Integration of Chitosan and Biopesticides to Suppress Pre-Harvest Diseases of Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chitosan Products
2.2. Objective 1. Research Orchard Trials
2.2.1. Experiment 1. Research Orchard Trials—2021
2.2.2. Experiment 2. Research Orchard Trials—2022
2.2.3. Objective 1. Disease Assessments
2.3. Objective 2. On-Farm Trials
2.3.1. Experiment 3. On-Farm Site #1
2.3.2. Experiment 4. On-Farm Site #2
2.3.3. Objective 2. Disease Assessments
2.4. Objective 3. Evaluation of Chitosan to Reduce Overwintering of V. inaequalis in Orchard Leaf Litter
2.5. Data Analysis
3. Results
3.1. Objective 1. Experiment 1–2
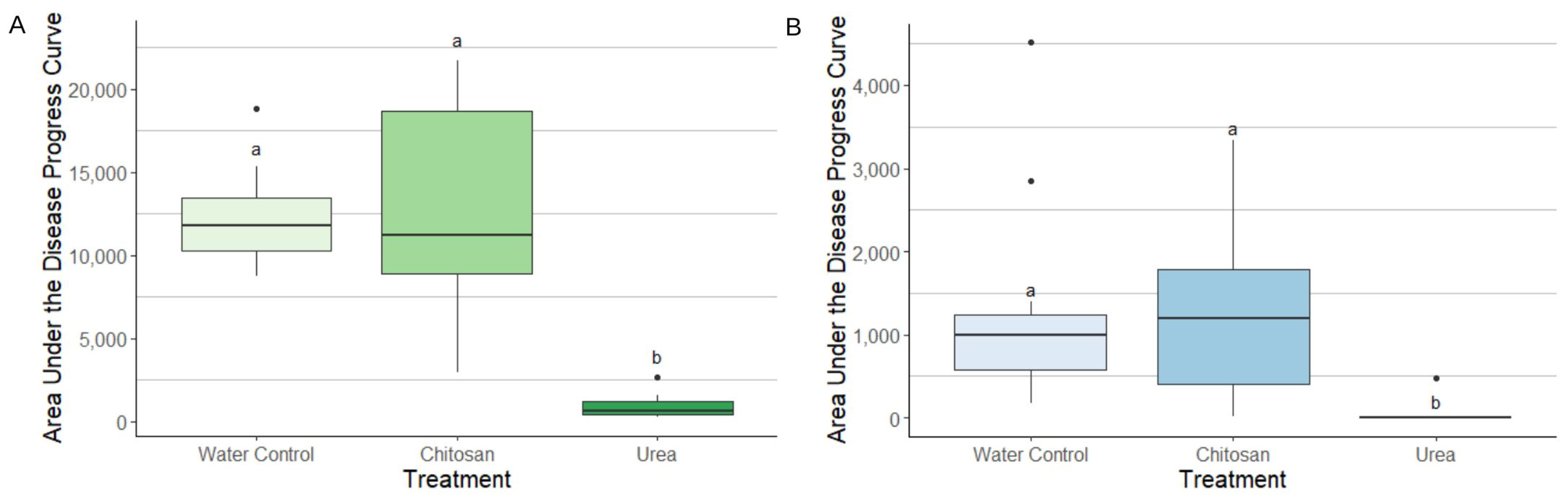
3.2. Objective 1. Experiment 1. FREC Research Trials—2021
3.3. Objective. Experiment 2. FREC Research Trials—2022
3.4. Objective 2. Experiment 3. NH On-Farm Site #1
3.5. Objective 2. Experiment 4. NH On-Farm Trial #2
3.6. Objective 3. Evaluation of Chitosan to Reduce Overwintering of V. inaequalis in Orchard Leaf Litter
4. Discussion
4.1. Chitosan Can Reduce Disease When Applied as Part of a Conventional Fungicide Program
4.2. Synergisms between Chitosan and Biopesticides Varied by Site, Cultivar, and Pathogen
4.3. Chitosan Did Not Reduce Overwintering Spores of V. inaequalis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C. Why Farmers Continue to Use Pesticides despite Environmental, Health and Sustainability Costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Ekström, G.; Ekbom, B. Pest Control in Agro-Ecosystems: An Ecological Approach. CRC Crit. Rev. Plant Sci. 2011, 30, 74–94. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental Impact of Different Agricultural Management Practices: Conventional vs. Organic Agriculture. CRC Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for Novel Biocontrol Agents Applicable in Plant Disease Management—A Review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty Years of Postharvest Biocontrol Research: Is It Time for a New Paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological Control of Postharvest Diseases of Fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [Green Version]
- Fravel, D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.; Shimizu, M. Improving Performance of Microbial Biocontrol Agents against Plant Diseases. J. Gen. Plant Pathol. 2019, 85, 329–336. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Mark Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have Biopesticides Come of Age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.F.; Backman, P.A.; Rodriguez-Kabana, R.; Kokalis-Burelle, N. Biological Control of Apple Fruit Diseases by Chaetomium globosum Formulations Containing Cellulose. Biol. Control 1992, 2, 118–123. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Backman, P.A.; Rodriguez-kabana, R.; Ploper, L.D. Potential for Biological Control of Early Leafspot of Peanut Using Bacillus cereus and Chitin as Foliar Amendments. Biol. Control 1992, 2, 321–328. [Google Scholar] [CrossRef]
- Beauséjour, J.; Clermont, N.; Beaulieu, C. Effect of Streptomyces melanosporofaciens Strain EF-76 and of Chitosan on Common Scab of Potato. Plant Soil 2003, 256, 463–468. [Google Scholar] [CrossRef]
- Yu, T.; Yu, C.; Chen, F.; Sheng, K.; Zhou, T.; Zunun, M.; Abudu, O.; Yang, S.; Zheng, X. Integrated Control of Blue Mold in Pear Fruit by Combined Application of Chitosan, a Biocontrol Yeast and Calcium Chloride. Postharvest Biol. Technol. 2012, 69, 49–53. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Dhillon, G.S. The Versatile Biopolymer Chitosan: Potential Sources, Evaluation of Extraction Methods and Applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Jia, X.; Zeng, H.; Wang, W.; Zhang, F.; Yin, H. Chitosan Oligosaccharide Induces Resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana by Activating Both Salicylic Acid– and Jasmonic Acid–Mediated Pathways. Mol. Plant-Microbe Interact. 2018, 31, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- DeGenring, L.; Dickson, R.; Poleatewich, A. Inhibition of Botrytis cinerea Growth and Suppression of Gray Mold on Petunia Leaves Using Chitosan. Plant Dis. 2023, 107, 840–848. [Google Scholar] [CrossRef] [PubMed]
- El Ghaouth, A.; Arul, J.; Asselin, A. Antifungal Activity of Chitosan on Two Post-Harvest Pathogens of Strawberry Fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velázquez-del Valle, M.G.; Méndez-Montealvo, M.G.; Sánchez-Rivera, M.M.; Bello-Pérez, L.A. Antifungal Effects of Chitosan with Different Molecular Weights on in Vitro Development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr. Polym. 2008, 73, 541–547. [Google Scholar] [CrossRef]
- Benhamou, N.; Theriault, G. Treatment with Chitosan Enhances Resistance of Tomato Plants to the Crown and Root Rot Pathogen Fusarium oxysporum f. sp. radicis-lycopersici. Physiol. Mol. Plant Pathol. 1992, 41, 33–52. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E. Chapter 6—Use of Chitosan to Control Postharvest Decay of Temperate Fruit: Effectiveness and Mechanisms of Action. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 155–177. ISBN 978-0-12-802735-6. [Google Scholar]
- Zhang, H.; Li, R.; Liu, W. Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a Potential Natural Compound to Control Pre and Postharvest Diseases of Horticultural Commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Poleatewich, A.M. Development of Biological Control Strategies for Integrated Management of Pre- and Postharvest Diseases of Apple in Pennsylvania. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2010. [Google Scholar]
- Lafontaine, P.J.; Benhamou, N. Chitosan Treatment: An Emerging Strategy for Enhancing Resistance of Greenhouse Tomato Plants to Infection by Fusarium oxysporum f.sp. radicis-lycopersici. Biocontrol Sci. Technol. 1996, 6, 111–124. [Google Scholar] [CrossRef]
- Benhamou, N.; Lafontaine, P.J.; Nicole, M. Induction of Systemic Resistance to Fusarium Crown and Root Rot in Tomato Plants by Seed Treatment with Chitosan. Phytopathology 1994, 84, 1432–1444. [Google Scholar] [CrossRef]
- Sharathchandra, R.G.; Raj, S.N.; Shetty, N.P.; Amruthesh, K.N.; Shetty, H.S. A Chitosan Formulation ElexaTM Induces Downy Mildew Disease Resistance and Growth Promotion in Pearl Millet. Crop Prot. 2004, 23, 881–888. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Ardi, R.; Pinto, R.; Aki, C.; Fallik, E. Controlling Gray Mould Caused by Botrytis cinerea in Cucumber Plants by Means of Chitosan. Crop Prot. 2003, 22, 285–290. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Belkacemi, K.; Corcuff, R.; Arul, J. Effect of Pre-Harvest Chitosan Sprays on Post-Harvest Infection by Botrytis cinerea and Quality of Strawberry Fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Tuzun, S. Induction of Resistance against Fusarium Wilt of Tomato by Combination of Chitosan with an Endophytic Bacterial Strain: Ultrastructure and Cytochemistry of the Host Response. Planta 1998, 204, 153–168. [Google Scholar] [CrossRef]
- Lu, H.; Lu, L.; Zeng, L.; Fu, D.; Xiang, H.; Yu, T.; Zheng, X. Effect of Chitin on the Antagonistic Activity of Rhodosporidium paludigenum against Penicillium expansum in Apple Fruit. Postharvest Biol. Technol. 2014, 92, 9–15. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Yin, Y.; Wang, Y.; Zheng, X. Effect of Chitin on the Antagonistic Activity of Cryptococcus laurentii against Penicillium expansum in Pear Fruit. Int. J. Food Microbiol. 2008, 122, 44–48. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, H.; Chen, K.; Ma, L.; Xu, Z. Effect of Chitin on the Antagonistic Activity of Rhodotorula glutinis against Botrytis cinerea in Strawberries and the Possible Mechanisms Involved. Food Chem. 2010, 120, 490–495. [Google Scholar] [CrossRef]
- MacHardy, W.E. Apple Scab: Biology, Epidemilogy and Management; American Phytopathological Society Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Ma, Z.; Michailides, T.J. Advances in Understanding Molecular Mechanisms of Fungicide Resistance and Molecular Detection of Resistant Genotypes in Phytopathogenic Fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Holb, I.J. Fungal Disease Management in Environmentally Friendly Apple Production—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Springer: Dordrecht, The Netherland, 2009; pp. 219–292. [Google Scholar]
- Turechek, W.W.; Köller, W. Managing Resistance of Venturia inaequalis to the Strobilurin Fungicides. Plant Health Prog. 2004, 5, 3. [Google Scholar] [CrossRef]
- Poleatewich, A.M.; Ngugi, H.K.; Backman, P.A. Assessment of Application Timing of Bacillus spp. to Suppress Pre- and Postharvest Diseases of Apple. Plant Dis. 2012, 96, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Travis, J.W.; Halbrendt, N.O.; Rytter, J.; Lehman, B.; Jarjour, B. Evaluation of Organic Alternatives for Control of Apple Scab. Biol. Cult. Tests 2005, 21, N009. [Google Scholar]
- Yoder, K.S.; Cochran, A.E., II; Royston, W.S., Jr.; Kilmer, S.W. Fungal Disease Control by Organic and Biocontrols on Jonagold Apple. Fungic. Nematic. Tests 2006, PF061. [Google Scholar] [CrossRef]
- Cromwell, M.L.; Berkett, L.P.; Darby, H.M.; Ashikaga, T. Alternative Organic Fungicides for Apple Scab Management and Their Non-Target Effects. HortScience 2011, 46, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Díaz Arias, M.M.; Batzer, J.C.; Harrington, T.C.; Wong, A.W.; Bost, S.C.; Cooley, D.R.; Ellis, M.A.; Hartman, J.R.; Rosenberger, D.A.; Sundin, G.W.; et al. Diversity and Biogeography of Sooty Blotch and Flyspeck Fungi on Apple in the Eastern and Midwestern United States. Phytopathology 2010, 100, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Holb, I.J.; Abonyi, F.; Buurma, J.; Heijne, B. On-Farm and on-Station Evaluations of Three Orchard Management Approaches against Apple Scab and Apple Powdery Mildew. Crop Prot. 2017, 97, 109–118. [Google Scholar] [CrossRef]
- Turley, N.E.; Biddinger, D.J.; Joshi, N.K.; López-Uribe, M.M. Six Years of Wild Bee Monitoring Shows Changes in Biodiversity within and across Years and Declines in Abundance. Ecol. Evol. 2022, 12. [Google Scholar] [CrossRef]
- National Cooperative Soil Survey Arendtsville Series. 2003. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/A/ARENDTSVILLE.html (accessed on 31 May 2023).
- NEWA Network for Environment and Weather Application. Available online: https://newa-cornell-edu.unh.idm.oclc.org/ (accessed on 9 October 2023).
- Adaskaveg, J.E.; Förster, H.; Gubler, W.D.; Teviotdale, B.L.; Thompson, D.F. Reduced-Risk Fungicides Help Manage Brown Rot and Other Fungal Diseases of Stone Fruit. Calif. Agric. (Berkeley) 2005, 59, 109–114. [Google Scholar] [CrossRef] [Green Version]
- NH GRANIT. Soil Survey Geographic (SSURGO) Database for New Hampshire; Earth Systems Research Center, University of New Hampshire: Durham, NH, USA, 2020; Available online: https://www.nhgeodata.unh.edu/datasets/NHGRANIT::soil-survey-geographic-ssurgo-database-for-new-hampshire/about (accessed on 31 May 2023).
- National Cooperative Soil Survey Hollis Series. 2016. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/H/HOLLIS.html (accessed on 31 May 2023).
- National Cooperative Soil Survey Gilmanton Series. 2016. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/G/GILMANTON.html (accessed on 31 May 2023).
- Sutton, D.K.; MacHardy, W.E.; Lord, W.G. Effects of Shredding or Treating Apple Leaf Litter with Urea on Ascospore Dose of Venturia inaequalis and Disease Buildup. Plant Dis. 2000, 84, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Frey, C.N.; Keitt, G.W. Studies of Spore Dissemination of Venturia inaequalis (Cke.) Wint. in Relation to Seasonal Development of Apple Scab. J. Agric. Res. 1925, 30, 529–540. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Shaner, G.; Finney, R. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, N.; Vaillancourt, L.J.; Hartman, J.R. Apple Scab. Plant Health Instr. 2018. [Google Scholar] [CrossRef]
- Marine, S.; Yoder, K.; Baudoin, A. Powdery Mildew of Apple. Plant Health Instr. 2010. [Google Scholar] [CrossRef]
- Koetter, R.; Grabowski, M. Cedar-Apple and Related Rust Diseases; University of Minnesota Extension: St Paul, MN, USA, 2019; Available online: https://extension.umn.edu/plant-diseases/cedar-apple-rust (accessed on 31 May 2023).
- Peter, K. Apple Diseases–Sooty Blotch and Flyspeck; Penn State Extension: State College, PA, USA, 2023; Available online: https://extension.psu.edu/apple-diseases-sooty-blotch-and-flyspeck (accessed on 31 May 2023).
- Mauch, T.; Rodriguez Salamanca, L. Frog-Eye/Black Rot of Apples and Pears; Iowa State University Extension and Outreach: Ames, IA, USA, 2022. Available online: https://hortnews.extension.iastate.edu/frog-eye-black-rot-apples-and-pears (accessed on 31 May 2023).
- Muñoz, Z.; Moret, A. Sensitivity of Botrytis Cinerea to Chitosan and Acibenzolar-S-Methyl. Pest Manag. Sci. 2010, 66, 974–979. [Google Scholar] [CrossRef]
- Felipini, R.B.; Boneti, J.I.; Katsurayama, Y.; Neto, A.C.R.; Veleirinho, B.; Maraschin, M.; di Piero, R.M. Apple Scab Control and Activation of Plant Defence Responses Using Potassium Phosphite and Chitosan. Eur. J. Plant Pathol. 2016, 145, 929–939. [Google Scholar] [CrossRef]
- Benhamou, N. Elicitor-Induced Plant Defence Pathways. Trends Plant Sci. 1996, 1, 233–240. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; de Masi, L.; de Palma, M.; Lorito, M. The Beneficial Effect of Trichoderma spp. on Tomato Is Modulated by the Plant Genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Algam, S.A.E.; Xie, G.; Li, B.; Yu, S.; Su, T.; Larsen, J. Effects of Paenibacillus Strains and Chitosan on Plant Growth Promotion and Control of Ralstonia Wilt in Tomato. J. Plant Pathol. 2010, 92, 593–600. [Google Scholar]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Benhamou, N.; Asselin, A.; Belanger, R.R. Effect of Chitosan on Cucumber Plants: Suppression of Pythium aphanidermatum and Induction of Defense Reactions. Phytopathology 1994, 84, 313. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Liu, F.; Yang, Y.; Wu, Z.; Cai, H.; Zhang, Q.; Wang, Y.; Li, P. Effects of Chitosan on Control of Postharvest Blue Mold Decay of Apple Fruit and the Possible Mechanisms Involved. Sci. Hortic. 2015, 186, 77–83. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of Chitosan on Control of Postharvest Diseases and Physiological Responses of Tomato Fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Zahid, N.; Maqbool, M.; Siddiqui, Y.; Manickam, S.; Ali, A. Regulation of Inducible Enzymes and Suppression of Anthracnose Using Submicron Chitosan Dispersions. Sci. Hortic. 2015, 193, 381–388. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Smilanick, J.L.; Wilson, C.L. Enhancement of the Performance of Candida saitoana by the Addition of Glycolchitosan for the Control of Postharvest Decay of Apple and Citrus Fruit. Post 2000, 19, 103–110. [Google Scholar] [CrossRef]
- Ait Barka, E.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan Improves Development, and Protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- El Ghaouth, A.; Arul, J.; Wilson, C.; Benhamou, N. Ultrastructural and Cytochemical Aspects of the Effect of Chitosan on Decay of Bell Pepper Fruit. Physiol. Mol. Plant Pathol. 1994, 44, 417–432. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer with Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered Chitosan Based Nanomaterials: Bioactivities, Mechanisms and Perspectives in Plant Protection and Growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, Mode of Action, and in Vivo Activity of Chitosan and Its Micro- and Nanoparticles as Antimicrobial Agents: A Review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ayer, K.M.; Strickland, D.A.; Choi, M.; Cox, K.D. Optimizing the Integration of a Biopesticide (Bacillus subtilis QST 713) with a Single-Site Fungicide (Benzovindiflupyr) to Reduce Reliance on Synthetic Multisite Fungicides (Captan and Mancozeb) for Management of Apple Scab. Plant Dis. 2021, 105, 3545–3553. [Google Scholar] [CrossRef]
- Köhl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W. Toward an Integrated Use of Biological Control by Cladosporium cladosporioides H39 in Apple Scab (Venturia inaequalis) Management. Plant Dis. 2015, 99, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Carisse, O.; Philion, V.; Rolland, D.; Bernier, J. Effect of Fall Application of Fungal Antagonists on Spring Ascospore Production of the Apple Scab Pathogen, Venturia inaequalis. Phytopathology 2000, 90, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Wang, L.; Qin, Y.; Li, P. Activity of Chitin/Chitosan/Chitosan Oligosaccharide against Plant Pathogenic Nematodes and Potential Modes of Application in Agriculture: A Review. Carbohydr. Polym. 2023, 306, 120592. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Mac an tSaoir, S.; Cooke, L.R.; Mc Cracken, A.R. The Effects of Leaf Litter Treatments, Post-Harvest Urea and Omission of Early Season Fungicide Sprays on the Overwintering of Apple Scab on Bramley’s Seedling Grown in a Maritime Environment. Ir. J. Agric. Food Res. 2010, 49, 55–66. [Google Scholar]


| Treatment | Trade Name (Active Ingredient) | Rate (per acre) | Timing 1 |
|---|---|---|---|
| Water Control | Water | -- | TC-10C |
| Grower Standard (GS) | Manzate Pro-Stick (Mancozeb) | 1361 g (3 lb) | TC-1C |
| Captan Gold (Captan) | 1134 g (2.5 lb) | TC, 2C-10C | |
| Luna Sensation (Fluopyram and Trifloxystrobin) | 184 mL (5 fl oz) | P, FB | |
| Inspire Super (Difenoconazole and Cyprodinil) | 355 mL (12 fl oz) | PF, 1C | |
| LI 700 (Penetrant) | 473 mL (1 pint) | 2C-10C | |
| Chitosan (C) | Tidal Grow (2% Chitosan) | 473 mL | TC-10C |
| Reduced Risk (RR) | Microthiol Disperss (Sulfur 80%) | 4536 g (10 lb) | TC-PF |
| Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qt) | 1C-10C | |
| Reduced Risk + Chitosan (RR + C) | Microthiol Disperss (Sulfur 80%) | 4536 g (10 lb) | TC-PF |
| Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qt) | 1C-10C | |
| Tidal Grow (2% Chitosan) | 473 mL | TC-10C |
| Treatment | Trade Name (Active Ingredient) | Rate (per acre) | Timing 1 |
|---|---|---|---|
| Water Control | Water | -- | TC-11C |
| Grower Standard (GS) | Manzate Pro-Stick (Mancozeb) | 1361 g (3 lb) | P-11C |
| Captan Gold (Captan) | 1134 g (2.5 lb) | P | |
| Inspire Super (Difenoconazole and Cyprodinil) | 355 mL (12 fl oz) | P, 1C | |
| Miravis (Pydiflumetofen) | 101 mL (3.42 fl oz) | B. PF | |
| Chitosan (C) | Tidal Grow (2% Chitosan) | 1893 mL | P-11C |
| Reduced Risk (RR) | Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qt) | P-11C |
| Reduced Risk + Chitosan (RR + C) | Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qt) | P-11C |
| Tidal Grow (2% Chitosan) | 1893 mL | P-11C |
| Treatment | Trade Name (Active Ingredient) | Rate (per acre) | Timing 1 |
|---|---|---|---|
| Grower Standard (GS) | Koverall Fungicide (Mancozeb) | 1700 g (3.75 lbs) | GT-P |
| Captan Gold (Captan) | 2612 mL (0.69 gal) | FB-1C | |
| Captan Gold (Captan) | 3785 mL (1 gal) | 1C-5C | |
| Agro Mos (Copper 4%) | 1892 mL (0.5 gal) | 3C-5C | |
| Grower Standard + Chitosan (GS + C) | Koverall Fungicide (Mancozeb) | 1700 g (3.75 lbs) | GT-P |
| Captan Gold (Captan) | 2612 mL (0.69 gal) | FB-1C | |
| Captan Gold (Captan) | 3785 mL (1 gal) | 1C-5C | |
| Agro Mos (Copper 4%) | 1892 mL (0.5 gal) | 3C-5C | |
| ARMOUR Zen (15% Chitosan) | 3785 mL (4 qts) | P-5C | |
| Grower Standard + Biopesticide + Chitosan (GS + B + C) | Koverall Fungicide (Mancozeb) | 1700 g (3.75 lbs) | GT-P |
| Captan Gold (Captan) | 2612 mL (0.69 gal) | FB-1C | |
| Captan Gold (Captan) | 3785 mL (1 gal) | 1C-5C | |
| Agro Mos (Copper 4%) | 1892 mL (0.5 gal) | 3C-5C | |
| Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qts) | P-5C | |
| ARMOUR Zen (15% Chitosan) | 3785 mL (4 qts) | P-5C |
| Treatment | Trade Name (Active Ingredient) | Rate (per acre) | Timing 1 |
|---|---|---|---|
| Grower Standard (GS) | Kocide 3000 (Copper Hydroxide 46.1%) | 1814 g (4 lbs) | GT |
| Koverall Fungicide (Mancozeb) | 1361 g (3 lbs) | P-PF | |
| Captan Gold (Captan) | 3785 mL (4 qts) | FB-3C | |
| Pristine Fungicide (Pyraclostrobin and Boscalid) | 454 g (16 oz) | 4C | |
| Grower Standard + Chitosan (GS + C) | Kocide 3000 (Copper Hydroxide 46.1%) | 1814 g (4 lbs) | GT |
| Koverall Fungicide (Mancozeb) | 1361 g (3 lbs) | P-PF | |
| Captan Gold (Captan) | 3785 mL (4 qts) | FB-3C | |
| Pristine Fungicide (Pyraclostrobin and Boscalid) | 454 g (16 oz) | 4C | |
| ARMOUR Zen (15% Chitosan) | 3785 mL (4 qts) | P-5C | |
| Grower Standard + Biopesticide + Chitosan (GS + B + C) | Kocide 3000 (Copper Hydroxide 46.1%) | 1814 g (4 lbs) | GT |
| Koverall Fungicide (Mancozeb) | 1361 g (3 lbs) | P-PF | |
| Captan Gold (Captan) | 3785 mL (4 qts) | FB-3C | |
| Pristine Fungicide (Pyraclostrobin and Boscalid) | 454 g (16 oz) | 4C | |
| Serenade ASO (Bacillus subtilis strain QST 713) | 3785 mL (4 qts) | P-5C | |
| ARMOUR Zen (15% Chitosan) | 3785 mL (4 qts) | P-5C |
| Water Control | GS | C | RR | RR + C | |
|---|---|---|---|---|---|
| Scab Incidence (%) | 56.2 ± 6.1 (a) | 2.0 ± 1.4 (b) | 39.3 ± 6.3 (a) | 6.7 ± 1.7 (b) | 11.3 ± 3.3 (b) |
| Number of Scab Lesions | 4.6 ± 0.7 (a) | 0.0 ± 0.0 (c) | 1.8 ± 0.3 (b) | 0.2 ± 0.1 (c) | 0.4 ± 0.2 (bc) |
| Scab Severity (0–6) | 0.9 ± 0.1 (a) | 0.02 ± 0.01 (b) | 0.5 ± 0.1 (a) | 0.07 ± 0.02 (b) | 0.2 ± 0.04 (b) |
| Powdery Mildew Incidence (%) | 54.6 ± 5.6 (a) | 26.0 ± 4.9 (b) | 40.0 ± 4.0 (ab) | 28.1 ± 6.1 (b) | 28.0 ± 4.0 (b) |
| Russet Severity (0–6) | 1.8 ± 0.2 (a) | 1.1 ± 0.1 (b) | 1.3 ± 0.1 (ab) | 0.8 ± 0.1 (b) | 0.9 ± 0.1 (b) |
| Sooty Blotch Incidence (%) | 57.6 ± 3.8 (a) | 14.7 ± 2.5 (c) | 32.0 ± 6.3 (bc) | 35.4 ± 5.1 (b) | 31.3 ± 6.8 (bc) |
| Flyspeck Incidence (%) | 24.5 ± 9.0 (a) | 1.3 ± 1.3 (b) | 33.3 ± 9.6 (a) | 19.2 ± 4.2 (ab) | 18.3 ± 5.2 (a) |
| Water Control | GS | C | RR | RR + C | ||
|---|---|---|---|---|---|---|
| Leaves | Scab Severity (0–6) | 0.41 ± 0.07 (ab) | 0.01 ± 0.01 (b) | 0.42 ± 0.09 (ab) | 0.66 ± 0.10 (a) | 0.45 ± 0.09 (ab) |
| Powdery Mildew Incidence (%) | 39.0 ±2.75 (a) | 21.1 ± 2.9 (b) | 48.9 ± 2.0 (a) | 52.3 ± 2.1 (a) | 51.2 ± 2.1 (a) | |
| Powdery Mildew Shoot Incidence | 12.4 ± 3.9 | 7.2 ± 2.4 | 10.0 ± 1.6 | 9.8 ± 1.7 | 15.0 ± 1.2 | |
| Rust Incidence (%) | 8.2 ± 1.3 (a) | 0.0 ± 0.0 (c) | 2.4 ± 1.8 (bc) | 5.4 ± 1.0 (ab) | 6.7 ± 1.5 (ab) | |
| Harvested Fruit | Scab Incidence (%) | 29.6 ± 8.8 (a) | 0.8 ± 0.8 (b) | 16 ± 5.2 (a) | 24.8 ± 4.1 (a) | 39.2 ± 6.0 (a) |
| Number of Scab Lesions | 2.3 ± 0.7 (ab) | 0.01 ± 0.01 (b) | 2.1 ± 1.2 (ab) | 1.3 ± 0.4 (ab) | 3.6 ± 0.7 (a) | |
| Scab Severity (0–6) | 0.62 ± 0.2 (a) | 0.01 ± 0.01 (b) | 0.28 ± 0.1 (ab) | 0.36 ± 0.1 (a) | 0.76 ± 0.2 (a) | |
| Powdery Mildew Incidence (%) | 68.8 ± 6.5 | 72.0 ± 10.0 | 71.2 ± 7.1 | 67.2 ± 4.6 | 68.8 ± 8.2 | |
| Russet Severity (0–6) | 1.9 ± 0.8 | 1.3 ± 0.5 | 0.9 ± 0.2 | 1.2 ± 0.08 | 1.0 ± 0.2 | |
| Rust Incidence (%) | 9.6 ± 4.3 (a) | 0.0 ± 0.0 (b) | 0.8 ± 0.8 (b) | 11.2 ± 5.0 (a) | 4.8 ± 3.2 (ab) | |
| Sooty Blotch Incidence (%) | 40.8 ± 3.9 (a) | 3.2 ± 0.8 (b) | 21.6 ± 6.4 (ab) | 33.6 ± 5.9 (a) | 41.6 ± 4.1 (a) | |
| Flyspeck Incidence (%) | 20.0 ± 7.2 (a) | 0.0 ± 0.0 (b) | 6.4 ± 2.0 (ab) | 13.6 ± 6.5 (a) | 21.6 ± 4.7 (a) |
| Macoun | McIntosh | ||||||
|---|---|---|---|---|---|---|---|
| GS | GS + C | GS + B + C | GS | GS + C | GS + B + C | ||
| Leaves | Scab Incidence | 45.5 ± 8.7 (ab) | 36.5 ± 9.2 (a) | 49.0 ± 7.4 (b) | 226.0 ± 26.0 | 213.7 ± 43.7 | 236.3 ± 22.4 |
| Scab Severity | 1.42 ± 0.7 | 1.1 ± 0.4 | 3.9 ± 0.6 | 2.8 ± 0.7 | 3.8 ± 1.0 | 1.2 ± 0.4 | |
| Powdery Mildew Incidence | 54.0 ± 18.5 (a) | 37.5 ± 7.9 (ab) | 27.5 ± 5.9 (b) | 7.1 ± 1.8 | 6.3 ± 1.5 | 9.4 ± 5.5 | |
| Rust Incidence | 67.0 ± 23.2 | 74.5 ± 26.3 | 73.25 ± 27.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.7 ± 0.8 | |
| Rust Severity | 1.4 ± 0.5 | 2.0 ± 0.6 | 1.6 ± 0.6 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.05 ± 0.05 | |
| Frog Eye Incidence | 148.5 ± 41.1 | 129.5 ± 32.1 | 132.0 ± 30.0 | 42.3 ± 10.4 (b) | 104.6 ± 35.2 (a) | 48.0 ± 9.9 (b) | |
| Frog Eye Severity | 5.6 ± 2.0 | 3.8 ± 1.4 | 3.9 ± 1.3 | 1.0 ± 0.3 | 0.8 ± 0.1 | 3.1 ± 1.5 | |
| Harvested Fruit | Scab Incidence (%) | 50.4 ± 14.1 | 46.3 ± 10.1 | 46.3 ± 15.8 | 57.5 ± 15.8 (a) | 56.0 ± 16.3 (a) | 37.6 ± 5.2 (b) |
| Number of Scab Lesions | 1.4 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.1 | 3.9 ± 0.7 (ab) | 6.6 ± 1.2 (a) | 1.4 ± 0.4 (b) | |
| Scab Severity (0–6) | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.5 ± 0.1 | |
| Powdery Mildew Incidence (%) | 68 ± 9.2 | 68.6 ± 4.6 | 75.5 ± 8.7 | 90.2 ± 2.4 (a) | 59.6 ± 7.5 (b) | 78.9 ± 4.1 (ab) | |
| Russet Score (0–6) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.6 ± 0.2 | 2.0 ± 0.2 (a) | 0.7 ± 0.1 (b) | 2.0 ± 0.2 (a) | |
| Sooty Blotch Incidence (%) | 1.6 ± 1.6 | 4.0 ± 1.7 | 1.0 ± 1.0 | 1.3 ± 1.3 | 0.8 ± 0.8 | 0.8 ± 0.8 | |
| Flyspeck Incidence (%) | 19.2 ± 5.4 | 18.3 ± 4.4 | 15.0 ± 6.0 | 8.0 ± 5.4 | 8.8 ± 4.0 | 23.9 ± 9.7 | |
| Kingston Black | Dabinett | Wickson | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GS | GS + C | GS + B + C | GS | GS + C | GS + B + C | GS | GS + C | GS + B + C | |
| Powdery Mildew Incidence | 45.5 ± 11 (ab) | 61.5 ± 20 (a) | 24.0 ± 11 (b) | 10.0 ± 2.7 | 17.5 ± 6.2 | 27.0 ± 10 | 13.0 ± 6.0 | 10.0 ± 5.0 | 13.0 ± 4.7 |
| Lesion Incidence 3 | 53.0 ± 12 (a) | 24.0 ± 1.6 (b) | 31.0 ± 4.4 (ab) | 6.0 ± 2.6 | 11.0 ± 6.0 | 18.0 ± 4.8 | 4.0 ± 1.6 | 9.0 ± 4.1 | 11.0 ± 3.4 |
| Lesion Severity | 4.8 ± 1.7 | 2.0 ± 0.7 | 1.5 ± 0.7 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 |
| Frog Eye Incidence | 18.0 ± 6.6 (ab) | 9.5 ± 2.4 (b) | 37.0 ± 13.3 (a) | 7.5 ± 1.9 | 13.0 ± 2.6 | 9.0 ± 1.3 | 6.5 ± 3.9 | 5.0 ± 2.1 | 8.0 ± 2.8 |
| Frog Eye Severity | 0.3 ± 0.1 (b) | 0.1 ± 0.0 (b) | 0.9 ± 0.2 (a) | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeGenring, L.; Peter, K.; Poleatewich, A. Integration of Chitosan and Biopesticides to Suppress Pre-Harvest Diseases of Apple. Horticulturae 2023, 9, 707. https://doi.org/10.3390/horticulturae9060707
DeGenring L, Peter K, Poleatewich A. Integration of Chitosan and Biopesticides to Suppress Pre-Harvest Diseases of Apple. Horticulturae. 2023; 9(6):707. https://doi.org/10.3390/horticulturae9060707
Chicago/Turabian StyleDeGenring, Liza, Kari Peter, and Anissa Poleatewich. 2023. "Integration of Chitosan and Biopesticides to Suppress Pre-Harvest Diseases of Apple" Horticulturae 9, no. 6: 707. https://doi.org/10.3390/horticulturae9060707









