Development of Cleaved Amplified Polymorphic Sequence Marker for Cap Color Identification in Pleurotus cornucopiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Primer Design and Synthesis
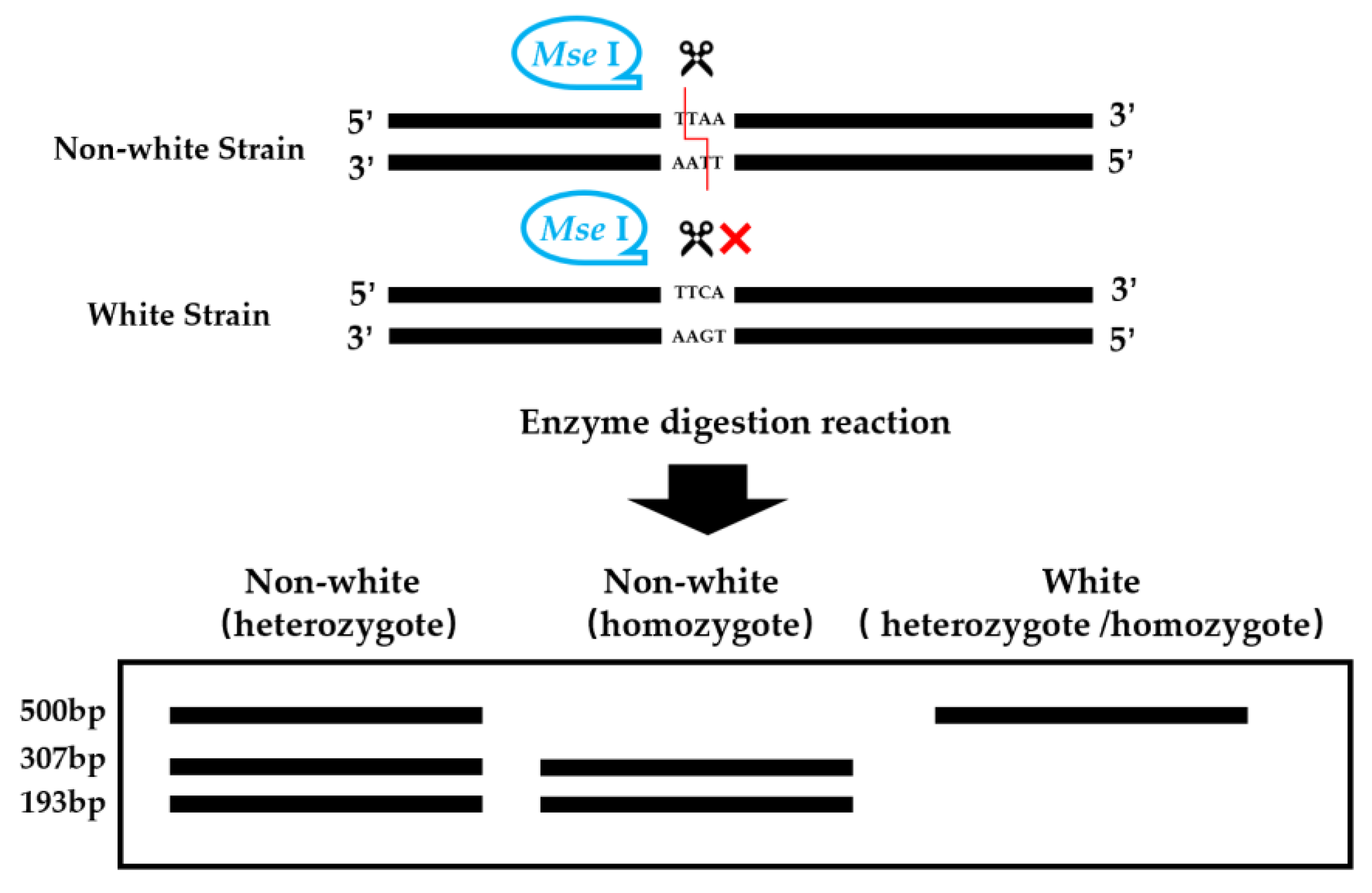
2.2.2. CAPS/dCAPS Marker Development and Restriction Enzyme Selection
2.2.3. PCR Amplification System and Program
2.2.4. Enzyme Digestion System and Electrophoresis Detection of the Digestion Products
3. Results
3.1. Primer Design and PCR Validation
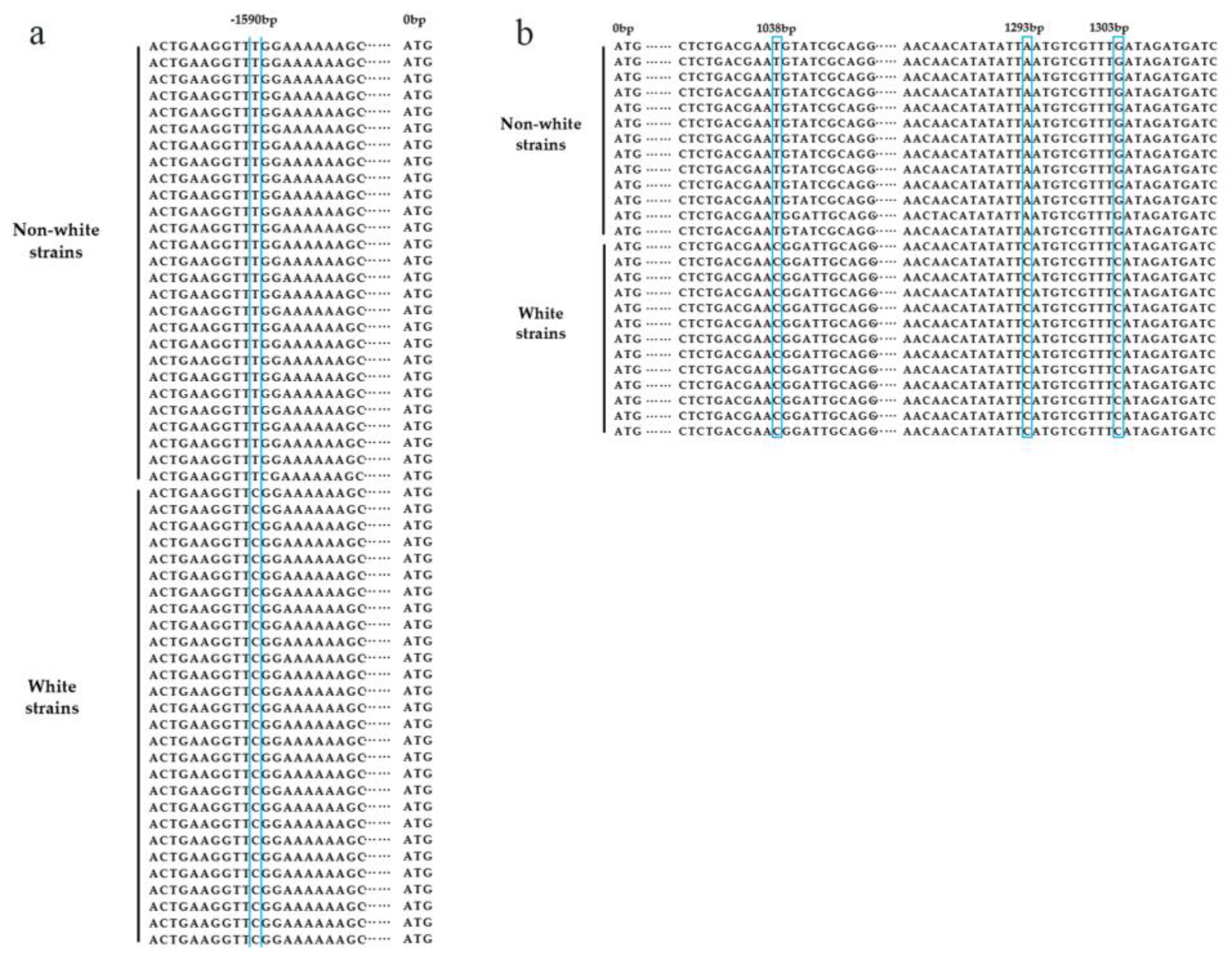
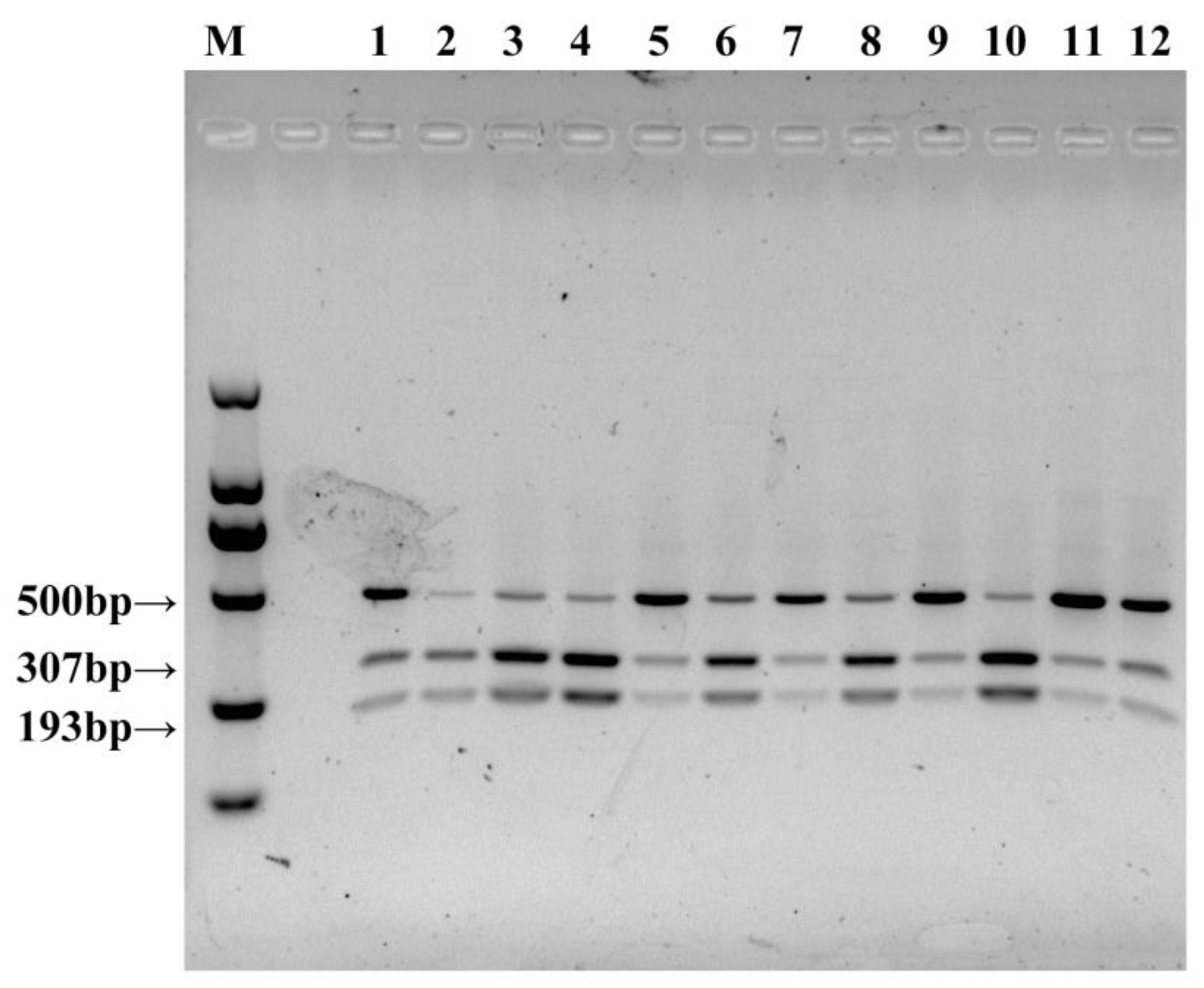
3.2. CAPS Marker Development and Screening
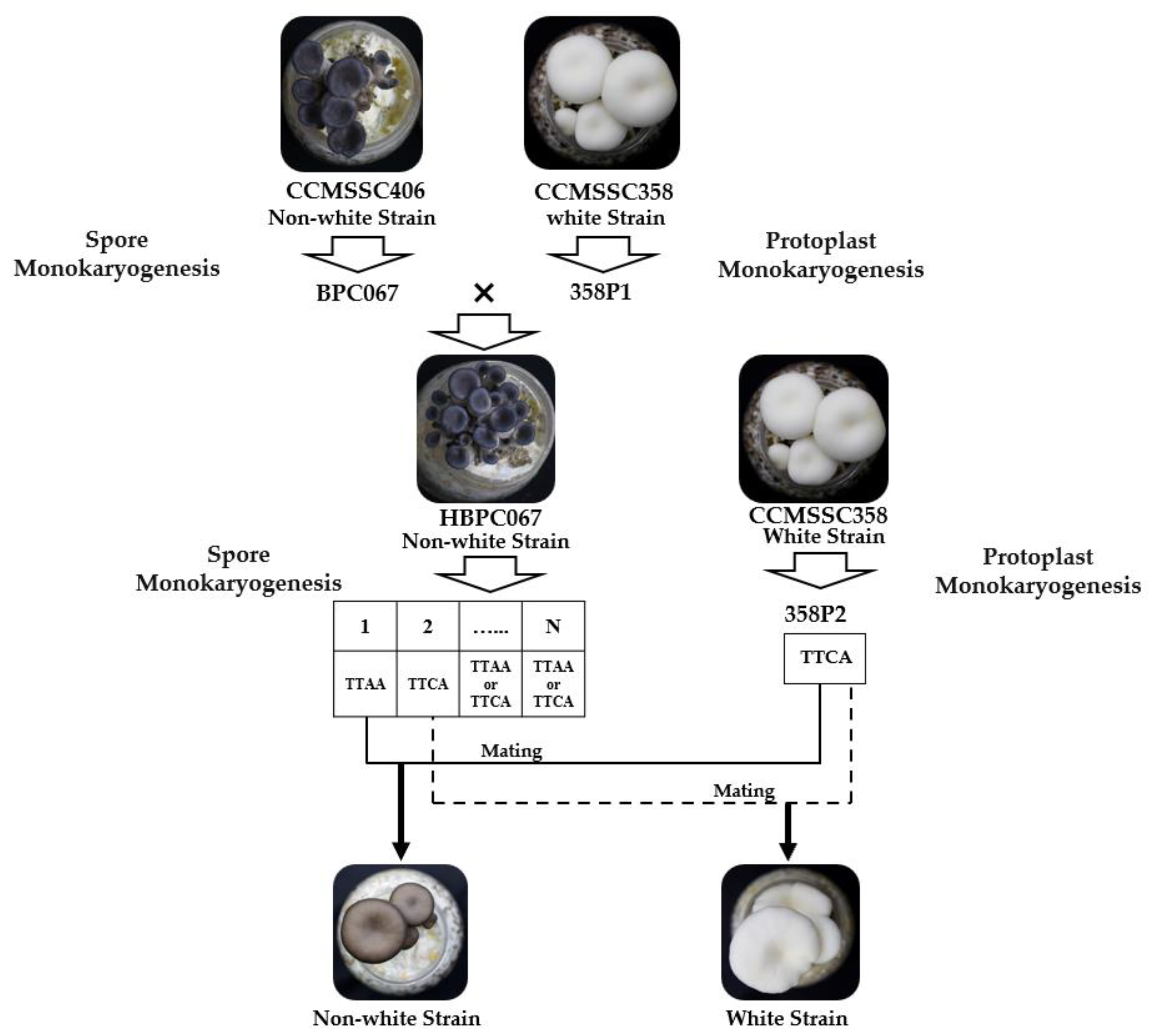
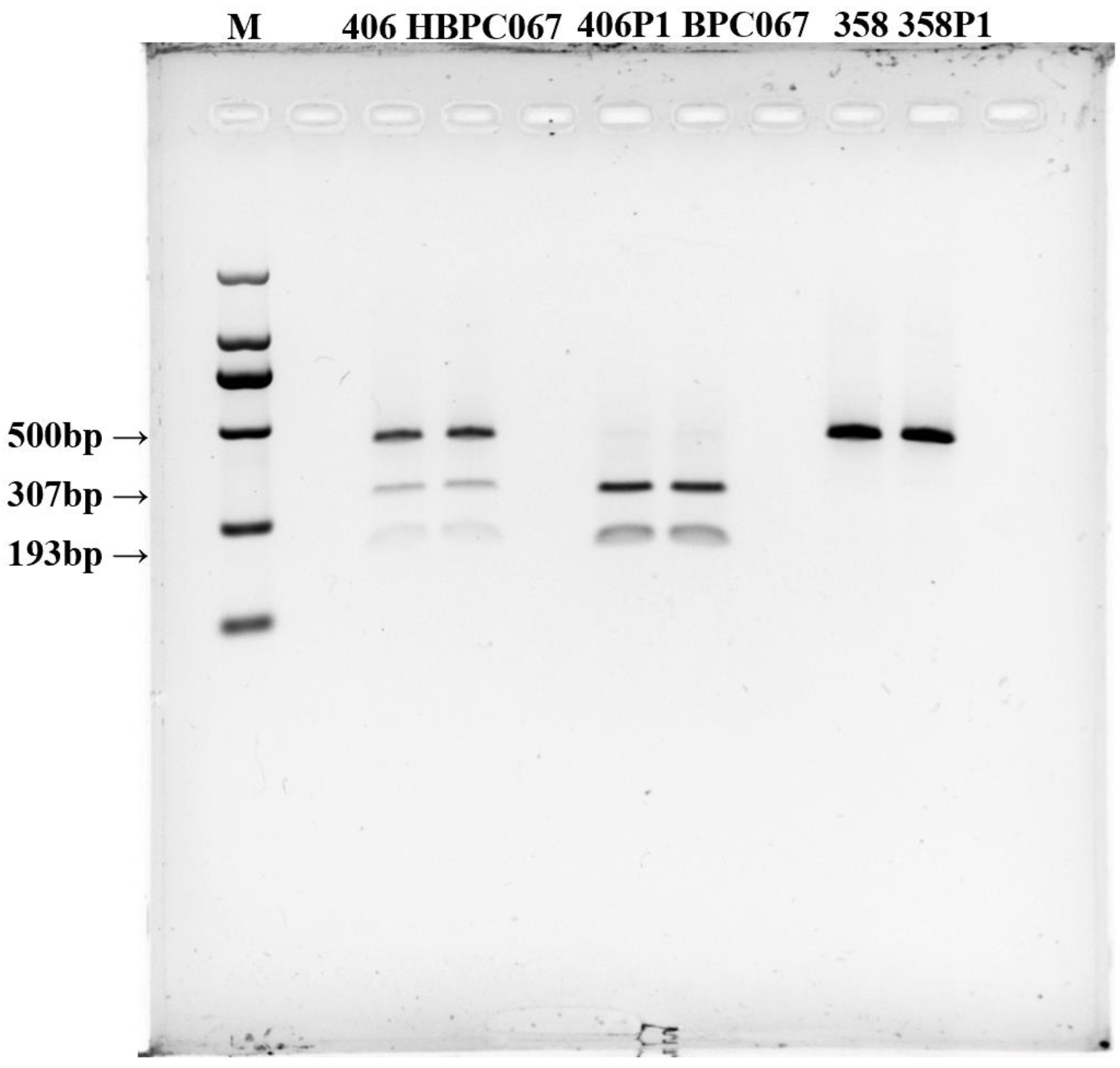
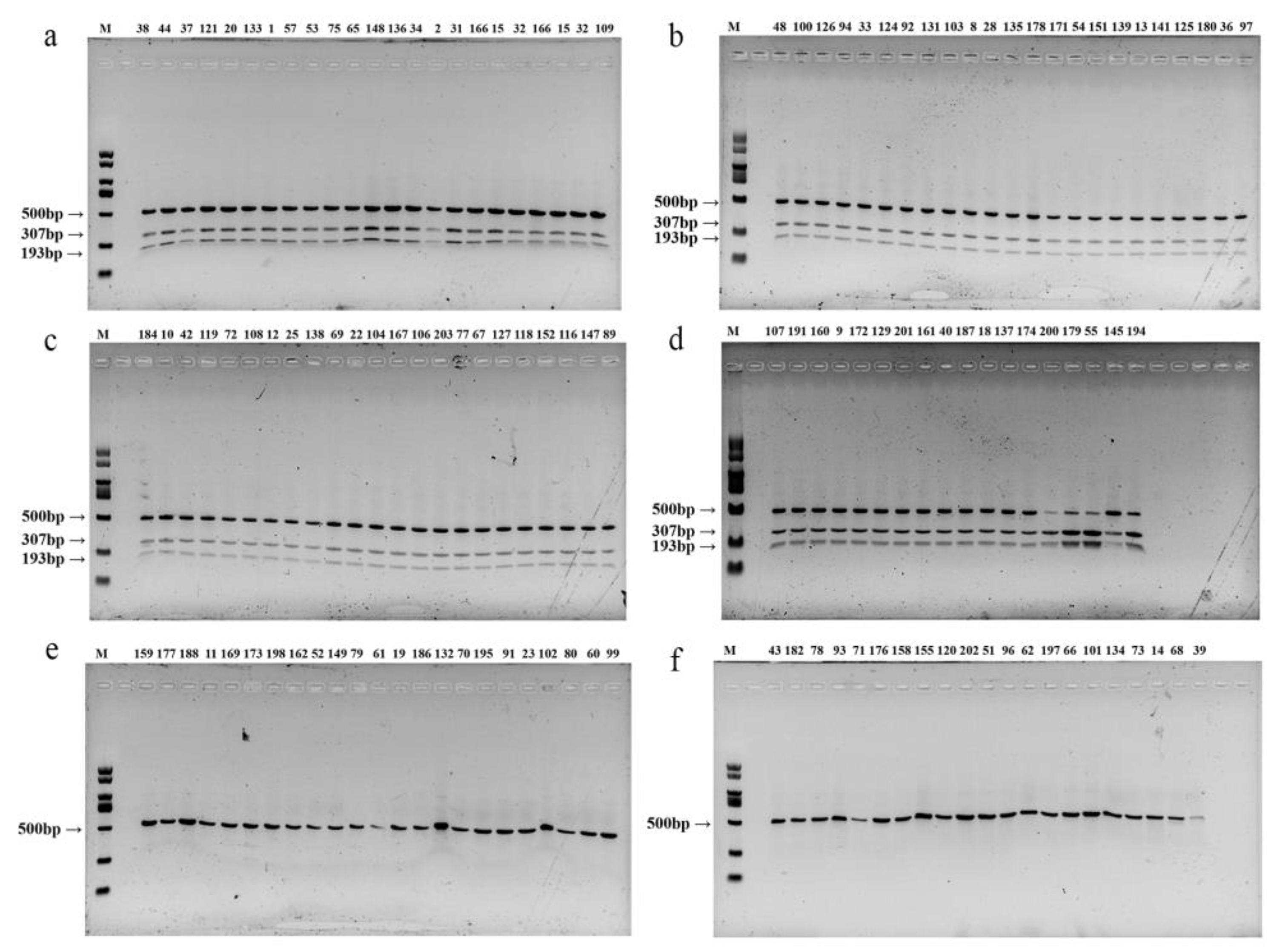
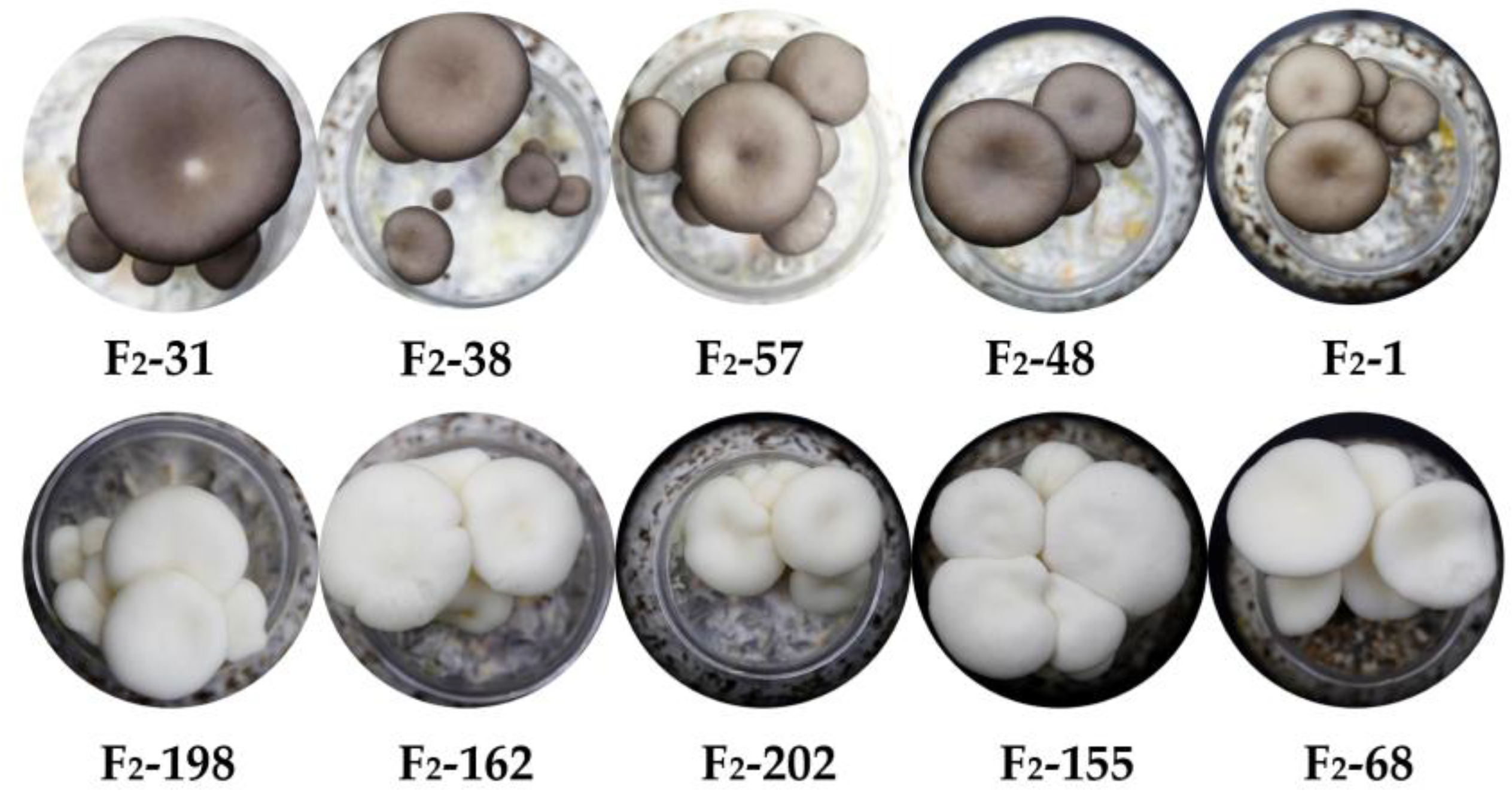
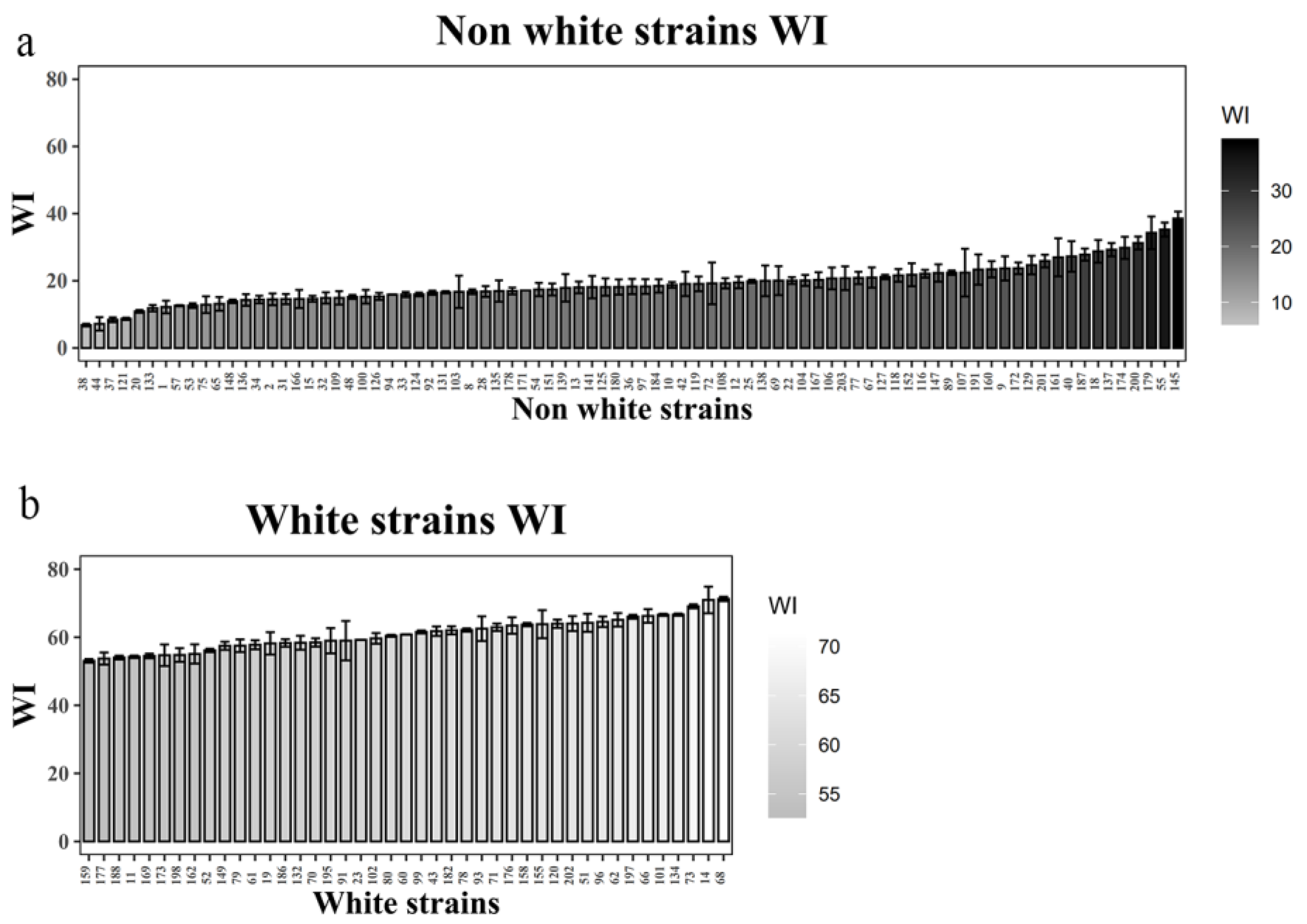
3.3. Validation of CAPS/dCAPS Markers Co-Segregating with Cap Color
3.4. Validation of CAPS/dCAPS Markers in Other Dark-Colored Oyster mushrooms Varieties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.Y.; Zhang, J.X.; Wang, H.X.; Huang, C.Y. Polyphasic taxonomy of cultivated oyster mushrooms in China. Edible Fungi China 2003, 3, 3–6. [Google Scholar]
- Papaspyridi, L.M.; Aligiannis, N.; Christakopoulos, P.; Skaltsounis, A.L.; Fokialakis, N. Production of bioactive metabolites with pharmaceutical and nutraceutical interest by submerged fermentation of Pleurotus ostreatus in a batch stirred tank bioreactor. In Proceedings of the 11th International Congress on Engineering and Food (ICEF), Athens, Greece, 22–26 May 2011; pp. 1746–1752. [Google Scholar]
- Barbosa, J.R.; Freitas, M.M.D.; Martins, L.H.D.; de Carvalho, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.T.; Pandey, I.; Hachenberger, Y.; Krause, B.C.; Haidar, R.; Laux, P.; Luch, A.; Singh, M.P.; Singh, A.V. Emerging paradigm against global antimicrobial resistance via bioprospecting of mushroom into novel nanotherapeutics development. Trends Food Sci. Technol. 2020, 106, 333–344. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising anticancer activity of polysaccharides and other macromolecules derived from oyster mushroom (Pleurotus sp.): An updated review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Tao, Q.Q.; Wang, K.; Han, J.J.; Bao, L.; Liu, H.W. Research advances on the secondary metabolites produced by edible mushroom Pleurotus and their bioactivities. Mycosystema 2015, 34, 569–580. [Google Scholar]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and identification of pigments from oyster mushrooms with black, yellow and pink caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Zeng, H.L.; Huang, J.H.; Lei, L.; Tong, X.L.; Li, S.; Zhou, Y.; Guo, H.R.; Khan, M.; Luo, L.P.; et al. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Sonnenberg, A.; Chen, Q.; Zhang, J.X.; Huang, C.Y. Genetic linkage and physical mapping for an oyster mushroom (Pleurotus cornucopiae) and quantitative trait locus analysis for cap color. Appl. Environ. Microbiol. 2021, 87, e00953-21. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.Y.; van Peer, A.F.; Sonnenberg, A.S.M.; Zhao, M.W.; Gao, W. Fine mapping and functional analysis of the gene PcTYR, involved in control of cap color of Pleurotus cornucopiae. Appl. Environ. Microbiol. 2022, 88, e02173-21. [Google Scholar] [CrossRef]
- Durstewitz, G.; Polley, A.; Plieske, J.; Luerssen, H.; Graner, E.M.; Wieseke, R.; Ganal, M.W. SNP discovery by amplicon sequencing and multiplex SNP genotyping in the allopolyploid species Brassica Napus. Genome 2010, 53, 948–956. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, Q.; Han, B.; Gong, R.; Jia, S.; Wang, L.; Qiao, X.; Cui, W.; Xu, Y.; Li, Y.; et al. Molecular differentiation of different pathogenic phenotypes of infectious bursal disease viruses by RT-PCR combined with restriction fragment length polymorphism (RFLP) assay. J. Northeast Agric. Univ. (Engl. Ed.) 2019, 26, 37–45. [Google Scholar]
- Xu, J.L.; Wang, Y.; Hou, M.; Li, Q. Progresson detection methods of SNP. Mol. Plant Breed. 2015, 13, 475–482. [Google Scholar]
- Michaels, S.D.; Amasino, R.M. A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 1998, 14, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M.; Neff, J.D.; Chory, J.; Pepper, A.E. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 1998, 14, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, G.; Du, L.; Guo, W.; Wang, J. A novel Pro197Glu substitution in acetolactate synthase (ALS) confers broad-spectrum resistance across ALS inhibitors. Pestic. Biochem. Physiol. 2014, 117, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Krenz, B.; Gerhards, R. Target-site resistance to ALS-inhibiting herbicides in Apera spica-venti populations is conferred by documented and previously unknown mutations. Weed Res. 2011, 51, 294–303. [Google Scholar] [CrossRef]
- Chi, Y.Y.; Gao, P.; Zhu, Z.C.; Luan, F.S.; Li, G.Y.; Yu, P. The QTL analysis of fruit and seed associated traits in watermelon based on CAPS markers. Sci. Agric. Sin. 2017, 50, 1282–1293. [Google Scholar]
- Chen, L.; Zhang, J.F.; Hu, M.L.; Pu, H.M.; Long, W.H.; Gao, J.Q.; Chen, F.; Zhou, X.Y.; Zhang, W.; Chen, S. Development and application of the CAPS marker for herbicide-resistant gene in M342. Jiangsu J. Agric. Sci. 2019, 35, 241–247. [Google Scholar]
- Huang, J.; Tan, Q.; Bao, D.P. Location of important functional genes of Lentinula edodes by using the SNP-CAPS molecular markers. Acta Agriculyurae Shanghai 2015, 31, 13–17. [Google Scholar]
- An, H.; Lee, H.Y.; Shim, D.; Choi, S.H.; Cho, H.; Hyun, T.K.; Jo, I.H.; Chung, J.W. Development of CAPS markers for evaluation of genetic diversity and population structure in the germplasm of button mushroom (Agaricus bisporus). J. Fungi 2021, 7, 375. [Google Scholar] [CrossRef]
- Tao, A.F.; You, Z.Y.; Xu, J.T.; Lin, L.H.; Zhang, L.W.; Qi, J.M.; Fang, P.P. Development and verification of CAPS markers based on SNPs from transcriptome of jute (Corchorus L.). Acta Agron. Sin. 2020, 46, 987–996. [Google Scholar]
- Xu, C. SVM-Based Model for Predicting the Effect of Regulatory SNPs on Gene Expression. Master’s Thesis, Suzhou University, Suzhou, China, 2012. [Google Scholar]
- Chasman, D.; Adams, R.M. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms: Structure-based assessment of amino acid variation 1. J. Mol. Biol. 2001, 307, 683–706. [Google Scholar] [CrossRef] [PubMed]
- Capon, F.; Allen, M.H.; Ameen, M.; Burden, A.D.; Tillman, D.; Barker, J.N.; Trembath, R.C. A synonymous SNP of the corneodesmosin gene leads to increased mRNA stability and demonstrates association with psoriasis across diverse ethnic groups. Hum. Mol. Genet. 2004, 13, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (Synonymous) SNPs: Should we care about them? In Single Nucleotide Polymorphisms: Methods and Protocols, 2nd ed.; Komar, A.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; pp. 23–39. [Google Scholar]
- Song, N.N.; Chai, Z.X.; Zhong, J.C. Research advances on long non-coding RNA. Biotechnol. Bull. 2016, 32, 23–31. [Google Scholar]
- Flynt, A.S.; Greimann, J.C.; Chung, W.J.; Lima, C.D.; Lai, E.C. MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol. Cell 2010, 38, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Chen, S.T.; Li, R.C. Present research on breeding methods of edible fungi and the problems in It as well as the prospects. J. Anhui Agric. Sci. 2012, 40, 5850–5852. [Google Scholar]
- Li, Y.; Wang, J.K.; Qiu, L.J.; Ma, Y.Z.; Li, X.H.; Wan, J.M. Crop molecular breeding in China: Current status and perspectives. Acta Agron. Sin. 2010, 36, 1425–1430. [Google Scholar]
- Wang, Y.Q.; Sun, Z.Q.; Zheng, Z.; Huang, B.Y.; Dong, W.Z.; Tang, F.S.; Zhang, X.Y. Status and prospect of crop molecular marker assisted selection breeding. Jiangsu Agric. Sci. 2018, 46, 6–12. [Google Scholar]
- Lestari, P.; Koh, H. Development of new CAPS/dCAPS and SNAP markers for rice eating quality. Hayati J. Biosci. 2013, 20, 15–23. [Google Scholar] [CrossRef]
- Gupta, P.K.; Roy, J.K.; Prasad, M. Single nucleotide polymorphisms: A new paradigm for molecular marker technology and DNA polymorphism detection with emphasis on their use in plants. Curr. Sci. 2001, 80, 524–535. [Google Scholar]
- Drenkard, E.; Richter, B.G.; Rozen, S.; Stutius, L.M.; Angell, N.A.; Mindrinos, M.; Cho, R.J.; Oefner, P.J.; Davis, R.W.; Ausubel, F.M. A simple procedure for the analysis of single nucleotide polymorphisms facilitates map-based cloning in Arabidopsis. Plant Physiol. 2000, 124, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, Y.P.; Jiang, L.; Xu, C.W.; Lu, J.F.; Xu, M.L. Functional analysis of starch-synthesis genes in determining rice eating and cooking qualities. Mol. Breed. 2006, 18, 277–290. [Google Scholar] [CrossRef]
| Marker | SNP | Primer Sequence (5′-3′) | Restriction Endonuclease |
|---|---|---|---|
| TYR-CAPS-1-1 | 1: −1590 bp T>C | F: GCCGAATAACGAACCAAAATGCGCG R: AGCAGTCGGAGCGTTCAAGTGGTTA | Hpy188 I (TCNGA) |
| TYR-CAPS-1-2 | 1: −1590 bp T>C | F: GCCGAATAACGAACCAAAATGCGCG R: AGCAGTCGGAGCGTTCAAGTGGTTA | Nla IV (GGNNCC) |
| TYR-dCAPS-2 | 2: 1038 bp T>C | F: TCTACATATATAAAGTTTAACTCTGACGACTGGATT R: ACTTTGAGCCGTCTTTGCATTCCTC | Nla III (CATG) |
| TYR-CAPS-3-1 | 3: 1273 bp A>C | F: GTGCACGGATTGATACATTTGATA R: ATATCCGCCAGTCGTATTCATAA | Nla III (CATG) |
| TYR-CAPS-3-2 | 3: 1273 bp A>C | F: GTGCACGGATTGATACATTTGATA R: ATATCCGCCAGTCGTATTCATAA | Mse I (TTAA) |
| TYR-dCAPS-4 | 4: 1303bp G>C | F: ATCAACAACATCAACTACATATATTAATGTCGTCT R: GGGCCTCCATTAGGGTATCGTTCAT | Hpy188 I (TCNGA) |
| Strain Number | Species |
|---|---|
| CCMSSC00328 | Pleurotus ostreatus |
| CCMSSC00329 | Pleurotus ostreatus |
| CCMSSC00363 | Pleurotus sp. |
| CCMSSC00364 | Pleurotus sp. |
| CCMSSC00499 | Pleurotus pulmonarius |
| CCMSSC00630 | Pleurotus sp. |
| CCMSSC03989 | Pleurotus sp. |
| CCMSSC04195 | Pleurotus ostreatus |
| CCMSSC00387 | Pleurotus sp. |
| CCMSSC00322 | Pleurotus ostreatus |
| CCMSSC04976 | Pleurotus sp. |
| CCMSSC04977 | Pleurotus sp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Y.; Huang, C.; Chen, Q.; Gao, W. Development of Cleaved Amplified Polymorphic Sequence Marker for Cap Color Identification in Pleurotus cornucopiae. Horticulturae 2023, 9, 1238. https://doi.org/10.3390/horticulturae9111238
Yang Y, Zhang Y, Huang C, Chen Q, Gao W. Development of Cleaved Amplified Polymorphic Sequence Marker for Cap Color Identification in Pleurotus cornucopiae. Horticulturae. 2023; 9(11):1238. https://doi.org/10.3390/horticulturae9111238
Chicago/Turabian StyleYang, Yashu, Yan Zhang, Chenyang Huang, Qiang Chen, and Wei Gao. 2023. "Development of Cleaved Amplified Polymorphic Sequence Marker for Cap Color Identification in Pleurotus cornucopiae" Horticulturae 9, no. 11: 1238. https://doi.org/10.3390/horticulturae9111238






