Arbuscular Mycorrhizal Fungi, Especially Rhizophagus intraradices as a Biostimulant, Improve Plant Growth and Root Columbin Levels in Tinospora sagittata
Abstract
:1. Introduction
2. Materials and Methods
2.1. AMF Inoculum Preparation
2.2. Plant Culture
2.3. Experimental Design
2.4. Determination of Plant Growth and Root Mycorrhizal Colonization Rate
2.5. Determination of Leaf Photosynthetic Properties
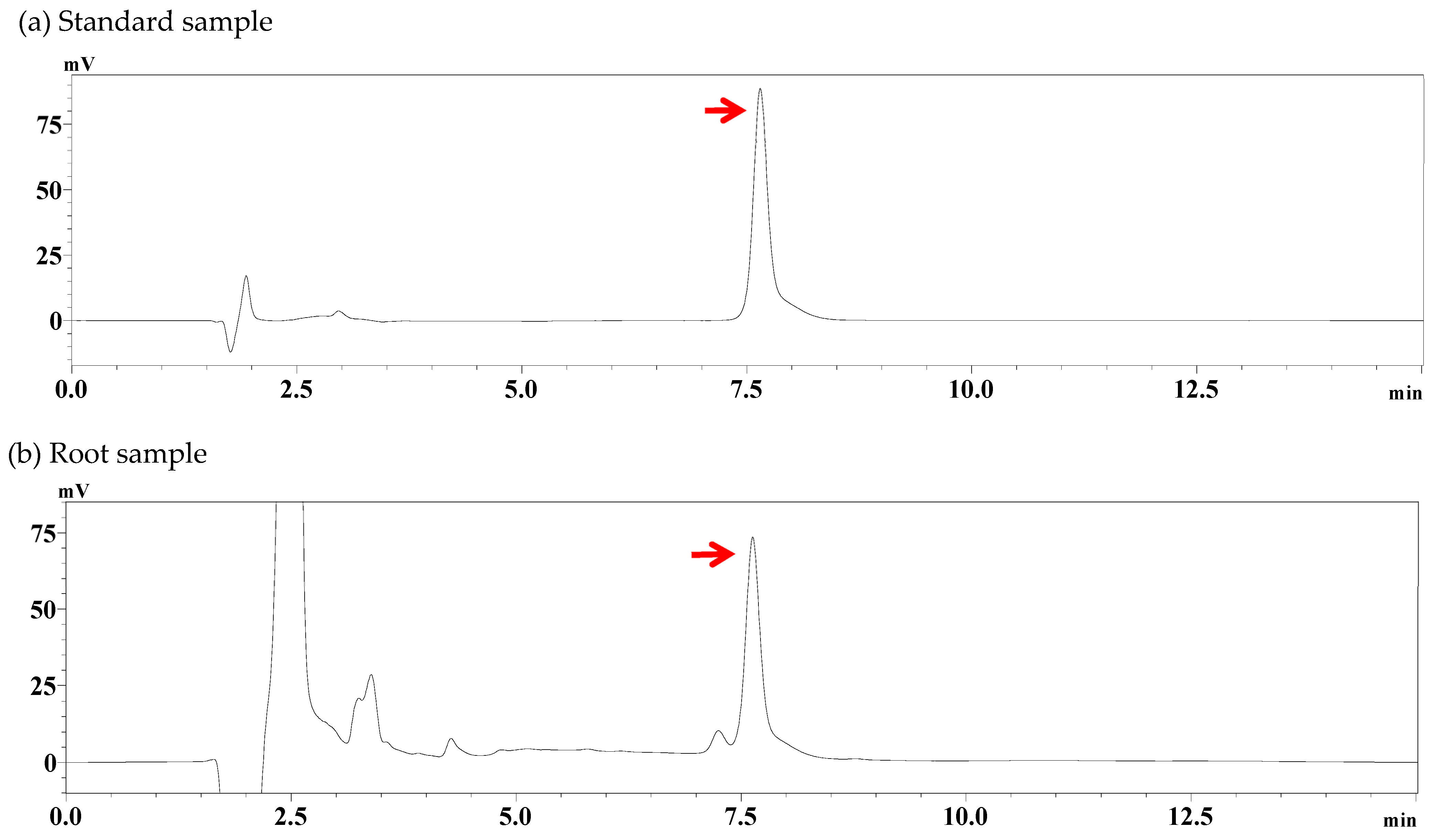
2.6. Determination of Columbin Levels in Roots
2.7. Statistical Analysis
3. Results
3.1. Changes in Mycorrhizal Status
3.2. Changes in Plant Growth Behavior
3.3. Changes in Root Morphological Variables
3.4. Changes in Chlorophyll Index, Nitrogen Balance Index, and Gas Exchange in Leaves
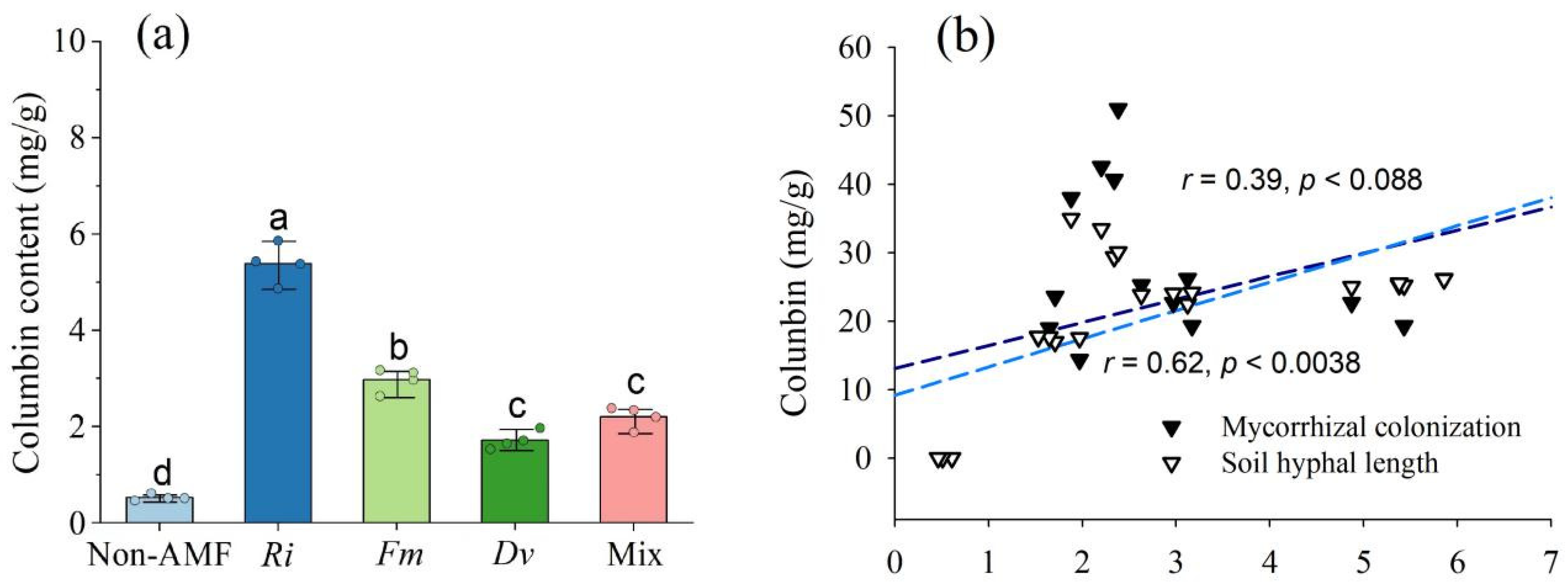
3.5. Changes in Columbin Concentrations in Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Ma, H.; Hu, S.; Xu, H.; Yang, B.; Yang, Q.; Xue, Y.; Cheng, L.; Jiang, J.; Zhang, J.; et al. Clerodane-type diterpenoids from tuberous roots of Tinospora sagittata (Oliv.) Gagnep. Fitoterapia 2016, 110, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, A.; Gupta, P.; Kailash, C.; Mukesh, S.; Rakesh, M.; Anuradha, D. Evaluation of antil-eishmanial potential of Tinospora sinensis against experi-mental visceral leishmaniasis. Parasitol. Res. 2008, 102, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Dai, Y.; Huang, X.Z.; Zhu, Y.; Dai, J.H.; Liu, X.F.; Wang, J. Chemical constituents from roots of Tinospora sagittata var. yunnanensis. Chin. Tradit. Herb. Drugs 2010, 41, 689–692. [Google Scholar]
- Atta-ur-Rahman; Ahmad, S. A furanoid diterpene, 10 α-hydroxycolumbin, from Tinospora malabarica. Phytochemistry 1988, 27, 1882–1884. [Google Scholar] [CrossRef]
- Lu, L.J.; Wu, Q.H.; Zhou, Y.F.; Ren, C.X.; Zhu, X.L.; Yan, Y.H.; Wei, M.; Liu, S.B.; Zhou, T.; Pei, J. Research progress on chemical composition and pharmacological effects of Tinosporae radix and predictive analysis on quality marker. Chin. Tradit. Herb. Drugs 2022, 53, 6245–6257. [Google Scholar] [CrossRef]
- Li, H.B.; Hu, J.; Chen, J.C.; Qin, M.H. Chemical constituents of Tinospora craveniana. Nat. Prod. Res. Dev. 2005, 17, 3. [Google Scholar]
- Yuan, S.H.; Fang, J.M.; Tu, Q.M.; Tu, Z.L.; Nie, G.; Chen, L. Chemical constituents and antimicrobial effect of Tinospora sagittata (Oliv). Chin. Pharm. J. 2010, 45, 733–735. [Google Scholar]
- Zhou, X.X.; Shu, Y.; Shu, S.; Wang, C.H.; Yang, X.; Zhan, Z.L. Research progress on the chemical constituents and pharmacological effects of Tinosporae radix and other medicinal plants in the same genus. Mod. Chin. Med. 2023, 25, 1135–1146. [Google Scholar] [CrossRef]
- Liu, X.H.; Hu, Z.L.; Shi, Q.S.; Zeng, H.W.; Shen, Y.H.; Jin, H.Z.; Zhang, W.D. Anti-inflammatory and anti-nociceptive activities of compounds from Tinospora sagittata (Oliv.) Gagnep. Arch. Pharm. Res. 2010, 33, 981. [Google Scholar] [CrossRef]
- Li, R.W.; David, L.G.; Myers, S.P.; Leach, D.N. Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol. 2003, 85, 61–67. [Google Scholar] [CrossRef]
- Huang, X.Z.; Cheng, C.M.; Dai, Y.; Fu, G.M.; Guo, J.M.; Yin, Y.; Liang, H. A novel 18-norclerodane diterpenoid from the roots of Tinospora sagittata var. yunnanensis. Molecules 2010, 15, 8360. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M. Theory and technology frontiers of ecological planting of Chinese medicinal materials. J. Jilin Agric. Univ. 2020, 42, 355–363. [Google Scholar] [CrossRef]
- Mahobiya, D.; Kulkarni, P. Biodiversity of arbuscular mycorrhizal (AM) fungi associated with cultivated medicinal plants. Adv. Plant Sci. 2018, 24, 125–128. [Google Scholar]
- Sun, R.T.; Zhang, Z.Z.; Zhou, N.; Srivastava, A.K.; Kuca, K.; Abd-Allah, E.F.; Hashem, A.; Wu, Q.S. A review of the interaction of medicinal plants and arbuscular mycorrhizal fungi in the rhizosphere. Not. Bot. Horti Agrobo. 2021, 49, 12454. [Google Scholar] [CrossRef]
- Zubek, S.; Błaszkowski, J. Medicinal plants as hosts of arbuscular mycorrhizal fungi and dark septate endophytes. Phytochem. Rev. 2009, 8, 571–580. [Google Scholar] [CrossRef]
- Mi, F.Z. Vesicular arbuscular mycorrhizal (VAM) fungi in relation to 8 kinds of medical plants in Shangluo of Shannxi. Shannxi For. Sci. Technol. 2012, 5, 37–39. [Google Scholar]
- Wang, Y.; Zou, Y.N.; Shu, B.; Wu, Q.S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Yan, Q.X.; Li, X.Y.; Xiao, X.F.; Chen, J.Z.; Liu, J.M.; Lin, C.H.; Guan, R.T. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Cinnamomum migao by enhancing physio-biochemical responses. Ecol. Evol. 2022, 12, e9091. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. Bot. 2022, 204, 105089. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, X.; Zhao, R.H.; Yu, J.; Gu, W.; Li, R.; Cao, G.H.; He, S. Effect and mechanism of arbuscular mycorrhizal fungi in herbs. Chin. J. Exp. Trad. Med. Form. 2020, 26, 217–226. [Google Scholar] [CrossRef]
- Huang, J.H.; Tan, J.F.; Jie, H.K.; Zeng, R.S. Effects of inoculating arbuscular mycorrhizal fungi on Artemisia annua growth and its officinal components. Chin. J. Appl. Ecol. 2011, 22, 1443–1449. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.P.; Zhou, X.F.; Jiang, X.L.; Xu, L.J.; Wu, S.L.; Zhao, A.H.; Zhang, X.; Chen, B.D. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.T.; Feng, X.C.; Zhang, Z.Z.; Zhou, N.; Feng, H.D.; Liu, Y.M.; Hashem, A.; Al-Arjani, A.F.; Abd_Allah, E.F.; Wu, Q.S. Root endophytic fungi regulate changes in sugar and medicinal compositions of Polygonum cuspidatum. Front. Plant Sci. 2022, 13, 818909. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.T.; Zhang, Z.Z.; Feng, X.C.; Zhou, N.; Feng, H.D.; Liu, Y.M.; Harsonowati, W.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Endophytic fungi accelerate leaf physiological activity and resveratrol accumulation in Polygonum cuspidatum by up-regulating expression of associated genes. Agronomy 2022, 12, 1220. [Google Scholar] [CrossRef]
- Sun, R.T.; Zhang, Z.Z.; Liu, M.Y.; Feng, X.C.; Zhou, N.; Feng, H.D.; Hashem, A.; Abd-Allah, E.F.; Harsonowati, W.; Wu, Q.S. Arbuscular mycorrhizal fungi and phosphorus supply accelerate main medicinal component production of Polygonum cuspidatum. Front. Microbiol. 2022, 13, 1006140. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Kumar, S.; Khaliq, A.; Pandey, A. Heavy metals and arbuscular mycorrhizal (AM) fungi can alter the yield and chemical composition of volatile oil of sweet basil (Ocimum basilicum L.). Biol. Fert. Soils 2011, 47, 853–861. [Google Scholar] [CrossRef]
- Liu, J.L.; Liu, C.; Dong, Q.; Yao, W.; Wu, C. Effects of Piriformospora indica inoculation on gas exchange, biomass accumulation and contents of secondary metabolites of Ocimum gratissimum. Acta. Agric. Jiangxi 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Yu, Y. Corresponding Analysis on Camptothecin Content and Arbuscular Mycorrhiza Formation in Camptotheca acuminata Seedlings. Master’s Thesis, Northeast Forestry University, Harbin, China, 2010. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Li, H.; Zuo, Y.L.; He, X.L.; Hou, Y.T.; Li, M.; Li, B.K. Plant identity and soil variables shift the colonization and species composition of dark septate endophytes associated with medicinal plants in a northern farmland in China. Appl. Soil. Ecol. 2021, 167, 104042. [Google Scholar] [CrossRef]
- Jia, H.M.; Wang, Q.; Zhang, S.H.; Yan, Z.Y.; Liu, M. Effects of AM fungi on growth and rhizosphere soil enzyme activities of Salvia miltiorrhiza. Acta. Pratacul. Sin. 2020, 29, 83–92. [Google Scholar] [CrossRef]
- Yang, W.W.; Guo, D.Q.; Cao, W.G.; Pan, X.J.; Xue, Y.B.; Zhang, J.; Zhou, N. Effects of 27 strains of arbuscular mycorrhizal fungi inoculation on physiology and biochemistry and major components of terpenoids in potted Saussurea costus. Chin. J. Trop. Crops. 2020, 41, 1822–1830. [Google Scholar] [CrossRef]
- Watanarojanaporn, N.; Boonkerd, N.; Wongkaew, S.; Prommanop, P.; Teaumroong, N. Selection of arbuscular mycorrhizal fungi for citrus growth promotion and Phytophthora suppression. Sci. Hortic. 2011, 128, 423–433. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Sun, Q.; Niu, T.; Zhang, J. Arbuscular mycorrhizal fungus alleviates anthracnose disease in tea seedlings. Front. Plant Sci. 2023, 13, 1058092. [Google Scholar] [CrossRef] [PubMed]
- Ramia, J.; Bernd, K.K.; Jens, D.; Rainer, G.J. Impact of pea growth and arbuscular mycorrhizal fungi on the decomposition of 15 N-labeled maize residues. Biol. Fert. Soils 2012, 48, 547–560. [Google Scholar] [CrossRef]
- Ravnskov, S.; Larsen, J. Functional compatibility in cucumber mycorrhizas in terms of plant growth performance and foliar nutrient composition. Plant Biol. 2016, 18, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil. 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, L.P.; Wang, J.Y.; Cui, X.M.; Yang, G.; Wang, Y.Y.; Huang, L.Q. Relationship between intensity of the mycorrhizal colonisation in root system and biomass or saponins content of Panax notoginseng. Mod. Chin. Med. 2015, 17, 1253–1257. [Google Scholar] [CrossRef]
- Xie, L.Y.; Zhang, Y.; Xiong, B.Q.; Gan, B.C. Effect of polyamine on growth and development of arbuscular mycorrhizal fungi and host plant in symbiotic culture condition. Chin. J. Ecol. Agric. 2009, 17, 1216–1220. [Google Scholar] [CrossRef]
- Cosme, M.; Wurst, S. Interactions between arbuscular mycorrhizal fungi, rhizobacteria, soil phosphorus and plant cytokinin deficiency change the root morphology, yield and quality of tobacco. Soil Biol. Biochem. 2013, 57, 436–443. [Google Scholar] [CrossRef]
- Gao, Y.L.; Li, J.M.; Yan, M. Effects of AMF inoculation on the growth and chlorophyll fluorescence of Sorghum bicolor × S. sudanense in coal wastes. Guihaia 2016, 36, 539–547. [Google Scholar] [CrossRef]
- Baslam, M.; Esteban, R.; García-Plazaola, J.I.; Goicoechea, N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013, 97, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New. Phytol. 2005, 168, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.M.; Chen, S.M.; Zou, Y.N.; Srivastava, A.K.; Rahman, M.M.; Wu, Q.S.; Kuča, K. Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth. Regul. 2021, 93, 311–318. [Google Scholar] [CrossRef]
- Li, X.M.; Wang, J.J. Effects of arbuscular mycorrhizal fungi on photosynthesis and antioxidant enzyme activity of osmanthus seedlings under nickel stress. Jiangsu Agric. Sci. 2019, 47, 223–227. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, L.; Chen, B.D.; Hao, Z.P.; Wang, J.Y.; Huang, L.Q.; Yang, G.; Cui, X.M.; Yang, L.; Wu, Z.X.; et al. Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: Current research status and prospectives. Mycorrhiza 2013, 23, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Silva, F.S.B.; Hijri, M.; Kapoor, R. Editorial: Arbuscular mycorrhiza-mediated augmentation of plant secondary metabolite production. Front. Plant Sci. 2023, 13, 1150900. [Google Scholar] [CrossRef]
- Mandal, S.; Upadhyay, S.; Wajid, S.; Ram, M.; Jain, D.C.; Singh, V.P.; Abdin, M.Z.; Kapoor, R. Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 2015, 25, 345–357. [Google Scholar] [CrossRef]
- Thangavel, S.; Nisha, M.C.; Sevanan, R. Effect of indigenous arbuscular mycorrhizal fungi on some growth parameters and phytochemical constituents of Pogostemon patchouli Pellet. Maejo Int. J. Sci. Technol. 2009, 3, 222–234. [Google Scholar]
- Liang, X.F.; Tang, M.J.; Lu, L.X.; Zhao, X.Y.; Dai, C.C. Effects of three arbuscular mycorrhizal fungi (AMF) species on the growth, physiology, and major components of essential oil of Atractylodes lancea. J. Ecol. 2018, 37, 1871. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Hu, Y.N.; Peng, Y.L.; Sun, M.L.; Zhang, Y.H.; Liu, T. Effects of three arbuscular mycorrhizas on different provenances of amur cork Seedings. Bull. Bot. Res. 2015, 35, 92–100. [Google Scholar] [CrossRef]
- Wagg, C.; Barendregt, C.; Jansa, J.; van der Heijden, M.G. Complementarity in both plant and mycorrhizal fungal communities are not necessarily increased by diversity in the other. J. Ecol. 2015, 103, 1233–1244. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi and water stress on ultrastructural change of glandular hairs and essential oil compositions in Ocimum gratissimum. Chem. Biol. Technol. Agric. 2017, 4, 20. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.P.; Guo, L.P.; Zhang, X.; Zhang, S.B.; Wang, Y.S.; Chen, B.D. Research advances in terpenoids synthesis and accumulation in plants as influenced by arbuscular mycorrhizal symbiosis. Biotechnol. Bull. 2020, 36, 49–63. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]


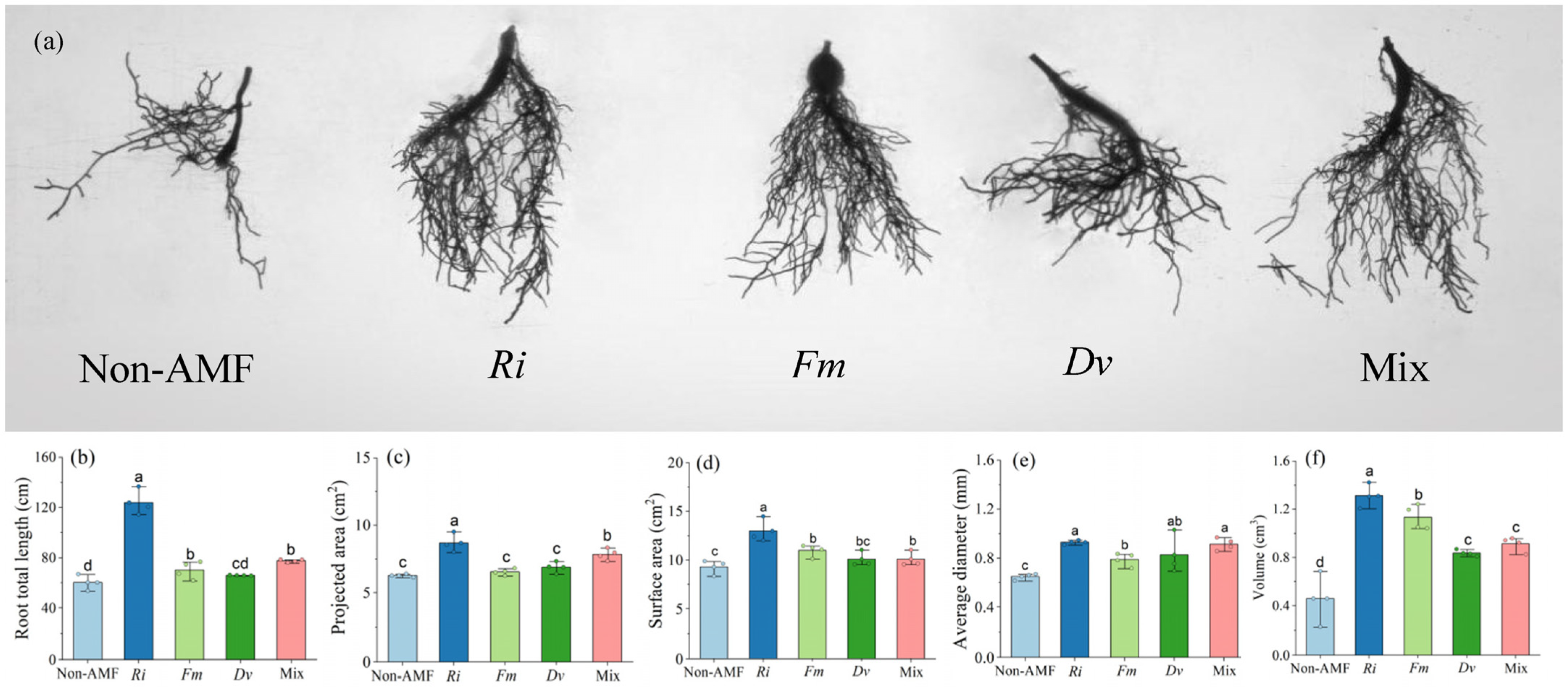
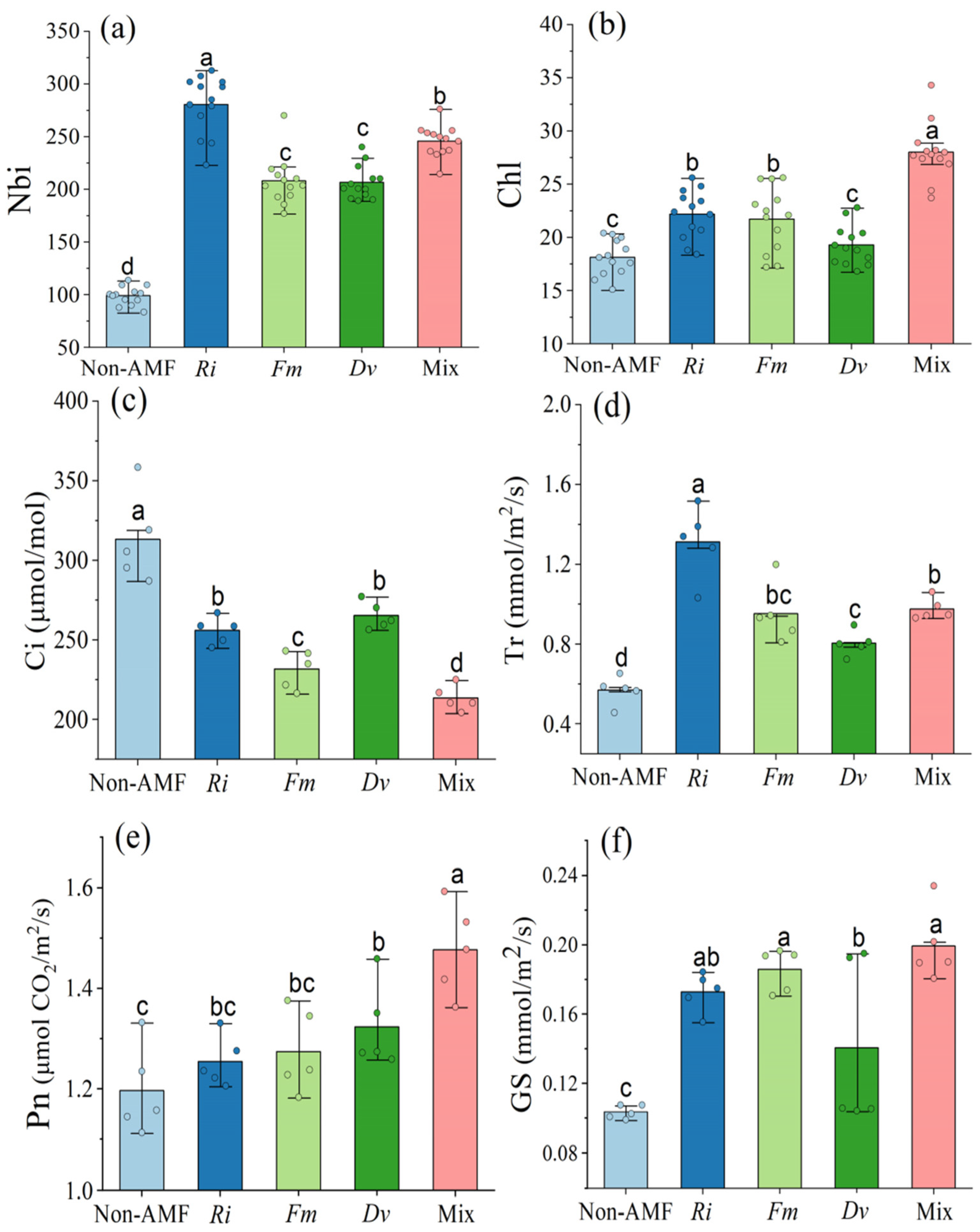
| Root Total Length | Root Projected Area | Root Surface Area | Root Volume | Root Average Diameter | |
|---|---|---|---|---|---|
| Root columbin | 0.92 ** | 0.73 ** | 0.87 ** | 0.89 ** | 0.64 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.-L.; Xu, F.-Q.; Zhang, Z.-Z.; Alqahtani, M.D.; Tashkandi, M.A.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi, Especially Rhizophagus intraradices as a Biostimulant, Improve Plant Growth and Root Columbin Levels in Tinospora sagittata. Horticulturae 2023, 9, 1350. https://doi.org/10.3390/horticulturae9121350
Meng L-L, Xu F-Q, Zhang Z-Z, Alqahtani MD, Tashkandi MA, Wu Q-S. Arbuscular Mycorrhizal Fungi, Especially Rhizophagus intraradices as a Biostimulant, Improve Plant Growth and Root Columbin Levels in Tinospora sagittata. Horticulturae. 2023; 9(12):1350. https://doi.org/10.3390/horticulturae9121350
Chicago/Turabian StyleMeng, Lu-Lu, Fu-Qi Xu, Ze-Zhi Zhang, Mashael Daghash Alqahtani, Manal A. Tashkandi, and Qiang-Sheng Wu. 2023. "Arbuscular Mycorrhizal Fungi, Especially Rhizophagus intraradices as a Biostimulant, Improve Plant Growth and Root Columbin Levels in Tinospora sagittata" Horticulturae 9, no. 12: 1350. https://doi.org/10.3390/horticulturae9121350






