Quality of Bokashi-Type Biofertilizer Formulations and Its Application in the Production of Vegetables in an Ecological System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Bokashi-Type Biofertilizer Formulations
2.2. Production of Beet and Cabbage Seedlings in a Greenhouse Environment
2.3. Preparation of the Cultivation Area, Experimental Design, and Transplanting of Cultures to the Field
2.4. Preparation of Samples of Leaf Tissue of Crops for Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Characterization of Bokashi-Type Biofertilizer Formulations
3.2. Metagenomic Analysis of Bokashi-Type Biofertilizer Formulations
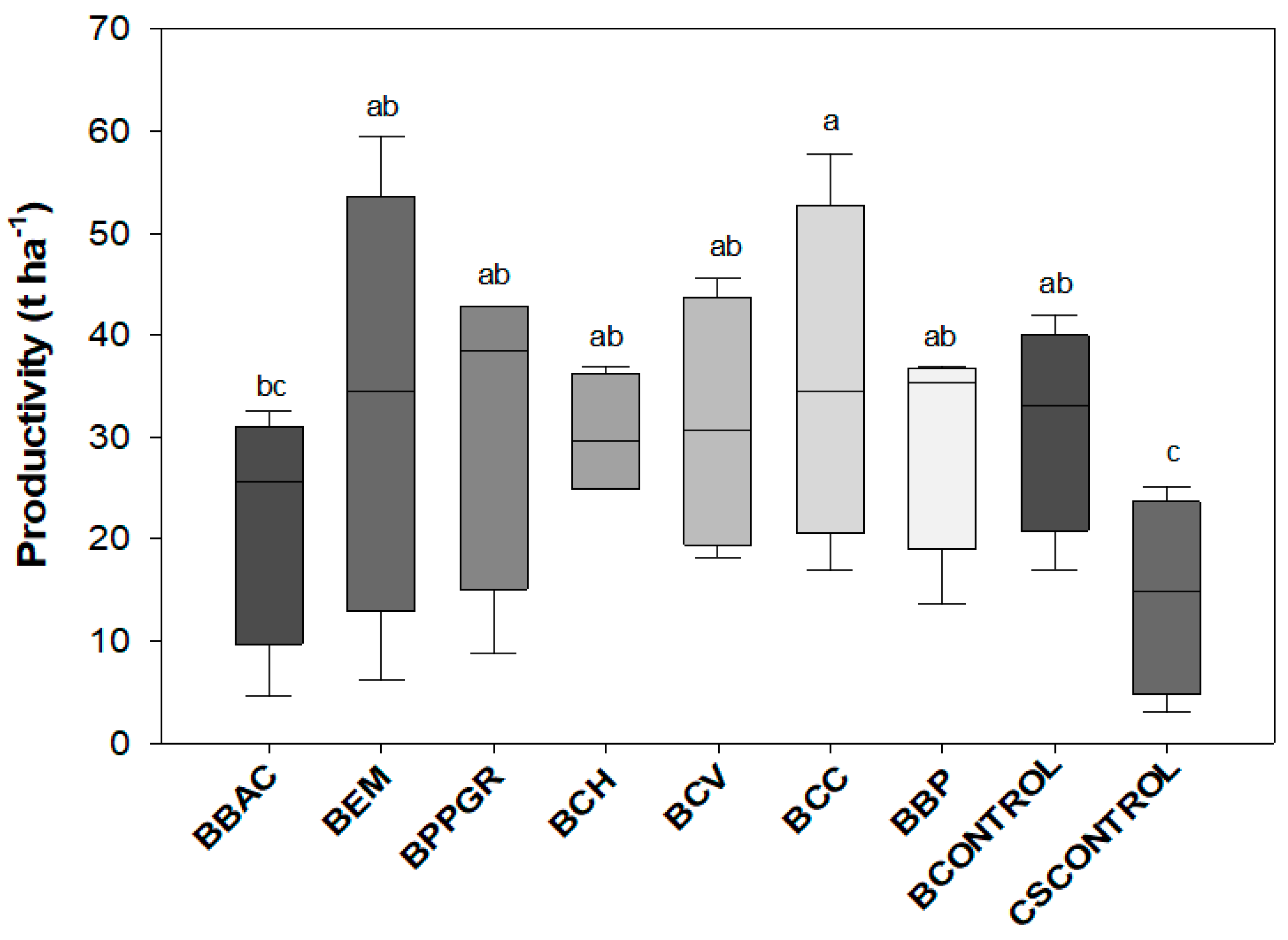
3.3. Agronomic Variables of Beet and Cabbage Crops after Transplanting in the Field
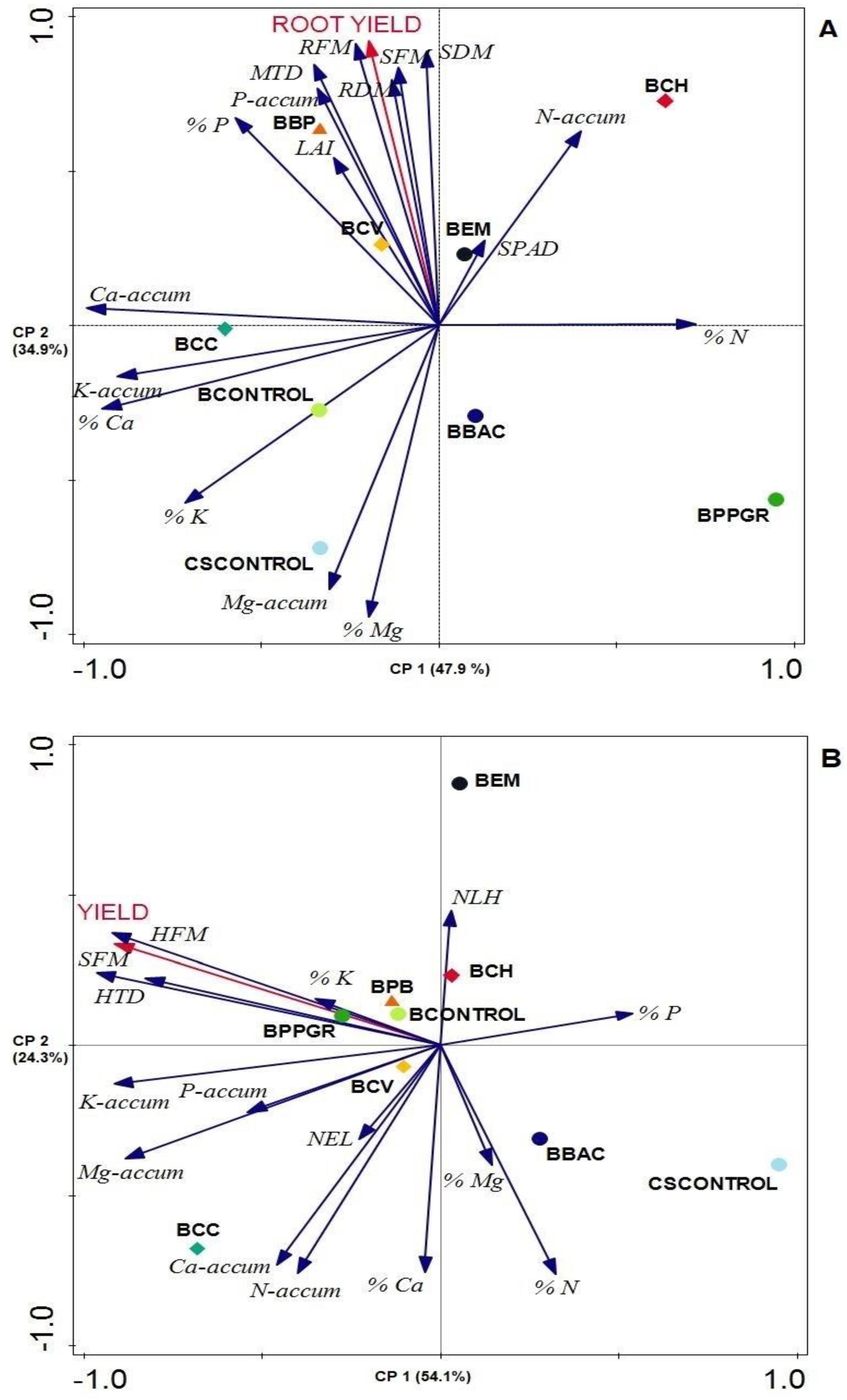
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.-K.; Pineda, A.; Van Der Wurff, A.W.G.; Raaijmakers, C.; Bezemer, T.M. Plant Soil feedback Effects on Growth, Defense and susceptibility to a Soil-Borne Disease in a Cut Flower Crop: Species and Functional Group Effects. Front. Plant Sci. 2017, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Thiessen-Mertens, J.R.; Entz, M.H.; Wonneck, M.D. Review: Redesingning Canadian Prairie cropping systems for profitability, sustainability, and resilience. Can. J. Plant Sci. 2015, 95, 1049–1072. [Google Scholar] [CrossRef]
- Tilman, D.; Blazer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- ANDA—Associação Nacional para Difusão de Adubos. Pesquisa Setorial 2022, Macro Indicadores do Mercado de Fertilizantes No Brasil. Available online: http://anda.org.br/pesquisa_setorial/ (accessed on 15 June 2023).
- Metson, G.S.; Macdonald, G.K.; Habermana, D.; Nesme, T.; Bennett, E.M. Feeding the corn belt: Opportunities for phosphorus recycling in U.S. agriculture. Sci. Total Environ. 2016, 542, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- IBGE–Instituto Brasileiro de Geografia e Estatística. Sidra. Censo Agropecuário Brasileiro. 2017. Available online: https://censoagro2017.ibge.gov.br/ (accessed on 15 June 2023).
- Brainer, M.S.C.P. Produção de hortaliças na área de atuação do BNB. Cad. Setorial ETENE 2021, 180, 1–14. [Google Scholar]
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Al Abboud, M.A.; Ghany, T.A.; Alawlaqi, M.M. Role of biofertilizers in agriculture: A brief review. Mycopath 2013, 11, 95–101. [Google Scholar]
- Rao, K.M.; Singh, K.; Ryingkhun, H.B.K.; Maying, B. Use of bio-fertilizers in vegetable production. Indian Hortic. J. 2014, 4, 73–76. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Biofertilizers Market by Microorganism (Bacillus, Azotobacter, Rhizobium, Azospirillum, VAM, Pseudomonas, and Others), by Type (Phosphate Solubilizers, Nitrogen Fixing, and Others), by Crop Type (Cereals, Pulses & Oilseeds, Fruits & Vegetables, and Others), by Application (Soil Treatment, and Seed Treatment), and by Region—Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022—2028. 2022. Available online: https://www.zionmarketresearch.com/report/biofertilizers-market (accessed on 14 May 2023).
- FAO. Organización de las Naciones Unidas para la Alimentación y la Agricultura. Aboneras Tipo Bocashi. Colección “Buenas Prácticas”; Programa Extraordinario de Apoyo a la Seguridad Alimentaria y Nutricional (Food Facility) FAO/Unión Europea: Ciudad de Guatemala, Guatemala, 2011; p. 13. [Google Scholar]
- Wijayanto, T.; Zulfikar, M.; Tufaila, M.; Alam, S.M.; Zamrun, M.F. Agricultural wastes based-organic fertilizers (Bokashi) improve the growth and yield of soybean (Glycine max (L.) Merrill). Int. J. Agric. Sci. 2016, 1, 27–32. [Google Scholar]
- Lasmini, S.A.; Nasir, B.; Hayati, N.; Edy, N. Improvement of soil quality using bokashi composting and NPK fertilizer to increase shallot yield on dry land. Aust. J. Crop Sci. 2018, 12, 1743–1749. [Google Scholar] [CrossRef]
- Salisu, M.A.; Sulaiman, Z.; Rus, R.C.; Samad, M.Y.A. Water use efficiency, plant growth and vegetative traits of rubber (Hevea brasiliensis) seedlings grown using different growing media and water levels. Aust. J. Crop Sci. 2020, 14, 1497–1505. [Google Scholar] [CrossRef]
- Jusoh, M.L.; Manaf, L.A.; Latiff, A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. J. Environ. Health Sci. Eng. 2013, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Arora, A.; Shah, R.; Singh, A.; Pranaw, K.; Nain, L. Insights into rapid composting of paddy straw augmented with efficient microorganism consortium. Int. J. Recycl. Org. Waste Agric. 2014, 3, 54. [Google Scholar] [CrossRef]
- Silva, J.W.; Rodríguez, W.; Rosas, G. Caracterización física y química de bokashi y lombricompost y su evaluación agronómica en plantas de maíz. Ing. Amazon. 2015, 7, 5–16. [Google Scholar]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.R. How effective are ‘effective microorganisms® (EM)’? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as an amendment and source of nitrogen in sustainable agricultural systems: A Review. J. Soil Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Hata, F.T.; da Silva, D.C.; Yassunaka-Hata, N.N.; Cancian, M.A.d.Q.; Sanches, I.A.; Poças, C.E.P.; Ventura, M.U.; Spinosa, W.A.; Macedo, R.B. Leafy vegetables’ agronomic variables, nitrate, and bioactive compounds have different responses to bokashi, mineral fertilization, and boiled chicken manure. Horticulturae 2023, 9, 194. [Google Scholar] [CrossRef]
- Christel, D.M. The Use of Bokashi as a Soil Fertility Amendment in Organic Spinach Cultivation. Master’s Thesis, University of Vermont, Burlington, VT, USA, 2017; 162p. [Google Scholar]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. Rev. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- de Paula Vicente, N.F.; Marafeli, É.A.M.; de Castro Oliveira, J.A.; Tomita, J.L.C.; Piccoli, R.H. Uma revisão bibliográfica sobre bokashi dos últimos 20 anos. Res. Soc. Dev. 2020, 9, 10. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Olle, M. Review: Bokashi technology as a promising technology for crop production in Europe. J. Hortic. Sci. Biotechnol. 2020, 96, 145–152. [Google Scholar] [CrossRef]
- Silva, N.L.; Lanna, N.B.L.; Cardoso, A.I.I. Doses de Bokashi em cobertura na produção de beterraba. Rev. Agric. Neotrop. 2018, 5, 28–34. [Google Scholar] [CrossRef]
- Xavier, M.C.G.; Santos, C.A.; Costa, E.S.P.; Carmo, M.G.F. Produtividade de repolho em função de doses de bokashi. Rev. De Agric. Neotrop. 2019, 6, 17–22. [Google Scholar] [CrossRef]
- França, F.C.T.; da Silva, E.C.; Pedrosa, M.W.; de Almeida Carlos, L. Adubos orgânicos no cultivo e nutrição mineral de tomateiro. Ambiência 2017, 13, 235–244. [Google Scholar]
- Goulart RG, T.; dos Santos, C.A.; de Oliveira, C.M.; Costa ES, P.; de Oliveira, F.A.; de Andrade, N.F.; do Carmo MG, F. Desempenho agronômico de cultivares de alface sob adubação orgânica em Seropédica—RJ. Rev. Bras. De Agropecuária Sustentável 2018, 8, 66–72. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Sousa, V.; Fregonezi, G.A.F. Low-cost organic fertilizations and bioactivator for arugula-radish intercropping. Emir. J. Food Agric. 2019, 31, 773–778. [Google Scholar] [CrossRef]
- Lima, C.E.P.; Fontenelle, M.R.; Silva, L.R.B.; Soares, D.C.; Moita, A.W.; Zandonadi, D.B.; Souza, R.B.; Lopes, C.A. Short-term changes in fertility attributes and soil organic matter caused by the addition of EM Bokashis in two tropical soils. Int. J. Agron. 2015, 2015, 754298. [Google Scholar] [CrossRef]
- Quiroz, M.; Flores, F. Nitrogen availability, maturity and stability of bokashi-type fertilizers elaborated with different feedstocks of animal origin. Arch. Agron. Soil Sci. 2018, 65, 867–875. [Google Scholar] [CrossRef]
- Wrege, M.S.; Steinmetz, S.; Junior, C.R.; Almeida, I.R. Atlas Climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul; Brasília DF Embrapa: Pelotas, Brazil, 2012. [Google Scholar]
- Bonfim, F.P.G.; Honório, I.C.G.; Reis, I.L.; Pereira, A.D.J.; Souza, D.D.B. Caderno dos Microrganismos Eficientes (EM): Instruções Práticas Sobre o uso Ecológico e Social do EM; Universidade Federal de Viçosa, Departamento de Fitotecnia: Viçosa, Brazil, 2011. [Google Scholar]
- Breitwieser, F.P.; Lu, J.; Salzberg, S.L. A review of methods and databases for metagenomic classification and assembly. Brief Bioinform. 2017, 20, 1125–1136. [Google Scholar] [CrossRef]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The metagenomics RAST server—A public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G. Sistema Brasileiro de Classificação de Solos; EMBRAPA: Brasilia, Brazil, 2018; p. 356. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análise de solo Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul, (Boletim Técnico, 5): Porto Alegre, Brazil, 1995. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Laviola, B.G.; Dias, L.A.S. Teor e acúmulo de nutrientes em folhas e frutos de pinhão-manso. Rev. Bras. De Ciência Do Solo 2008, 32, 1969–1975. [Google Scholar] [CrossRef]
- Leblanc, A.; Cerrato, M.; Vélex, L. Comparación del contenido de nutrientes de Bocashis elaborados con desechos de fincas del trópico húmedo de Costa Rica. Tierra Trop. 2005, 2, 149–159. [Google Scholar]
- Leblanc, H.; Cerrato, M.; Miranda, A.; Valle, G.Y. Determinación de la calidad de abonos orgánicos a través de bioensayos. Tierra Trop. 2007, 3, 97–107. [Google Scholar]
- Trani, P.E.; Terra, M.M.; Tecchio, M.A.; Teixeira LA, J.; Hanasiro, J. Adubação Orgânica de Hortaliças e Frutíferas; Instituto Agronômico de Campinas: Campinas, Brazil, 2013. Available online: http://www.iac.sp.gov.br/imagem_informacoestecnologicas/83.pdf (accessed on 8 June 2023).
- Turinek, M. Biodynamic soil fertility management in fruit crops. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 28; pp. 393–400. [Google Scholar]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2013; p. 954. [Google Scholar]
- Curi, N.; Ker, J.C.; Novais, R.F.; Vidal-Torrado; Schaefer, C.E.G.R. Pedologia: Solos dos Biomas Brasileiros; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2017. [Google Scholar]
- Garcia-Gómez, A.; Bernal, M.P.; Roig, A. Organic matter fractions involved in degradation and humification processes during composting. Compost. Sci. Util. 2005, 13, 127–135. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Guerrero Lopez, F.; Villas Boas, R.L.; Silva, R.B. Transformação da matéria orgânica em substâncias húmicas durante a compostagem de resíduos vegetais. Rev. Bras. De Agroecol. 2009, 4, 59–66. [Google Scholar]
- Iglesias-Jimenez, E.; Perez-Garcia, V. Determination or maturity indexes for city refuse composts. Agric. Ecosyst. Environ. 1992, 38, 331–343. [Google Scholar] [CrossRef]
- Jodice, R. Parametri chimici e biologici per la valutazione della qualità del compost. In Proceedings of the COMPOST Production and Use International Symposium, S. Michelle all’Adige, Italy, 20–23 June 1989; Volume 20–23, pp. 363–384. [Google Scholar]
- Bernal, M.P.; Paredes, C.; Sánchez-Monedero, M.A.; Cegarra, J. Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour. Technol. 1998, 63, 91–99. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa Nº 61, 8 de julho de 2020. Estabelece as Regras sobre Definições, Exigências, Especificações, Garantias, Tolerâncias, Registro, Embalagem e Rotulagem dos Fertilizantes Orgânicos e dos Biofertilizantes, Destinados à Agricultura. 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-61-de-8-7-2020-organicos-e-biofertilizantes-dou-15-7-20.pdf (accessed on 15 June 2023).
- Kjellberg, K. Supervision of the Sanitary Quality of Composting in the Nordic Countries; TemaNord—Nordic Council of Ministers: Copenhagen, Denmark, 2002; Volume 567. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Malik, A. Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Maki, Y.; Soejima, H.; Kitamura, T.; Sugiyama, T.; Sato, T.; Watahiki, M.K.; Yamaguchi, J. 3-Phenyllactic acid, a root-promoting substance isolated from Bokashi fertilizer, exhibits synergistic effects with tryptophan. Plant Biotechnol. 2021, 38, 9–16. [Google Scholar] [CrossRef]
- Siqueira, A.P.P.; Siqueira, M.F.B. Bokashi: Adubo Orgânico Fermentado; Programa Rio Rural: Niterói, Brazil, 2013; Volume 40, pp. 1–16. [Google Scholar]
- Hata, F.T.; Spagnuolo, F.A.; de Paula, M.T.; Moreira, A.A.; Ventura, M.U.; Fregonezi, G.A.F.; de Oliveira, A.L.M. Bokashi compost and biofertilizer increase lettuce agronomic variables in protected cultivation and indicates substrate microbiological changes. Emir. J. Food Agric. 2020, 32, 640–646. [Google Scholar] [CrossRef]
- Scotton, J.C.; da Silva Pereira, J.; Campos, A.A.B.; Pinto, D.F.P.; Costa, W.L.F.; Homma, S.K. Different sources of inoculum to the bokashi provides distinct effects on the soil quality. Braz. J. Sustain. Agric. 2017, 7, 32. [Google Scholar] [CrossRef]
- Santos, F.T.; Ludwig, F.; Costa, L.A.M.; Costa, M.S.S.M. Nutrition and growth of potted gerbera according to mineral and organic fertilizer. Rev. Bras. De Hortic. Ornam. 2015, 21, 251–258. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Effects of a microbial inoculant and organic fertilizers on the growth, photosynthesis and yield of sweet corn. J. Crop Prod. 2001, 3, 183–214. [Google Scholar] [CrossRef]
- Xu, H.; Wang, R.; Mridha, A. Effects of organic fertilizers and a microbial inoculant on leaf photosynthesis and fruit yield and quality of tomato plants. J. Crop Prod. 2001, 3, 173–182. [Google Scholar] [CrossRef]
- Zaman, A.; Ahmed, M.; Gogoi, P. Effect of bokashi on plant growth, yield and essential oil quantity and quality in patchouli (Pogostemon cablin Benth.). Biosci. Biotech. Res. Asia 2010, 7, 383–387. [Google Scholar]
- Murillo-Amador, B.; Morales-Prado, L.E.; Troyo-Diéguez, E.; Córdoba-Matson, M.V.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; Nieto-Garibay, A. Changing environmental conditions and applying organic fertilizers in Origanum vulgare L. Front. Plant Sci. 2015, 6, 549. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, G.P. Adubação Orgânica e Biodinâmica na Produção de Chicória (Cichorium endivia) e de Beterraba (Beta vulgaris), em Sucessão. Master’s Thesis, UNESP, Botucatu, Brazil, 2009. [Google Scholar]
- Ferreira, S.; Assis, R.P.; Souza, R.J.; Gomes, L.A.A. Avaliação da adição de bokashi no cultivo de brócolis Lord Summer. Rev. Agrogeoambiental 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Saiter, O.; Oliveira, L.A.A.; Oliveira, E.A.G.; Araujo, D.B. Efeito do Adubo Orgânico Fermentado Bokashi no Desempenho Agronômico do Brócolis Americano; Program Rio Rural: Teresópolis, Brazil, 2016. [Google Scholar]
- Silva, N.L.; Lanna, N.B.L.; Cardoso, A.I.I. Produção de beterraba em função de doses de torta de mamona em cobertura. Hortic. Bras. 2016, 34, 416–421. [Google Scholar] [CrossRef]
- Condé, F.; Oliveira, D.M.; Oliveira, J.E.Z. Incidência e severidade de hérnia das crucíferas (Plasmodiophora brassicae W.) em repolho (Brassica oleracea L. var. capitata) em solo tratado com biofertilizante tipo bokashi. Ciência E Nat. 2017, 39, 7–15. [Google Scholar] [CrossRef]
- Ferreira, S.; Souza, R.J.; Gomes, L.A.A. Produtividade de brócolis de verão com diferentes doses de bokashi. Rev. Agrogeoambiental 2013, 5, 31–38. [Google Scholar] [CrossRef]
- Piva, R.; Botelho, R.V.; de Lima PC, G.; Rambolà, A.D. Desenvolvimento, fisiologia e ocorrência de míldio em videiras cv. BRS Margot tratadas com preparados biodinâmicos. Rev. De Ciências Agrárias 2019, 42, 472–482. [Google Scholar]
- Leite, A.B.; Polli, H.Q. Agricultura orgânica no Brasil com enfoque na agricultura biodinâmica. Interface Tecnológica 2020, 17, 417–430. [Google Scholar] [CrossRef]



| Clay | pH H2O | SMP | P | K | O.M | Al | Ca | Mg | H + Al |
|---|---|---|---|---|---|---|---|---|---|
| % | ------- mg dm−3---- | % | ------------ cmolc dm−3-------- | ||||||
| 0.00–0.20 m | |||||||||
| 36 | 5.53 | 6.19 | 29.35 | 146.5 | 2.9 | 0 | 11.92 | 5.51 | 3.51 |
| Treatments | FA | HA | DM | TOC | OM | N | P | K | Ca | Mg | Na | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ---------------------------------------------- % ------------------------------------------------------- | ||||||||||||
| BBAC | 3.79 | 4.93 | 83.8 | 15.19 | 33.41 | 1.43 | 0.74 | 1.86 | 3.68 | 1.76 | 0.31 | 0.36 |
| BEM | 10.24 | 5.85 | 85.5 | 14.14 | 31.12 | 1.32 | 0.72 | 1.89 | 3.33 | 1.61 | 0.31 | 0.36 |
| BPPGR | 36.43 | 3.72 | 83.9 | 12.36 | 27.19 | 1.43 | 0.84 | 1.97 | 4.28 | 2.02 | 0.34 | 0.39 |
| BCH | 4.9 | 4.52 | 85.2 | 19.68 | 43.31 | 1.44 | 0.91 | 2.03 | 4.19 | 2.01 | 0.36 | 0.42 |
| BCV | 3.56 | 3.56 | 85.7 | 16.76 | 36.86 | 1.34 | 0.77 | 1.91 | 3.2 | 1.7 | 0.33 | 0.37 |
| BCC | 4.46 | 5.45 | 87.3 | 18.52 | 40.75 | 1.38 | 0.74 | 1.89 | 3.58 | 1.74 | 0.33 | 0.36 |
| BBP | 5.74 | 3.98 | 84.2 | 13.71 | 30.17 | 1.31 | 0.69 | 1.82 | 3.63 | 2.05 | 0.3 | 0.34 |
| BCONTROL | 6.77 | 3.21 | 84.4 | 18.2 | 40.03 | 1.31 | 0.76 | 1.86 | 3.51 | 1.71 | 0.32 | 0.36 |
| Mean | 9.5 | 4.4 | 85 | 16.1 | 35.4 | 1.4 | 0.8 | 1.9 | 3.7 | 1.8 | 0.3 | 0.4 |
| CV (%) | 116.9 | 21.4 | 1.3 | 16.2 | 4.1 | 4.1 | 9.2 | 3.5 | 10.3 | 9.4 | 5.9 | 6.2 |
| Treatments | B | Cu | Mn | Zn | Fe | Ds | pH H2O | pH CaCl2 | Ec H2O | Ec CaCl2 | C/N | |
| -------------- mg kg−1------------- % g cm−3 ------- ms−1 ----- | ||||||||||||
| BBAC | 42.1 | 437.1 | 552.1 | 284.8 | 5.79 | 0.45 | 8.33 | 8.16 | 2.9 | 3.67 | 10.6 | |
| BEM | 40.8 | 415.8 | 578.9 | 273.3 | 5.82 | 0.49 | 8.32 | 8.14 | 2.92 | 3.62 | 10.7 | |
| BPPGR | 38.8 | 462.8 | 610.4 | 308.8 | 5.31 | 0.48 | 8.35 | 8.16 | 2.58 | 3.6 | 8.6 | |
| BCH | 39.9 | 489.7 | 651.2 | 327.3 | 5.15 | 0.49 | 8.24 | 8.12 | 2.97 | 3.64 | 13.6 | |
| BCV | 41.7 | 427.1 | 565.4 | 288.3 | 5.43 | 0.43 | 8.3 | 8.14 | 2.82 | 3.55 | 12.5 | |
| BCC | 38.1 | 437.5 | 561.3 | 288.2 | 5.72 | 0.54 | 8.27 | 8.15 | 2.97 | 3.69 | 13.4 | |
| BBP | 39.5 | 402.7 | 560 | 269.2 | 6.66 | 0.44 | 8.28 | 8.11 | 2.82 | 3.45 | 10.4 | |
| BCONTROL | 40.7 | 425.4 | 574.1 | 281.3 | 5.2 | 0.43 | 8.27 | 8.14 | 2.83 | 3.62 | 13.4 | |
| Mean | 40.2 | 437.3 | 581.7 | 290.2 | 5.6 | 0.5 | 8.3 | 8.1 | 2.9 | 3.6 | 11.7 | |
| CV (%) | 3.5 | 6.2 | 5.7 | 6.6 | 8.7 | 8.7 | 0.4 | 0.2 | 4.4 | 2.1 | 16.1 | |
| Treatments | Reads | Genera | Species | Bacteria % | Eukaryota % | Other % * | ||
|---|---|---|---|---|---|---|---|---|
| BBAC | 197,097 | 1187 | 4038 | 99.63 | 0.15 | 0.23 | ||
| BEM | 230,084 | 1296 | 4872 | 99.61 | 0.16 | 0.22 | ||
| BPPGR | 261,919 | 1403 | 5169 | 99.62 | 0.23 | 0.15 | ||
| BCH | 327,408 | 1550 | 6024 | 99.65 | 0.13 | 0.22 | ||
| BCV | 285,633 | 1422 | 5360 | 99.66 | 0.17 | 0.17 | ||
| BCC | 353,064 | 1587 | 6359 | 99.67 | 0.13 | 0.19 | ||
| BBP | 458,348 | 1728 | 7286 | 99.63 | 0.17 | 0.19 | ||
| BCONTROL | 348,092 | 1507 | 6081 | 99.68 | 0.17 | 0.15 | ||
| Treatments | BBAC | BEM | BPPGR | BCH | BCV | BCC | BBP | BCONTROL |
| Bacteria (genus) 1 | --------------------------- % DNA g−1 --------------------------- | |||||||
| Marinobacter sp. | 22.72 | 21.01 | 17.91 | 22.2 | 27.3 | 23.88 | 21.08 | 25.37 |
| Halomonas sp. | 9.09 | 9.96 | 7.5 | 7.63 | 7.3 | 8.88 | 8.03 | 9.19 |
| Galbibacter sp. | 7.19 | 2.86 | 11.58 | 9.71 | 5.95 | 7.89 | 7.97 | 4.44 |
| Alcanivorax sp. | 6.12 | 7.24 | 7.28 | 6.65 | 6.01 | 6.07 | 5.95 | 7.28 |
| Azospirillum sp. | 1.42 | 2.44 | 1.59 | 1.67 | 1.79 | 1.72 | 1.51 | 2.2 |
| Bacillus sp. | 2.88 | 2.19 | 2.12 | 2.14 | 2.06 | 2.45 | 2.32 | 2.33 |
| Bradyhizobium sp. | 0.14 | 0.18 | 0.26 | 0.15 | 0.39 | 0.12 | 0.12 | 0.26 |
| Burkholderia sp. | 0.12 | 0.15 | 0.12 | 0.12 | 0.13 | 0.12 | 0.11 | 0.13 |
| Cupriavidus sp. | 0.06 | 0.07 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Curtobacterium sp. | 0.08 | 0.15 | 0.08 | 0.08 | 0.11 | 0.09 | 0.08 | 0.11 |
| Paenibacillus sp. | 0.13 | 0.22 | 0.30 | 0.17 | 0.1 | 0.16 | 0.21 | 0.1 |
| Paraburkholderia sp. | 0.08 | 0.1 | 0.08 | 0.08 | 0.08 | 0.09 | 0.07 | 0.09 |
| Pseudomonas sp. | 2.2 | 2.96 | 2.2 | 2.32 | 2.45 | 2.27 | 2.02 | 2.31 |
| Rhizobium sp. | 0.12 | 0.17 | 0.12 | 0.12 | 0.11 | 0.13 | 0.11 | 0.1 |
| Streptomyces sp. | 2.08 | 2.87 | 2 | 2.12 | 2.4 | 2.03 | 1.81 | 2.35 |
| Idiomarina sp. | 1.49 | 1.46 | 2.14 | 1.85 | 0.82 | 1.33 | 1.3 | 0.69 |
| Brachybacterium sp. | 1.48 | 1.92 | 1.04 | 1.05 | 1.59 | 1.38 | 1.32 | 1.95 |
| Georgina sp. | 1.47 | 2.24 | 1.2 | 1.24 | 1.82 | 1.59 | 1.4 | 2.02 |
| Others | 41.12 | 41.82 | 42.44 | 40.66 | 39.56 | 39.76 | 44.59 | 39.02 |
| Treatments | SFM | SDM | RFM | RDM | LAI | RD | Class |
|---|---|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (cm2) | (mm) | ||
| BBAC | 157.5 b | 80.0 ns | 217.0 bcd | 16.3 cd | 1355.3 b | 66.3 cd | 2A |
| BEM | 177.3 ab | 95.7 | 217.5 bcd | 17.7 bc | 1474.6 ab | 69.2 bcd | 2A |
| BPPGR | 169.1 ab | 85.9 | 167.0 d | 13.2 d | 1516.1 ab | 64.1 d | 2A |
| BCH | 198.5 ab | 99.6 | 271.3 ab | 21.1 ab | 1620.0 ab | 74.4 ab | 2A |
| BCV | 209.2 a | 97.7 | 253.8 ab | 14.7 cd | 1646.1 ab | 72.7 abc | 2A |
| BCC | 187.5 ab | 93.9 | 218.6 bcd | 17.0 bcd | 1958.6 a | 69.7 abcd | 2A |
| BBP | 198.3 ab | 102.7 | 282.0 a | 22.4 a | 1763.5 ab | 76.3 a | 2A |
| BCONTROL | 169.3 ab | 82.1 | 227.9 abc | 18.1 abc | 1421.9 b | 70.9 abc | 2A |
| CSCONTROL | 168.3 ab | 83.1 | 175.3 cd | 12.5 d | 1402.8 b | 68.0 bcd | 2A |
| CV (%) | 17.97 | 17.14 | 17.81 | 18.09 | 22.64 | 6.59 | - |
| Treatments | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| ---------------- % --------------- | |||||
| BBAC | 5.3 ab | 1.72 ns | 5.89 abc | 0.27 a | 0.17 ab |
| BEM | 5.29 ab | 1.83 | 4.40 c | 0.26 ab | 0.13 ab |
| BPPGR | 5.94 a | 1.71 | 4.98 bc | 0.13 c | 0.19 a |
| BCH | 5.45 ab | 1.84 | 4.37 c | 0.15 bc | 0.09 b |
| BCV | 5.15 b | 1.94 | 5.51 abc | 0.27 a | 0.13 ab |
| BCC | 5.26 b | 1.86 | 6.37 ab | 0.35 a | 0.16 ab |
| BBP | 5.0 bc | 2.0 | 5.61 abc | 0.27 a | 0.13 ab |
| BCONTROL | 4.37 c | 1.86 | 6.43 ab | 0.35 a | 0.21 a |
| CSCONTROL | 5.01 bc | 1.8 | 6.96 a | 0.34 a | 0.22 a |
| Ref. Values 1 (%) | 3.0–5.0 | 0.2–0.4 | 2.0–4.0 | 2.5–3.5 | 0.3–0.8 |
| CV (%) | 8.68 | 15.54 | 21.21 | 29.86 | 40.91 |
| Treatments | N accumulated | P accumulated | K accumulated | Ca accumulated | Mg accumulated |
| --------------- mg --------------- | |||||
| BBAC | 4252.5 ab | 1372.3 b | 4753.5 ns | 224.31 abc | 139.34 ns |
| BEM | 5092.9 ab | 1790.6 ab | 4229.6 | 247.72 ab | 127.31 |
| BPPGR | 5115.2 ab | 1473.6 ab | 4284.4 | 114.77 c | 172.85 |
| BCH | 5492.1 a | 1810.5 ab | 4346.3 | 150.38 bc | 103.09 |
| BCV | 5016.1 ab | 1913.0 ab | 5423.5 | 262.77 ab | 128.88 |
| BCC | 4974.6 ab | 1751.9 ab | 6114.1 | 352.72 a | 167.17 |
| BPB | 5215.4 a | 2083.8 a | 5726.5 | 283.37 a | 136.3 |
| BCONTROL | 3612.1 b | 1564.1 ab | 5352.2 | 279.58 a | 164.81 |
| CSCONTROL | 4226.0 ab | 1643.5 ab | 6273.3 | 278.01 ab | 182.99 |
| CV (%) | 22.52 | 35.31 | 32.56 | 36.17 | 45.9 |
| Treatments | SFM | NEL | HTD | NLH | HFM |
|---|---|---|---|---|---|
| (g) | (cm) | (g) | |||
| BBAC | 1609.8 bc | 13 b | 17.8 c | 25 ab | 884.5 bc |
| BEM | 2123.3 ab | 13 b | 20.6 abc | 30 a | 1343.2 ab |
| BPPGR | 2162.4 ab | 15 ab | 20.5 abc | 23 b | 1284.0 ab |
| BCH | 2010.5 ab | 17 a | 20.8 abc | 26 ab | 1208.9 ab |
| BCV | 2067.8 ab | 14 ab | 20.6 abc | 26 ab | 1250.3 ab |
| BCC | 2494.7 a | 16 ab | 22.1 a | 26 ab | 1433.4 a |
| BBP | 2038.0 ab | 14 ab | 21.5 a | 26 ab | 1213.7 ab |
| BCONTROL | 2101.3 ab | 15 ab | 21.3 ab | 31 a | 1250.8 ab |
| CSCONTROL | 1246.3 c | 15 ab | 18.2 bc | 26 ab | 587.1 c |
| CV (%) | 23.95 | 15.21 | 10.66 | 15.29 | 24.5 |
| Treatments | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| --------------- % --------------- | |||||
| BBAC | 3.5 ab | 1.3 c | 6.1 a | 0.40 ab | 0.29 ab |
| BEM | 2.5 c | 1.5 bc | 5.1 ab | 0.25 cd | 0.26 ab |
| BPPGR | 3.1 bc | 1.7 ab | 5.9 a | 0.27 cd | 0.26 ab |
| BCH | 3.6 ab | 1.4 c | 5.0 ab | 0.23 d | 0.25 b |
| BCV | 3.4 ab | 1.5 bc | 4.8 ab | 0.32 abcd | 0.28 ab |
| BCC | 3.6 ab | 1.3 c | 4.9 ab | 0.40 a | 0.28 ab |
| BBP | 2.7 c | 1.4 c | 5.6 ab | 0.36 abc | 0.30 a |
| BCONTROL | 3.5 ab | 1.4 c | 5.1 ab | 0.27 bcd | 0.26 ab |
| CSCONTROL | 4.0 a | 1.8 a | 4.3 b | 0.34 abcd | 0.28 ab |
| Ref. Values 1 (%) | 4.0–4.5 | 0.4–0.5 | 2.5–2.7 | 0.75 | 0.25 |
| CV (%) | 13.34 | 16.63 | 16.42 | 26.99 | 12.37 |
| Treatments | N accumulated | P accumulated | K accumulated | Ca accumulated | Mg accumulated |
| --------------- mg−1 --------------- | |||||
| BBAC | 263.4 b | 104.1 ns | 428.1 ab | 28.8 ab | 21.1 ab |
| BEM | 200.1 b | 118.8 | 388.6 ab | 18.4 b | 19.9 b |
| BPPGR | 278.8 ab | 155.1 | 542.1 a | 22.9 b | 22.8 ab |
| BCH | 289.9 ab | 119.7 | 416.6 ab | 18.8 b | 20.9 ab |
| BCV | 285.2 ab | 125.4 | 400.1 ab | 27.3 ab | 23.1 ab |
| BCC | 405.8 a | 147.8 | 558.2 a | 36.1 a | 28.2 a |
| BBP | 221.2 b | 120.1 | 450.5 ab | 28.6 ab | 24.6 ab |
| BCONTROL | 306.3 ab | 124.3 | 451.9 ab | 21.8 b | 22.1 ab |
| CSCONTROL | 275.5 ab | 124.4 | 298.5 b | 21.6 b | 18.4 b |
| CV (%) | 34.14 | 42.8 | 29.25 | 33.49 | 25.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruker, G.; Guidi, E.S.; Santos, J.M.d.S.d.; Mafra, Á.L.; Almeida, J.A.d. Quality of Bokashi-Type Biofertilizer Formulations and Its Application in the Production of Vegetables in an Ecological System. Horticulturae 2023, 9, 1314. https://doi.org/10.3390/horticulturae9121314
Kruker G, Guidi ES, Santos JMdSd, Mafra ÁL, Almeida JAd. Quality of Bokashi-Type Biofertilizer Formulations and Its Application in the Production of Vegetables in an Ecological System. Horticulturae. 2023; 9(12):1314. https://doi.org/10.3390/horticulturae9121314
Chicago/Turabian StyleKruker, Gregory, Eduardo Schabatoski Guidi, Juliano Muniz da Silva dos Santos, Álvaro Luiz Mafra, and Jaime Antonio de Almeida. 2023. "Quality of Bokashi-Type Biofertilizer Formulations and Its Application in the Production of Vegetables in an Ecological System" Horticulturae 9, no. 12: 1314. https://doi.org/10.3390/horticulturae9121314






