ATCC 4602 | 10.87 ± 0.69 | 12 | |
| Gram-negative bacteria | Escherichia coli | ATCC25922 | 17.73 ± 0.61 | 23 |
| Pseudomonas aeruginosa | ATCC 27653 | 15.49 ± 2.01 | 22 | |
| Bacteria Group | Bacterial Strains | Reference | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | ATCC 25923 | 1.95 | >50 |
| Enterococcus faecium | ATCC 4602 | 3.91 | >50 | |
| Gram-negative bacteria | Escherichia coli | ATCC25922 | 8.51 | >50 |
| Pseudomonas aeruginosa | ATCC 27653 | 2.50 | 50 |
| Rt (min) | MW | MS/MS | Compound Name | |
|---|---|---|---|---|
| 1 | 1.516 | 191 | 111 | Citric acid |
| 2 | 1.935 | 335 | 169 | Gallic acid derivative |
| 3 | 3.523 | 267 | 108, 153 | Dihydroxybenzoic acid derivative |
| 4 | 3.642 | 299 | 153 | Dihydroxybenzoic acid rhamnoside |
| 5 | 4. 42 | 315 | 108, 153 | Dihydroxybenzoic acid glucoside |
| 6 | 5.243 | 371 | 135, 191 | Caffeoylglucaric acid |
| 7 | 5.862 | 169 | 125 | Gallic acid |
| 8 | 6.338 | 341 | 108, 167 | Vanillyl quinic acid |
| 9 | 7.124 | 153 | 108 | 3,4-dihydroxybenzoic acid |
| 10 | 9.452 | 225 | 137 | Hydroxybenzoic acid glycerol |
| 11 | 10.921 | 353 | 135, 191 | Chlorogenic acid |
| 12 | 11.676 | 311 | 137 | Hydroxybenzoic acid quinyl ester |
| 13 | 12.289 | 329 | 153 | Dihydroxybenzoic acid glucuronide |
| 14 | 13.102 | 223 | 133,81 | Pellitorine |
| 15 | 15.075 | 181 | 107, 135 | Dihydrocaffeic acid |
| 16 | 17.482 | 367 | 191 | Feruloylquinic acid |
| 17 | 19.437 | 353 | 179, 191 | Cryptochlorogenic acid |
| 18 | 20.607 | 179 | 135 | Caffeic acid |
| 19 | 21.88 | 163 | 119 | p-Coumaric acid |
| 20 | 23.919 | 337 | 191 | p-Coumaroylquinic acid |
| 22 | 24.853 | 161 | 133 | Hydroxycoumarin |
| 22 | 27.796 | 515 | 179, 191 | Isochlorogenic acid b |
| 23 | 28.521 | 447 | 301 | Quercetin rhamnoside |
| 24 | 48.907 | 515 | 179, 191 | Isochlorogenic acid C |
| Sample/Element | K | Ca | Cl | P | S | Si | Al | Mg | Na | |
|---|---|---|---|---|---|---|---|---|---|---|
| AEAP | Weight % | 33.62 | 12.42 | 10.60 | 8.02 | 4.00 | 3.26 | 2.86 | 1.86 | 0.52 |
| Atomic % | 21.87 | 7.88 | 7.60 | 6.59 | 3.17 | 2.95 | 2.70 | 1.95 | 0.57 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baslam, A.; Aitbaba, A.; Aboufatima, R.; Agouram, F.; Boussaa, S.; Chait, A.; Baslam, M. Phytochemistry, Antioxidant Potential, and Antibacterial Activities of Anacyclus pyrethrum: Promising Bioactive Compounds. Horticulturae 2023, 9, 1196. https://doi.org/10.3390/horticulturae9111196
Baslam A, Aitbaba A, Aboufatima R, Agouram F, Boussaa S, Chait A, Baslam M. Phytochemistry, Antioxidant Potential, and Antibacterial Activities of Anacyclus pyrethrum: Promising Bioactive Compounds. Horticulturae. 2023; 9(11):1196. https://doi.org/10.3390/horticulturae9111196
Chicago/Turabian StyleBaslam, Abdelmounaim, Abdelfatah Aitbaba, Rachida Aboufatima, Fatimazahra Agouram, Samia Boussaa, Abderrahman Chait, and Marouane Baslam. 2023. "Phytochemistry, Antioxidant Potential, and Antibacterial Activities of Anacyclus pyrethrum: Promising Bioactive Compounds" Horticulturae 9, no. 11: 1196. https://doi.org/10.3390/horticulturae9111196