Phytopathogenic Pseudomonas syringae as a Threat to Agriculture: Perspectives of a Promising Biological Control Using Bacteriophages and Microorganisms
Abstract
:1. Introduction
2. Pseudomonas syringae and Its Damage to Agriculture and Ecosystem
2.1. Identification and Classification of Pseudomonas syringae
2.2. Pseudomonas syringae: A Threat to the Global Agriculture
3. Current Methods of Pseudomonas syringae Management and Its Principal Limitations
3.1. Antimicrobial Resistance of Pseudomonas syringae
3.1.1. Copper Resistance Mechanisms
3.1.2. Streptomycin Resistance Mechanisms
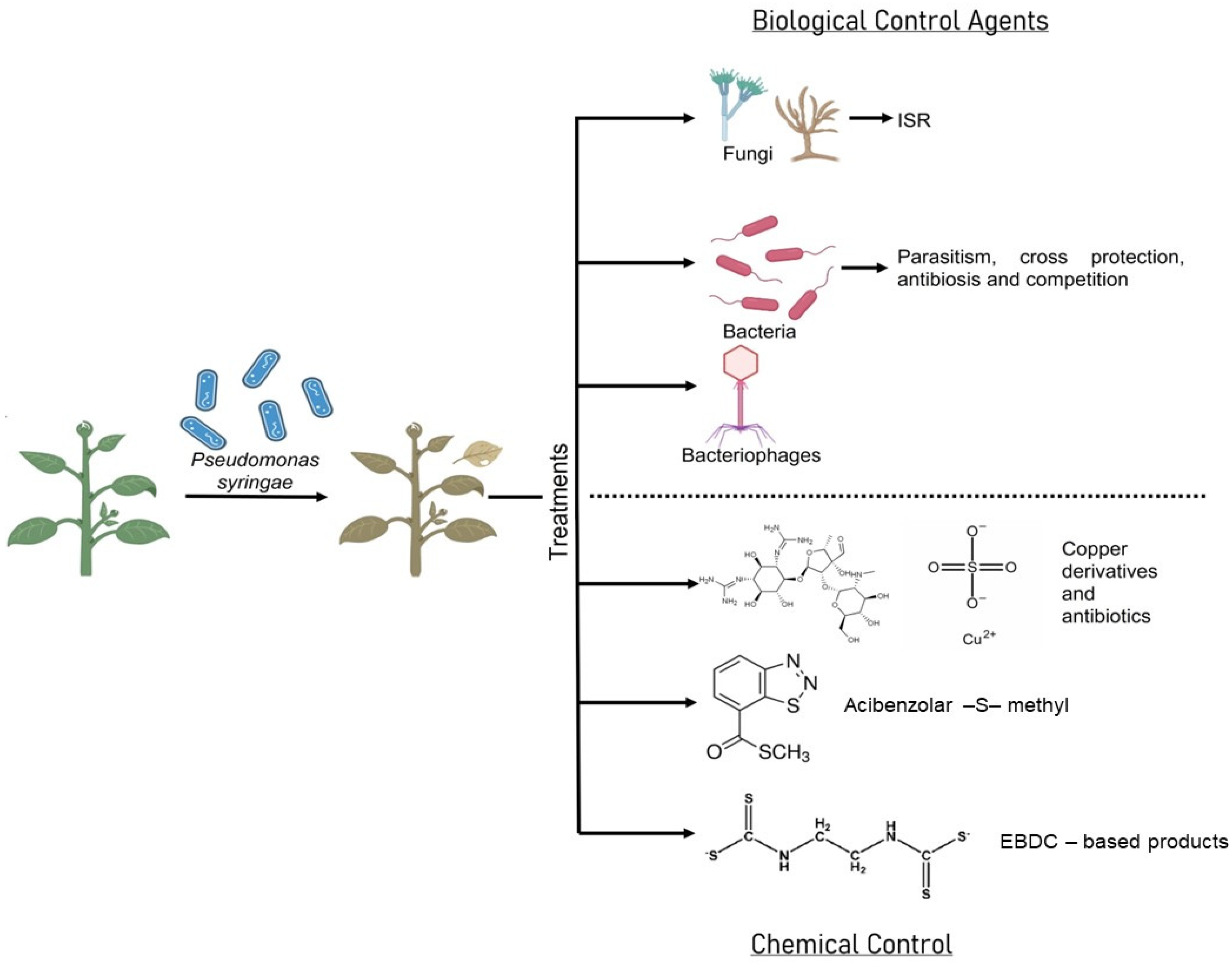
4. Biological Control of Pseudomonas syringae
4.1. Fungi
4.2. Bacteria
5. Bacteriophages in Pseudomonas syringae Control
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennelly, M.M.; Cazorla, F.M.; De Vicente, A.; Ramos, C.; Sundin, G.W. Pseudomonas syringae Diseases of Fruit Trees: Progress toward Understanding and Control. Plant Dis. 2007, 91, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.S.; Upper, C.D. Bacteria in the Leaf Ecosystem with Emphasis on Pseudomonas syringae—A Pathogen, Ice Nucleus, and Epiphyte. Microbiol. Mol. Biol. Rev. 2000, 64, 624–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteil, C.L.; Yahara, K.; Studholme, D.J.; Mageiros, L.; Méric, G.; Swingle, B.; Morris, C.E.; Vinatzer, B.A.; Sheppard, S.K. Population-Genomic Insights into Emergence, Crop Adaptation and Dissemination of Pseudomonas syringae Pathogens. Microb. Genom. 2016, 2, e000089. [Google Scholar] [CrossRef] [Green Version]
- Monteil, C.L.; Cai, R.; Liu, H.; Mechan Llontop, M.E.; Leman, S.; Studholme, D.J.; Morris, C.E.; Vinatzer, B.A. Nonagricultural Reservoirs Contribute to Emergence and Evolution of Pseudomonas syringae Crop Pathogens. New Phytol. 2013, 199, 800–811. [Google Scholar] [CrossRef]
- Ruinelli, M.; Blom, J.; Smits, T.H.M.; Pothier, J.F. Comparative Genomics and Pathogenicity Potential of Members of the Pseudomonas syringae Species Complex on Prunus spp. BMC Genom. 2019, 20, 172. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L.; Yu, J.; Cornish, D.A.; Oldham, J.M.; Spinelli, F.; Pattemore, D.E.; Moffat, B.; D’Accolti, A. Survival of Pseudomonas syringae pv. actinidiae in the Environment. Acta Hortic. 2015, 1095, 105–110. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Moore, L.W. Diseases Caused by Pseudomonas syringae. Available online: https://pnwhandbooks.org/plantdisease/pathogen-articles/pathogens-common-many-plants/bacteria-other-prokaryotes/diseases (accessed on 22 May 2022).
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, Infection Dynamics and Disease Epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical Control, Resistance Mechanisms and Possible Alternatives. Plant Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-Selection of Antibiotic and Metal Resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy Metal Driven Co-Selection of Antibiotic Resistance in Soil and Water Bodies Impacted by Agriculture and Aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [Green Version]
- Dy, R.L.; Rigano, L.A.; Fineran, P.C. Phage-Based Biocontrol Strategies and Their Application in Agriculture and Aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for Unifying the Terminology in Biological Control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for Using Bacteriophages for Plant Disease Control. Bacteriophage 2012, 2, e23857. [Google Scholar] [CrossRef] [Green Version]
- ANASAC Baciforte. Available online: https://www.anasac.cl/agropecuario/productos/baciforte/ (accessed on 31 May 2023).
- Bionativa Nacillus. Available online: http://www.bionativa.cl/web/productos/nacillus-pro-wp/#toggle-id-3 (accessed on 31 May 2023).
- BAYER Serenade® Max. Available online: https://www.cropscience.bayer.es/Hidden-Pages/Productos/Biologicos/Serenade-Max (accessed on 31 May 2023).
- James, S.L.; Rabiey, M.; Neuman, B.W.; Percival, G.; Jackson, R.W. Isolation, Characterization and Experimental Evolution of Phage That Infect the Horse Chestnut Tree Pathogen, Pseudomonas syringae pv. aesculi. Curr. Microbiol. 2020, 77, 1438–1447. [Google Scholar] [CrossRef] [Green Version]
- Cuppels, D.A. Isolation and Characterization of Phages Useful for Identifying Pseudomonas syringae pv. tomato. Phytopathology 1983, 73, 1376. [Google Scholar] [CrossRef]
- Frampton, R.A.; Taylor, C.; Holguín Moreno, A.v.; Visnovsky, S.B.; Petty, N.K.; Pitman, A.R.; Fineran, P.C. Identification of Bacteriophages for Biocontrol of the Kiwifruit Canker Phytopathogen Pseudomonas syringae pv. actinidiae. Appl. Environ. Microbiol. 2014, 80, 2216–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of Novel Bacteriophages for Biocontrol of Bacterial Blight in Leek Caused by Pseudomonas syringae pv. porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarillas, L.; Estrada-Acosta, M.; León-Chan, R.G.; López-Orona, C.; Lightbourn, L. Complete Genome Sequence of Phobos: A Novel Bacteriophage with Unusual Genomic Features That Infects Pseudomonas syringae. Arch. Virol. 2020, 165, 1485–1488. [Google Scholar] [CrossRef]
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of Bacteriophages against Pseudomonas syringae pv. actinidiae with Potential Use as Natural Antimicrobials in Kiwifruit Plants. Microorganisms 2020, 8, 974. [Google Scholar] [CrossRef]
- van Hall Pathogenicity and Identification of the Lilac Pathogen, Pseudomonas syringae pv. syringae. Ann. Appl. Biol. 1992, 118, 283–298. [CrossRef]
- Morris, C.E.; Sands, D.C.; Vinatzer, B.A.; Glaux, C.; Guilbaud, C.; Buffière, A.; Yan, S.; Dominguez, H.; Thompson, B.M. The Life History of the Plant Pathogen Pseudomonas syringae Is Linked to the Water Cycle. ISME J. 2008, 2, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapp, C. Handbuchdel Pflanzenkrank-Heiten. II Diepflanzlichen Parasiten; Sorauer, P., Ed.; Paul Parey: Berlin, Germany, 1928. [Google Scholar]
- Burkholder, W.H. The Bacterial Diseases of the Bean. Memoirs. Cornell Univ. Agric. Exp. Stn. 1930, 127, 1–88. [Google Scholar]
- Robbs, C.F. Uma Nova Doença Bacteriana Do Mamoeiro (Carica papaya L.). Rev. Soc. Bras. Agron. 1956, 12, 73–76. [Google Scholar]
- Psallidas, P.G.; Pamagopoulos, C.G. New Bacteriosis of Almond Caused by Pseudomonas amygdali sp. nov. Annales 1975, 11, 94–108. [Google Scholar]
- Ogimi, C. Studies on Bacterial Gall of Chinaberry (Melia Azedarach Lin.), Caused by Pseudomonas meliae n. sp. Sci. Bull. Coll. Agric. Univ. Ryukyu Jpn. 1977, 24, 497–556. [Google Scholar]
- Goto, M. Pseudomonas ficuserectae sp. nov., the Causal Agent of Bacterial Leaf Spot of Ficus erecta Thunb. Int. J. Syst. Evol. Microbiol. 1983, 33, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Janse, J.D.; Rossi, P.; Angelucci, L.; Scortichini, M.; Derks, J.H.J.; Akkermans, A.D.L.; De Vrijer, R.; Psallidas, P.G. Reclassification of Pseudomonas syringae pv. avellanae as Pseudomonas avellanae (spec. nov, the Bacterium Causing Canker of Hazelnut (Corylus avellana L.). Syst. Appl. Microbiol. 1996, 19, 589–595. [Google Scholar] [CrossRef]
- Cardan, L.; Shafik, H.; Belouin, S.; Broch, R.; Grimont, F.; Grimont, P.A.D. DNA Relatedness among the Pathovars of Pseudomonas syringae and Description of Pseudomonas tremae sp. nov. and Pseudomonas cannabina sp. nov. (Ex Sutic and Dowson 1959). Int. J. Syst. Bacteriol. 1999, 49, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Behrendt, U.; Ulrich, A.; Schumann, P. Fluorescent Pseudomonads Associated with the Phyllosphere of Grasses; Pseudomonas trivialis sp. nov., Pseudomonas poae sp. nov. and Pseudomonas congelans sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- González, A.J.; Cleenwerck, I.; De Vos, P.; Fernández-Sanz, A.M. Pseudomonas asturiensis sp. nov., Isolated from Soybean and Weeds. Syst. Appl. Microbiol. 2013, 36, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, M.; Willems, A.; Pothier, J.F.; Ruinelli, M.; Sobiczewski, P.; Puławska, J. Pseudomonas cerasi sp. nov. (Non Griffin, 1911) Isolated from Diseased Tissue of Cherry. Syst. Appl. Microbiol. 2016, 39, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Busquets, A.; Gomila, M.; Beiki, F.; Mulet, M.; Rahimian, H.; García-Valdés, E.; Lalucat, J. Pseudomonas caspiana sp. nov., a Citrus Pathogen in the Pseudomonas syringae Phylogenetic Group. Syst. Appl. Microbiol. 2017, 40, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Schaad, N.W.; Cunfer, B.M. Synonymy of Pseudomonas coronafaciens, Pseudomonas coronafaciens Pathovar zeae, Pseudomonas coronafaciens subsp. atropurpurea and Pseudomonas striafaciens. Int. J. Syst. Evol. Microbiol. 1979, 29, 213–221. [Google Scholar] [CrossRef]
- Gomila, M.; Busquets, A.; Mulet, M.; García-Valdés, E.; Lalucat, J. Clarification of Taxonomic Status within the Pseudomonas syringae Species Group Based on a Phylogenomic Analysis. Front. Microbiol. 2017, 8, 2422. [Google Scholar] [CrossRef] [Green Version]
- Bull, C.T.; de Boer, S.H.; Denny, T.P.; Firrao, G.; Fischer-Le Saux, M.; Saddler, G.S.; Scortichini, M.; Stead, D.E.; Takikawa, Y. Comprehensive list of names of plant pathogenic bacteria, 1980–2007. J. Plant Pathol. 2010, 92, 551–592. [Google Scholar]
- Berge, O.; Monteil, C.L.; Bartoli, C.; Chandeysson, C.; Guilbaud, C.; Sands, D.C.; Morris, C.E. A User’s Guide to a Data Base of the Diversity of Pseudomonas syringae and Its Application to Classifying Strains in This Phylogenetic Complex. PLoS ONE 2014, 9, e105547. [Google Scholar] [CrossRef]
- Pseudomonas syringae (Bacterial Blast). In PlantwisePlus Knowledge Bank 2022; CABI International: Wallingford, UK, 2022. [CrossRef]
- Lamichhane, J.R.; Varvaro, L.; Parisi, L.; Audergon, J.-M.M.; Morris, C.E. Disease and Frost Damage of Woody Plants Caused by Pseudomonas syringae: Seeing the Forest for the Trees. Adv. Agron. 2014, 126, 235–295. [Google Scholar]
- Lamichhane, J.R.; Messéan, A.; Morris, C.E. Insights into Epidemiology and Control of Diseases of Annual Plants Caused by the Pseudomonas syringae Species Complex. J. Gen. Plant Pathol. 2015, 81, 331–350. [Google Scholar] [CrossRef]
- Hulin, M.T.; Mansfield, J.W.; Brain, P.; Xu, X.; Jackson, R.W.; Harrison, R.J. Characterization of the Pathogenicity of Strains of Pseudomonas syringae towards Cherry and Plum. Plant Pathol. 2018, 67, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Bultreys, A.; Kaluzna, M. Minerview Bacterial Cankers Caused by (Pseudomonas syringae) on Stone Fruit Species with Special Emphasis on the (Pathovars syringae) and (morsprunorum) Race 1 and Race 2. J. Plant Pathol. 2010, 92, S21–S33. [Google Scholar]
- Ivanović, Ž.; Perović, T.; Popović, T.; Blagojević, J.; Trkulja, N.; Hrnčić, S. Characterization of Pseudomonas syringae pv. syringae, Causal Agent of Citrus Blast of Mandarin in Montenegro. Plant Pathol. J. 2017, 33, 21–33. [Google Scholar] [CrossRef]
- Aiello, D.; Ferrante, P.; Vitale, A.; Polizzi, G.; Scortichini, M.; Cirvilleri, G. Characterization of Pseudomonas syringae pv. syringae Isolated from Mango in Sicily and Occurrence of Copper-Resistant Strains. J. Plant Pathol. 2015, 97, 273–282. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Perveen, R.; Rehman, A.U.; Khan, T.; Malik, M.T.; Chohan, S.; Tariq, A.; Akram, S.; Abbas, S.H. Outbreak of Bacterial Apical Necrosis of Mango in Multan, Punjab, Pakistan. Pak. J. Phytopathol. 2016, 28, 107–113. [Google Scholar]
- Mazzaglia, A.; Studholme, D.J.; Taratufolo, M.C.; Cai, R.; Almeida, N.F.; Goodman, T.; Guttman, D.S.; Vinatzer, B.A.; Balestra, G.M. Pseudomonas syringae pv. actinidiae (Psa) Isolates from Recent Bacterial Canker of Kiwifruit Outbreaks Belong to the Same Genetic Lineage. PLoS ONE 2012, 7, e36518. [Google Scholar] [CrossRef]
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G. Pseudomonas syringae pv. actinidiae: A Re-Emerging, Multi-Faceted, Pandemic Pathogen. Mol. Plant Pathol. 2012, 13, 631–640. [Google Scholar] [CrossRef]
- Cameron, A.; De Zoysa, G.H.; Sarojini, V. Antimicrobial Peptides against Pseudomonas syringae pv. actinidiae and Erwinia amylovora: Chemical Synthesis, Secondary Structure, Efficacy, and Mechanistic Investigations. Biopolymers 2014, 102, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, A.; Talocci, S.; Pilotti, M.; Loreti, S. Real-Time and Qualitative PCR for Detecting Pseudomonas syringae pv. actinidiae Isolates Causing Recent Outbreaks of Kiwifruit Bacterial Canker. Plant Pathol. 2014, 63, 264–276. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Reglinski, T.; Yu, J.; Cornish, D.A. Multiplication and Movement of Pseudomonas syringae pv. actinidiae in Kiwifruit Plants. Acta Hortic. 2015, 1095, 117–122. [Google Scholar] [CrossRef]
- Prencipe, S.; Gullino, M.L.; Spadaro, D. Pseudomonas syringae pv. actinidiae Isolated from Actinidia chinensis Var. Deliciosa in Northern Italy: Genetic Diversity and Virulence. Eur. J. Plant Pathol. 2018, 150, 191–204. [Google Scholar] [CrossRef]
- Ferrante, P.; Scortichini, M. Redefining the Global Populations of Pseudomonas syringae pv. actinidiae Based on Pathogenic, Molecular and Phenotypic Characteristics. Plant Pathol. 2015, 64, 51–62. [Google Scholar] [CrossRef]
- Morris, C.E.; Monteil, C.L.; Berge, O. The Life History of Pseudomonas syringae: Linking Agriculture to Earth System Processes. Annu. Rev. Phytopathol. 2013, 51, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Basim, H.; Basim, E.; Yilmaz, S.; Dickstein, E.R.; Jones, J.B. An Outbreak of Bacterial Speck Caused by Pseudomonas syringae pv. tomato on Tomato Transplants Grown in Commercial Seedling Companies Located in the Western Mediterranean Region of Turkey. Plant Dis. 2004, 88, 1050. [Google Scholar] [CrossRef]
- Cruz, L.; Cruz, J.; Eloy, M.; Oliveira, H.; Vaz, H.; Tenreiro, R. First Report of Bacterial Speck of Tomato Caused by Pseudomonas syringae pv. tomato Race 1 in Portugal. Plant Dis. 2010, 94, 1504. [Google Scholar] [CrossRef]
- Takikawa, Y.; Takahashi, F. Bacterial Leaf Spot and Blight of Crucifer Plants (Brassicaceae) Caused by Pseudomonas syringae pv. maculicola and P. cannabina pv. alisalensis. J. Gen. Plant Pathol. 2014, 80, 466–474. [Google Scholar] [CrossRef]
- Shila, S.J.; Islam, M.R.; Ahmed, N.N.; Dastogeer, K.M.G.; Meah, M.B. Detection of Pseudomonas syringae pv. lachrymans Associated with the Seeds of Cucurbits. Univ. J. Agric. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Newberry, E.A.; Babu, B.; Roberts, P.D.; Dufault, N.S.; Goss, E.M.; Jones, J.B.; Paret, M.L. Molecular Epidemiology of Pseudomonas syringae pv. syringae Causing Bacterial Leaf Spot of Watermelon and Squash in Florida. Plant Dis. 2018, 102, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Martín-Sanz, A.; Pérez de la Vega, M.; Murillo, J.; Caminero, C. Genetic, Biochemical and Pathogenic Diversity of Pseudomonas syringae pv. pisi Strains. Plant Pathol. 2012, 61, 1063–1072. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What It Takes to Be a Pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Warneke, B.W.; Nackley, L.L. Efficacy of tank mixing biological fungicides with sulfur for management of grape powdery mildew. GRAPE (Vitis Vinifera ‘Chardonnay’). 2022. [Google Scholar]
- Hinrichs-Berger, J. Epidemiology of Pseudomonas syringae Pathovars Associated with Decline of Plum Trees in the Southwest of Germany. J. Phytopathol. 2004, 152, 153–160. [Google Scholar] [CrossRef]
- Iličić, R.; Balaž, J.; Ognjanov, V.; Popović, T. Epidemiology Studies of Pseudomonas syringae Pathovars Associated with Bacterial Canker on the Sweet Cherry in Serbia. Plant Prot. Sci. 2021, 57, 196–205. [Google Scholar] [CrossRef]
- Umiraliyeva, Z.Z.; Kopzhassarov, B.K.; Jaimurzina, A.A.; Niyazbekov, Z.B.; Issenova, G.Z.; Tursunova, A.K.; Berganayeva, G.E. Epidemiology of Fire Blight in Fruit Crops in Kazakhstan. Agrivita 2021, 43, 273–284. [Google Scholar] [CrossRef]
- Mauri, S.; Cellini, A.; Buriani, G.; Donati, I.; Costa, G.; Spinelli, F. Optimization of Cultural Practices to Reduce the Development of Pseudomonas syringae pv. actinidiae, Causal Agent of the Bacterial Canker of Kiwifruit. J. Berry Res. 2016, 6, 355–371. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L.; Poliakoff, F.; Audusseau, C.; Cornish, D.A.; Paillard, S.; Rivoal, C.; Yu, J. First Report of Pseudomonas syringae pv. actinidiae, the Causal Agent of Bacterial Canker of Kiwifruit in France. Plant Dis. 2011, 95, 1311. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.H.; Kim, G.H.; Jung, J.S.; Hur, J.S.; Koh, Y.J. Comparative Analysis of Korean and Japanese Strains of Pseudomonas syringae pv. actinidiae Causing Bacterial Canker of Kiwifruit. Plant Pathol. J. 2005, 21, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Altimira, F.; Yáñez, C.; Bravo, G.; González, M.; Rojas, L.A.; Seeger, M. Characterization of Copper-Resistant Bacteria and Bacterial Communities from Copper-Polluted Agricultural Soils of Central Chile. BMC Microbiol. 2012, 12, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.B.; Jackson, L.E.; Balogh, B.; Obradovic, A.; Iriarte, F.B.; Momol, M.T. Bacteriophages for Plant Disease Control. Annu. Rev. Phytopathol. 2007, 45, 245–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen Decades of Antimicrobial Copper Compounds Applied in Agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, L.A.M.; Pereira, C.; Frazão, C.; Balcão, V.M.; Almeida, A. Efficiency of Phage Φ6 for Biocontrol of Pseudomonas syringae pv. syringae: An in Vitro Preliminary Study. Microorganisms 2019, 7, 286. [Google Scholar] [CrossRef] [Green Version]
- Frampton, R.A.; Pitman, A.R.; Fineran, P.C. Advances in Bacteriophage-Mediated Control of Plant Pathogens. Int. J. Microbiol. 2012, 2012, 326452. [Google Scholar] [CrossRef] [Green Version]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of Phytobacteria with Bacteriophage Cocktails. Pest Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper Accumulation in Agricultural Soils: Risks for the Food Chain and Soil Microbial Populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.C.; Squitti, R.; Ventriglia, M.; Cerchiaro, G.; Daher, J.P.; Rocha, J.G.; Rongioletti, M.C.A.; Moonen, A.-C. Biomolecules Agricultural Use of Copper and Its Link to Alzheimer’s Disease. Biomolecules 2020, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Knowles, A. Recent Developments of Safer Formulations of Agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; Lojkowska, E. Isolation and Characterization of Novel Soilborne Lytic Bacteriophages Infecting Dickeya Spp. Biovar 3 (‘D. Solani’). Plant Pathol. 2013, 63, 758–772. [Google Scholar] [CrossRef]
- Conlin, K.C. Effectiveness of Selected Chemicals in Inhibiting Pseudomonas syringae pv. tomato in vitro and in Controlling Bacterial Speck. Plant Dis. 1983, 67, 639–644. [Google Scholar] [CrossRef] [Green Version]
- McLeod, A.; Masimba, T.; Jensen, T.; Serfontein, K.; Coertze, S. Evaluating Spray Programs for Managing Copper Resistant Pseudomonas syringae pv. tomato Populations on Tomato in the Limpopo Region of South Africa. Crop Prot. 2017, 102, 32–42. [Google Scholar] [CrossRef]
- Wilson, M.; Campbell, H.L.; Ji, P.; Jones, J.B.; Cuppels, D.A. Biological Control of Bacterial Speck of Tomato under Field Conditions at Several Locations in North America. Phytopathology 2002, 92, 1284–1292. [Google Scholar] [CrossRef] [Green Version]
- Belpoggi, F.; Soffritti, M.; Guarino, M.; Lambertini, L.; Cevolani, D.; Maltoni, C. Results of Long-Term Experimental Studies on the Carcinogenicity of Ethylene-Bis-Dithiocarbamate (Mancozeb) in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 123–136. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical Elicitors of Systemic Acquired Resistance—Salicylic Acid and Its Functional Analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Stroud, E.A.; Rikkerink, E.H.A.; Jayaraman, J.; Templeton, M.D. ActigardTM Induces a Defence Response to Limit Pseudomonas syringae pv. actinidiae in Actinidia chinensis var. chinensis ‘Hort16A’ Tissue Culture Plants. Sci. Hortic. 2022, 295, 110806. [Google Scholar] [CrossRef]
- Wurms, K.V.; Gould, E.; Ah Chee, A.; Taylor, J.; Curran, B.; Reglinski, T. Elicitor Induction of Defense Genes and Reduction of Bacterial Canker in Kiwifruit. N. Z. Plant Prot. 2017, 70, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Michelotti, V.; Lamontanara, A.; Buriani, G.; Orrù, L.; Cellini, A.; Donati, I.; Vanneste, J.L.; Cattivelli, L.; Tacconi, G.; Spinelli, F. Comparative Transcriptome Analysis of the Interaction between Actinidia chinensis var. chinensis and Pseudomonas syringae pv. actinidiae in Absence and Presence of Acibenzolar-S-Methyl. BMC Genom. 2018, 19, 585. [Google Scholar] [CrossRef] [Green Version]
- Stout, M.J.; Fidantsef, A.L.; Duffey, S.S.; Bostock, R.M. Signal Interactions in Pathogen and Insect Attack: Systemic Plant-Mediated Interactions between Pathogens and Herbivores of the Tomato, Lycopersicon Esculentum. Physiol. Mol. Plant Pathol. 1999, 54, 115–130. [Google Scholar] [CrossRef]
- Reglinski, T.; Vanneste, J.L.; Wurms, K.; Gould, E.; Spinelli, F.; Rikkerink, E. Using Fundamental Knowledge of Induced Resistance to Develop Control Strategies for Bacterial Canker of Kiwifruit Caused by Pseudomonas syringae pv. actinidiae. Front. Plant Sci. 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Hwang, M.S.H.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic Characterization of Virulence and Resistance Phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191. [Google Scholar] [CrossRef] [Green Version]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, D.A. Molecular Mechanisms of Copper Resistance and Accumulation in Bacteria. FEMS Microbiol. Rev. 1994, 14, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Behlau, F.; Canteros, B.I.; Jones, J.B.; Graham, J.H. Copper Resistance Genes from Different Xanthomonads and Citrus Epiphytic Bacteria Confer Resistance to Xanthomonas citri subsp. citri. Eur. J. Plant Pathol. 2012, 133, 949–963. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Q.; Zhou, M.G. Identification and Characterization of Integron-Mediated Antibiotic Resistance in the Phytopathogen Xanthomonas oryzae pv. oryzae. PLoS ONE 2013, 8, e55962. [Google Scholar] [CrossRef] [Green Version]
- Argüello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of Copper Homeostasis in Bacteria. Front. Cell. Infect. Microbiol. 2013, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Adaikkalam, V.; Swarup, S. Molecular Characterization of an Operon, CueAR, Encoding a Putative P1-Type ATPase and a MerR-Type Regulatory Protein Involved in Copper Homeostasis in Pseudomonas putida. Microbiology 2002, 148, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Merry, R.H.; Tiller, K.G.; Alston, A.M. Accumulation of Copper, Lead and Arsenic in Some Australian Orchard Soils. Aust. J. Soil Res. 1983, 21, 549–561. [Google Scholar] [CrossRef]
- Griffin, K.; Campbell, P.; Gambley, C. Genetic Basis of Copper-Tolerance in Australian Pseudomonas syringae pv. tomato. Australas. Plant Pathol. 2019, 48, 425–437. [Google Scholar] [CrossRef]
- Teixeira, E.C.; de Oliveira, J.C.F.; Novo, M.T.M.; Bertolini, M.C. The Copper Resistance Operon CopAB from Xanthomonas axonopodis Pathovar citri: Gene Inactivation Results in Copper Sensitivity. Microbiology 2008, 154, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Bondarczuk, K.; Piotrowska-Seget, Z. Molecular Basis of Active Copper Resistance Mechanisms in Gram-Negative Bacteria. Cell Biol. Toxicol. 2013, 29, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Bender, C.L.; Cooksey, D.A. Molecular Cloning of Copper Resistance Genes from Pseudomonas syringae pv. tomato. J. Bacteriol. 1987, 169, 470–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Smith, J.J.; Zhao, Y.; Jackson, R.W.; Arnold, D.L.; Murillo, J.; Sundin, G.W. Phylogenetic Analysis of the PPT23A Plasmid Family of Pseudomonas syringae. Appl. Environ. Microbiol. 2007, 73, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Barranquero, J.A.; de Vicente, A.; Carrión, V.J.; Sundin, G.W.; Cazorla, F.M. Recruitment and Rearrangement of Three Different Genetic Determinants into a Conjugative Plasmid Increase Copper Resistance in Pseudomonas syringae. Appl. Environ. Microbiol. 2013, 79, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Colombi, E.; Straub, C.; Künzel, S.; Templeton, M.D.; McCann, H.C.; Rainey, P.B. Evolution of Copper Resistance in the Kiwifruit Pathogen Pseudomonas syringae pv. actinidiae through Acquisition of Integrative Conjugative Elements and Plasmids. Environ. Microbiol. 2017, 19, 819–832. [Google Scholar] [CrossRef]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; de Vicente, A.; Sundin, G.W. Complete Sequence and Comparative Genomic Analysis of Eight Native Pseudomonas syringae Plasmids Belonging to the PPT23A Family. BMC Genom. 2017, 18, 365. [Google Scholar] [CrossRef] [Green Version]
- Sundin, G.W.; Bender, C.L. Expression of the StrA-StrB Streptomycin Resistance Genes in Pseudomonas syringae and Xanthomonas campestris and Characterization of IS6100 in X. campestris. Appl. Environ. Microbiol. 1995, 61, 2891–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Nam, H.; Koh, Y.; Hur, J.; Jung, J. Molecular Bases of High-Level Streptomycin Resistance in Pseudomonas marginalis and Pseudomonas syringae pv. actinidiae. J. Microbiol. 2003, 41, 16–21. [Google Scholar]
- Nakajima, M.; Yamashita, S.; Takikawa, Y.; Tsuyumu, S.; Hibi, T.; Goto, M. Similarity of Streptomycin Resistance Gene(s) in Pseudomonas syringae pv. actinidiae with StrA and StrB of Plasmid RSF1010. Jpn. J. Phytopathol. 1995, 61, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Collis, C.M.; Hall, R.M. Expression of Antibiotic Resistance Genes in the Integrated Cassettes of Integrons. Antimicrob. Agents Chemother. 1995, 39, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G.; Scortichini, M. Pseudomonas syringae pv. actinidiae Draft Genomes Comparison Reveal Strain-Specific Features Involved in Adaptation and Virulence to Actinidia Species. PLoS ONE 2011, 6, e27297. [Google Scholar] [CrossRef]
- Walsh, C. Molecular Mechanisms That Confer Antibacterial Drug Resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Archer, G.L. New Mechanisms of Bacterial Resistance to Antimicrobial Agents. N. Engl. J. Med. 1991, 324, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.L.; Voyle, M.D.; Yu, J.; Cornish, D.A.; Boyd, R.J.; Mclaren, G.F. Copper and Streptomycin Resistance in Pseudomonas Strains Isolated from Pipfruit and Stone Fruit Orchards in New Zealand. In Pseudomonas syringae Pathovars and Related Pathogens—Identification, Epidemiology and Genomics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 81–90. [Google Scholar] [CrossRef]
- Shenge, K.C.; Wydra, K.; Mabagala, R.B.; Mortensen, C.N. Assessment of Strains of Pseudomonas syringae pv. tomato from Tanzania for Resistance to Copper and Streptomycin. Arch. Phytopathol. Plant Prot. 2008, 41, 572–585. [Google Scholar] [CrossRef]
- Glibota, N.; Grande Burgos, M.J.; Gálvez, A.; Ortega, E. Copper Tolerance and Antibiotic Resistance in Soil Bacteria from Olive Tree Agricultural Fields Routinely Treated with Copper Compounds. J. Sci. Food Agric. 2019, 99, 4677–4685. [Google Scholar] [CrossRef]
- Córdova, P.; Rivera-González, J.P.; Rojas-Martínez, V.; Villarreal, P.; Zamorano, A.; Fiore, N.; San Martín, D.; Vera, F.; Gálvez, E.; Romero, J.; et al. Antimicrobial Multiresistant Phenotypes of Genetically Diverse Pseudomonas spp. Isolates Associated with Tomato Plants in Chilean Orchards. Horticulturae 2022, 8, 750. [Google Scholar] [CrossRef]
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic Resistance in Agriculture: Perspectives on Upcoming Strategies to Overcome Upsurge in Resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef]
- Freeland, G.; Hettiarachchy, N.; Atungulu, G.G.; Apple, J.; Mukherjee, S. Strategies to Combat Antimicrobial Resistance from Farm to Table. Food Rev. Int. 2021, 39, 27–40. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Anand Kumar, P.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Stenberg, J.A. A Conceptual Framework for Integrated Pest Management. Trends Plant Sci. 2017, 22, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruana, J.C.; Dhar, N.; Raina, R. Overexpression of Arabidopsis MicroRNA167 Induces Salicylic Acid-Dependent Defense against Pseudomonas syringae through the Regulation of Its Targets ARF6 and ARF8. Plant Direct 2020, 4, e00270. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nordeen, R.O.; Di, M.; Owens, L.D.; McBeath, J.H. Expression of an Engineered Cecropin Gene Cassette in Transgenic Tobacco Plants Confers Disease Resistance to Pseudomonas syringae pv. tabaci. Phytopathology 1997, 87, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Rooney, W.M.; Grinter, R.W.; Correia, A.; Parkhill, J.; Walker, D.C.; Milner, J.J. Engineering Bacteriocin-Mediated Resistance against the Plant Pathogen Pseudomonas syringae. Plant Biotechnol. J. 2020, 18, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Téllez, J.; Muñoz-Barrios, A.; Sopeña-Torres, S.; Martín-Forero, A.F.; Ortega, A.; Pérez, R.; Sanz, Y.; Borja, M.; de Marcos, A.; Nicolas, M.; et al. YODA Kinase Controls a Novel Immune Pathway of Tomato Conferring Enhanced Disease Resistance to the Bacterium Pseudomonas syringae. Front. Plant Sci. 2020, 11, 584471. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ma, W.; Liu, J.; Hu, J.; Cai, W. Overexpression of a Small GTP-Binding Protein Ran1 in Arabidopsis Leads to Promoted Elongation Growth and Enhanced Disease Resistance against P. syringae DC3000. Plant J. 2021, 108, 977–991. [Google Scholar] [CrossRef]
- Köhl, J.; Medeiros, F.H.V.; Lombaers-van der Plas, C.; Groenenboom-de Haas, L.; van den Bosch, T. Efficacies of Bacterial and Fungal Isolates in Biocontrol of Botrytis cinerea and Pseudomonas syringae pv. tomato and Growth Promotion in Tomato Do Not Correlate. Biol. Control 2020, 150, 104375. [Google Scholar] [CrossRef]
- El-Wakeil, N.; Saleh, M.; Abu-hashim, M. Conclusions and Recommendations of Biological Control Industry. In Cottage Industry of Biocontrol Agents and Their Applications: Practical Aspects to Deal Biologically with Pests and Stresses Facing Strategic Crops; Springer: Cham, Switzerland, 2019; pp. 451–466. [Google Scholar]
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage Biocontrol to Combat Pseudomonas syringae Pathogens Causing Disease in Cherry. Microb. Biotechnol. 2020, 13, 1428–1445. [Google Scholar] [CrossRef] [PubMed]
- OmniLytics The Phage Company. Available online: https://www.omnilytics.com/ (accessed on 15 April 2023).
- Biogard Amylo-X®. Available online: https://www.biogard.it/prodotto/amylo-x/ (accessed on 15 April 2023).
- Mougou, I.; Boughalleb-M’hamdi, N. Biocontrol of Pseudomonas syringae pv. syringae Affecting Citrus Orchards in Tunisia by Using Indigenous Bacillus Spp. and Garlic Extract. Egypt. J. Biol. Pest Control 2018, 28, 60. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Braun-Kiewnick, A.; Jacobsen, B.J.; Sands, D.C. Biological Control of Pseudomonas syringae pv. syringae, the Causal Agent of Basal Kernel Blight of Barley, by Antagonistic Pantoea agglomerans. Phytopathology 2000, 90, 368–375. [Google Scholar] [CrossRef] [Green Version]
- NuFarm Americas BlightBan® A506-US-Crop. Available online: https://nufarm.com/uscrop/product/blightbana506/ (accessed on 17 April 2023).
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. The Plant Growth-Promoting Fungus Penicillium simplicissimum GP17-2 Induces Resistance in Arabidopsis thaliana by Activation of Multiple Defense Signals. Plant Cell Physiol. 2007, 48, 1724–1736. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.J.; Shin, D.J.; Won, H.Y.; Song, J.; Sang, M.K. Aspergillus terreus JF27 Promotes the Growth of Tomato Plants and Induces Resistance against Pseudomonas syringae pv. tomato. Mycobiology 2018, 46, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.; Bostock, R.M. Induced Systemic Resistance (ISR) against Pathogens in the Context of Induced Plant Defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Schweizer, P.; Meuwly, P.; Métraux, J.P. Systemic Acquired Resistance in Plants. Plant Cell 1996, 168, 303–340. [Google Scholar]
- Botrel, D.A.; Laborde, M.C.F.; De Medeiros, F.H.V.; De Resende, M.L.V.; Ribeiro Júnio, P.M.; Pascholati, S.F.; Gusmão, L.F.P. Saprobic Fungi as Biocontrol Agents of Halo Blight (Pseudomonas syringae pv. garcae) in Coffee Clones. Coffee Sci. 2018, 13, 283–291. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The Fungal Dimension of Biodiversity: Magnitude, Significance, and Conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Schmit, J.P.; Mueller, G.M. An Estimate of the Lower Limit of Global Fungal Diversity. Biodivers. Conserv. 2007, 16, 99–111. [Google Scholar] [CrossRef]
- Shoda, M. Bacterial Control of Plant Diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. Protection of Tomato Seedlings against Infection by Pseudomonas syringae pv. tomato by Using the Plant Growth-Promoting Bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2002, 68, 2637–2643. [Google Scholar] [CrossRef] [Green Version]
- Ji, P.; Campbell, H.L.; Kloepper, J.W.; Jones, J.B.; Suslow, T.V.; Wilson, M. Integrated Biological Control of Bacterial Speck and Spot of Tomato under Field Conditions Using Foliar Biological Control Agents and Plant Growth-Promoting Rhizobacteria. Biol. Control 2006, 36, 358–367. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological Control of Bacterial Plant Diseases with Lactobacillus plantarum Strains Selected for Their Broad-Spectrum Activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Tscheka, C.; Edwards, K.; Karlsson, G.; Heerklotz, H. All-or-None Membrane Permeabilization by Fengycin-Type Lipopeptides from Bacillus subtilis QST713. Biochim. Biophys. Acta 2011, 1808, 2000–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological Control of Pseudomonas syringae pv. actinidiae (Psa), the Causal Agent of Bacterial Canker of Kiwifruit, Using Endophytic Bacteria Recovered from a Medicinal Plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Gentili, A.; Mariotti, E.; Vincenzi, A.; Mazzaglia, A.; Heydari, A.; Schaad, N.W.; Varvaro, L.; Balestra, G.M. Dieback (Moria) of Hazelnut: Isolation and Characterization of Two Potential Biocontrol Agents. J. Plant Pathol. 2009, 90, 383–386. [Google Scholar]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; De Salamone, I.E.G.; Nelson, L.M.; Novák, O.; Strnad, M.; Van Der Graaff, E.; Roitsch, T. Cytokinin Production by Pseudomonas fluorescens G20-18 Determines Biocontrol Activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Yu, S.M.; Lee, Y.H. Assessment of the Contribution of Antagonistic Secondary Metabolites to the Antifungal and Biocontrol Activities of Pseudomonas fluorescens NBC275. Plant Pathol. J. 2020, 36, 491–496. [Google Scholar] [CrossRef]
- Attia Abo-Zaid, G.; Abdel-Mohsen Soliman, N.; Salah Abdullah, A.; Ebrahim El-Sharouny, E.; Mohamed Matar, S.; Abdel-Fattah Sabry, S. Maximization of Siderophores Production from Biocontrol Agents, Pseudomonas aeruginosa F2 and Pseudomonas fluorescens JY3 Using Batch and Exponential Fed-Batch Fermentation. Processes 2020, 8, 455. [Google Scholar] [CrossRef] [Green Version]
- Morella, N.M.; Zhang, X.; Koskella, B. Tomato Seed-Associated Bacteria Confer Protection of Seedlings against Foliar Disease Caused by Pseudomonas syringae. Phytobiomes J. 2019, 3, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Akbaba, M.; Ozaktan, H. Biocontrol of Angular Leaf Spot Disease and Colonization of Cucumber (Cucumis sativus L.) by Endophytic Bacteria. Egypt. J. Biol. Pest Control 2018, 28, 14. [Google Scholar] [CrossRef] [Green Version]
- Atterbury, R.J.; Barrow, P.A. The Use of Bacteriophages in Veterinary Therapy. In Bacteriophages; Springer: Cham, Switzerland, 2021; pp. 953–987. [Google Scholar] [CrossRef]
- Kotan, R.; Şahin, F. Biological Control of Pseudomonas syringae pv. syringae and Nutritional Similarity in Carbon Source Utilization of Pathogen and Its Potential Biocontrol Agents. J. Turk. Phytopathol. 2006, 35, 1–13. [Google Scholar]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Fousia, S.; Paplomatas, E.J.; Tjamos, S.E. Bacillus subtilis QST 713 Confers Protection to Tomato Plants Against Pseudomonas syringae pv. tomato and Induces Plant Defence-Related Genes. J. Phytopathol. 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Völksch, B.; May, R. Biological Control of Pseudomonas syringae pv. glycinea by Epiphytic Bacteria under Field Conditions. Microb. Ecol. 2001, 41, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C. Crystal Ball. The Viriosphere: The Greatest Biological Diversity on Earth and Driver of Global Processes. Environ. Microbiol. 2005, 7, 481–482. [Google Scholar] [CrossRef]
- Fauquet, C.M.; Mayo, M.A.; Maniloff, J.; Desselberger, U.; Ball, L.A. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses; Elsevier Science: Amsterdam, The Netherlands, 2005; ISBN 9780080575483. [Google Scholar]
- Summers, W.C. Bacteriophage Therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Mallmann, W.L.; Hemstreet, C. Isolation of an Inhibitory Substance from Plants. Agric. Res. 1924, 28, 599–602. [Google Scholar]
- Coons, G.H. The Transmissible Lytic Principle (Bacteriophage) in Relation to Plant Pathogens. Phytopathology 1925, 15, 357–370. [Google Scholar]
- Kotila, J.E. Investigations of the Blackleg Disease of the Potato; Michigan Agricultural College: East Lansing, MI, USA, 1925; Volume 67, pp. 3–29. [Google Scholar]
- Thomas, R.C. A Bacteriophage in Relation to Stewart’s Disease of Corn. Phytopathology 1935, 25, 371–372. [Google Scholar]
- Okabe, N.; Goto, M. Bacteriophages of Plant Pathogens. Annu. Rev. Phytopathol. 1963, 1, 397–418. [Google Scholar] [CrossRef]
- McCallin, S.; Brüssow, H. Phage Therapy: An Alternative or Adjunct to Antibiotics? Emerg. Top. Life Sci. 2017, 1, 105–116. [Google Scholar] [CrossRef]
- Richards, G.P. Bacteriophage Remediation of Bacterial Pathogens in Aquaculture: A Review of the Technology. Bacteriophage 2014, 4, e975540. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of Commercial Phage-Based Products against Listeria monocytogenes for Improvement of Food Safety in Spanish Dry-Cured Ham and Food Contact Surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Jikia, D.; Chkhaidze, N.; Imedashvili, E.; Mgaloblishvili, I.; Tsitlanadze, G.; Katsarava, R.; Morris, J.G.; Sulakvelidze, A. The Use of a Novel Biodegradable Preparation Capable of the Sustained Release of Bacteriophages and Ciprofloxacin, in the Complex Treatment of Multidrug-Resistant Staphylococcus aureus—Infected Local Radiation Injuries Caused by Exposure to Sr90. Clin. Exp. Dermatol. 2005, 30, 23–26. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.; Iriarte, F.; Momol, M. Phage Therapy for Plant Disease Control. Curr. Pharm. Biotechnol. 2010, 11, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Svircev, A.M.; Obradović, A.Ž. Crop Use of Bacteriophages. In Bacteriophages; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroth, M.N. Streptomycin Resistance in Erwinia amylovora. Phytopathology 1979, 69, 565–568. [Google Scholar] [CrossRef]
- Stall, R.E.; Loschke, D.C.; Rice, R.W. Conjugational Transfer of Copper Resistance and Avirulence to Pepper within Strains of Xanthomonas campestris pv. vesicatoria. Phytopathology 1984, 74, 797. [Google Scholar]
- The Commission of the European Communities. EUR-Lex-02004D0129-20050820-EN-EUR-Lex. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02004D0129-20050820 (accessed on 15 April 2023).
- Di Lallo, G.; Evangelisti, M.; Mancuso, F.; Ferrante, P.; Marcelletti, S.; Tinari, A.; Superti, F.; Migliore, L.; D’Addabbo, P.; Frezza, D.; et al. Isolation and Partial Characterization of Bacteriophages Infecting Pseudomonas syringae pv. actinidiae, Causal Agent of Kiwifruit Bacterial Canker. J. Basic Microbiol. 2014, 54, 1210–1221. [Google Scholar] [CrossRef] [Green Version]
- van Charante, F.; Holtappels, D.; Blasdel, B.; Burrowes, B. Isolation of Bacteriophages. In Bacteriophages; Springer International: Cham, Switzerland, 2019; pp. 1–32. [Google Scholar]
- Loc-Carrillo, C.; Abedon, S.T. Pros and Cons of Phage Therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Balogh, B.; Olson, S.M. Management of Tomato Bacterial Spot in the Field by Foliar Applications of Bacteriophages and SAR Inducers. Plant Dis. 2004, 88, 736–740. [Google Scholar] [CrossRef] [Green Version]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Jackson, L.E.; Balogh, B.; Guven, K.; Iriarte, F.B. Integration of Biological Control Agents and Systemic Acquired Resistance Inducers Against Bacterial Spot on Tomato. Plant Dis. 2005, 89, 712–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [Green Version]
- Milho, C.; Silva, M.D.; Sillankorva, S.; Harper, D.R. Biofilm Applications of Bacteriophages. In Bacteriophages; Springer International: Cham, Switzerland, 2019; pp. 1–35. [Google Scholar]
- Ni, P.; Wang, L.; Deng, B.; Jiu, S.; Ma, C.; Zhang, C.; Almeida, A.; Wang, D.; Xu, W.; Wang, S. Combined Application of Bacteriophages and Carvacrol in the Control of Pseudomonas syringae pv. actinidiae Planktonic and Biofilm Forms. Microorganisms 2020, 8, 837. [Google Scholar] [CrossRef] [PubMed]
- Vidaver, A.K.; Koski, R.K.; Van Etten, J.L. Bacteriophage Phi6: A Lipid-Containing Virus of Pseudomonas phaseolicola. J. Virol. 1973, 11, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persley, Y.G.J.; Crosse, J.E. A Bacteriophage Specific to Race 2 of the Cherry Strain of Pseudomonas morsprunorum. Ann. Appl. Biol. 1978, 89, 219–222. [Google Scholar] [CrossRef]
- Nordeen, R.O.; Morgan, M.K.; Currier, T.C. Isolation and Partial Characterization of Bacteriophages of the Phytopathogen Pseudomonas syringae. Appl. Environ. Microbiol. 1983, 45, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Minor, S.M.; Nordeen, R.O.; Paschall, C.E.; Minor, S.M.; Nordeen, R.O. Partial Characterization of Bacteriophages of Pseudomonas syringae pv. Tomato. J. Ark. Acad. Sci. 1996, 50, 28. [Google Scholar]
- Yin, Y.; Ni, P.; Deng, B.; Wang, S.; Xu, W.; Wang, D. Isolation and Characterization of Phages against Pseudomonas syringae pv. actinidiae. Acta Agric. Scand. B. Soil Plant Sci. 2019, 69, 199–208. [Google Scholar] [CrossRef]
- Yu, J.G.; Lim, J.A.; Song, Y.R.; Heu, S.; Kim, G.H.; Koh, Y.J.; Oh, C.S. Isolation and Characterization of Bacteriophages against Pseudomonas syringae pv. actinidiae Causing Bacterial Canker Disease in Kiwifruit. J. Microbiol. Biotechnol. 2015, 26, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lim, J.-A.; Yu, J.-G.; Oh, C.-S. Genomic Features and Lytic Activity of the Bacteriophage PPPL-1 Effective against Pseudomonas syringae pv. actinidiae, a Cause of Bacterial Canker in Kiwifruit. J. Microbiol. Biotechnol. 2018, 28, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.A.M.; Pereira, C.; Barreal, M.E.; Gallego, P.P.; Balcão, V.M.; Almeida, A. Use of Phage Φ6 to Inactivate Pseudomonas syringae pv. actinidiae in Kiwifruit Plants: In Vitro and Ex Vivo Experiments. Appl. Microbiol. Biotechnol. 2020, 104, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Frezza, D.; Di Lallo, G.; Visconti, S. A Phage Therapy Model for the Prevention of Pseudomonas syringae pv. actinidiae Infection of Kiwifruit Plants. Plant Dis. 2023, 107, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, M.; Holtappels, D.; Fortuna, K.; Hajlaoui, M.R.; Lavigne, R.; Sadfi-Zouaoui, N.; Wagemans, J. Biological and Molecular Characterization of the Lytic Bacteriophage SoKa against Pseudomonas syringae pv. syringae, Causal Agent of Citrus Blast and Black Pit in Tunisia. Viruses 2022, 14, 1949. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Xia, H.; Zhong, C.; Li, L.; Zeng, C. Genomic Characterization of Two Nickie-like Bacteriophages That Infect the Kiwifruit Canker Phytopathogen Pseudomonas syringae pv. actinidiae. Arch. Virol. 2022, 167, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Korniienko, N.; Kharina, A.; Zrelovs, N.; Jindřichová, B.; Moravec, T.; Budzanivska, I.; Burketová, L.; Kalachova, T. Isolation and Characterization of Two Lytic Phages Efficient Against Phytopathogenic Bacteria from Pseudomonas and Xanthomonas Genera. Front. Microbiol. 2022, 13, 853593. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, Y.; Liu, M.; Luo, S.; Cheng, Y.; Li, G.; Liu, C.; Wen, S.; Xia, M.; He, X.; et al. Application of Phage Therapy against Red-Fleshed Kiwifruit Canker. Biol. Control 2022, 169, 104893. [Google Scholar] [CrossRef]
- Kazantseva, O.A.; Buzikov, R.M.; Pilipchuk, T.A.; Valentovich, L.N.; Kazantsev, A.N.; Kalamiyets, E.I.; Shadrin, A.M. The Bacteriophage Pf-10—A Component of the Biopesticide “Multiphage” Used to Control Agricultural Crop Diseases Caused by Pseudomonas syringae. Viruses 2022, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, M.; Hu, R.; Bai, J.; He, X.; Jin, Y. Isolation of the Novel Phage PHB09 and Its Potential Use against the Plant Pathogen Pseudomonas syringae pv. actinidiae. Viruses 2021, 13, 2275. [Google Scholar] [CrossRef]
- Martino, G.; Holtappels, D.; Vallino, M.; Chiapello, M.; Turina, M.; Lavigne, R.; Wagemans, J.; Ciuffo, M. Molecular Characterization and Taxonomic Assignment of Three Phage Isolates from a Collection Infecting Pseudomonas syringae pv. actinidiae and P. syringae pv. phaseolicola from Northern Italy. Viruses 2021, 13, 2083. [Google Scholar] [CrossRef]
- Akbaba, M.; Ozaktan, H. Evaluation of Bacteriophages in the Biocontrol of Pseudomonas syringae pv. syringae Isolated from Cankers on Sweet Cherry (Prunus avium L.) in Turkey. Egypt. J. Biol. Pest Control 2021, 31, 35. [Google Scholar] [CrossRef]
- Holtappels, D.; Kerremans, A.; Busschots, Y.; Vaerenbergh Van, J.V.; Maes, M.; Lavigne, R.; Wagemans, J. Preparing for the KIL: Receptor Analysis of Pseudomonas syringae pv. porri Phages and Their Impact on Bacterial Virulence. Int. J. Mol. Sci. 2020, 21, 2930. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, J.B.; Djurhuus, A.M.; Carstens, A.B.; Kot, W.; Neve, H.; Morris, C.E.; Hansen, L.H. Presentation of Three Novel Tailed Phages Targeting Multiple Strains of Pseudomonas syringae. Phage 2020, 1, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Mathieu, J.; Li, M.; Dai, Z.; Alvarez, P.J.J. Isolation of Polyvalent Bacteriophages by Sequential Multiple-Host Approaches. Appl. Environ. Microbiol. 2015, 82, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.D. Bacteriophages from Sewage Specific for Fluorescent Phytopathogenic Pseudomonads. Phytopathology 1983, 73, 403. [Google Scholar] [CrossRef]
- Brockhurst, M.A.; Koskella, B.; Zhang, Q.-G. Bacteria-Phage Antagonistic Coevolution and the Implications for Phage Therapy. In Bacteriophages; Springer International: Cham, Switzerland, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhou, Y.; Bao, H.; Wang, R.; Li, T.; Pang, M.; Sun, L.; Zhou, X. Application of a Phage in Decontaminating Vibrio parahaemolyticus in Oysters. Int. J. Food Microbiol. 2018, 275, 24–31. [Google Scholar] [CrossRef]
- Tom, E.F.; Molineux, I.J.; Paff, M.L.; Bull, J.J. Experimental Evolution of UV Resistance in a Phage. PeerJ 2018, 6, e5190. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.A.; Momol, M.T.; Jones, J.B.; Vallad, G.E. Soil-Based Systemic Delivery and Phyllosphere in vivo Propagation of Bacteriophages. Bacteriophage 2012, 2, e23530. [Google Scholar] [CrossRef] [Green Version]
- Kolozsváriné Nagy, J.; Schwarczinger, I.; Künstler, A.; Pogány, M.; Király, L. Penetration and Translocation of Erwinia amylovora Specific Bacteriophages in Apple—A Possibility of Enhanced Control of Fire Blight. Eur. J. Plant Pathol. 2015, 142, 815–827. [Google Scholar] [CrossRef]
- Rao, P.S.; Srivastava, H.C. Others Industrial Gums; Whistler, R.L., Ed.; Academic Press: Cambridge, MA, USA, 1973; pp. 372–411. [Google Scholar]
- Jackson, L.R.E. Bacteriophage Prevention and Control of Harmful Plant Bacteria. U.S. Patent US4828999A, 9 May 1989. [Google Scholar]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The Future of Phage Biocontrol in Integrated Plant Protection for Sustainable Crop Production This Review Comes from a Themed Issue on Nanobiotechnology-Phage Therapy. Curr. Opin. Biotechnol. 2021, 68, 60–71. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors Determining Phage Stability/Activity: Challenges in Practical Phage Application. Expert. Rev. Anti Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef]
- Lin, K.; Schulte, C.R.; Marr, L.C. Survival of MS2 and Φ6 Viruses in Droplets as a Function of Relative Humidity, PH, and Salt, Protein, and Surfactant Concentrations. PLoS ONE 2020, 15, e0243505. [Google Scholar] [CrossRef] [PubMed]
- Sieiro, C.; Areal-hermida, L.; Pichardo-gallardo, Á.; Almuiña-gonzález, R.; de Miguel, T.; Sánchez, S.; Sánchez-pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]

| Phylogenomic Branch | Phylogenomic Species | P. syringae Pathovars | Phylogroups 1 |
|---|---|---|---|
| I | P. congelans 2 | syringae | 2c |
| P. syringae 2 | aptata, avellanae, coryli, japonica, panici, pisi, syringae | 2b | |
| P. cerasi 2 | np | ni | |
| Phylogenomic species A 3 | aceris, syringae | 2d | |
| II | P. tomato’ 3 | tomato | 1a |
| P. avellanae 2 | actinidae, theae | 1b | |
| III | P. cannabina 2 | alisalensis | 5 |
| P. coriandricola’ 3 | coriandricola | ||
| Phylogenomic species B 3 | up | 10 | |
| P. coronafaciens’ 3 | np | 4 | |
| IV | P. amygdali 2 | np | 3 |
| P. caricapapayae 2 | helianthi, tagetis | 6 | |
| V | P. asturiensis 2 | np | ni |
| Phylogenomic species C 3 | up | 9 | |
| P. viridiflava 2 | np | 7 | |
| VI | P. cichorii 2 | np | 11 |
| Phylogenomic species D 3 | up | 13 | |
| P. caspiana 2 | np | ni | |
| Phylogenomic species E 3 | np | ni |
| Type | Mechanism | Biocontrol Agent | Studied P. syringae Pathovar 1 | Progress Status 2,3 | References |
|---|---|---|---|---|---|
| Direct antagonism | Predation | Bacteriophage | Psa | In vivo studies (1) | [27] |
| Psm | In vivo studies (1) | [134] | |||
| Pspo | Field trial (1) | [25] | |||
| Pss | In vivo studies (1) | [134] | |||
| Pst | Phage-based product available (1) | [135] | |||
| Mixed-path antagonism | Antibiotics | Bacteria (Bacillus amyloliquefaciens D747; B. subtilis QST713) | Psa | Bacteria-based product available (2) | [21,136] |
| Pss | In vivo studies (2) | [137,138] | |||
| Pst | Bacteria-based product available (1) | [21] | |||
| Undetermined | Bacteria | Pc | In vitro (1) | [132] | |
| Pss | Field trial (1) | [139] | |||
| Indirect antagonism | Competition | Bacteria Pseudomonas fluorescens A506 | Pst | Bacteria-based product available (1) | [140] |
| Induction of host resistance | Fungi | Psga | In vivo (1) | [122] | |
| Pst | In vivo (1) | [141] | |||
| In vivo(1) | [132] | ||||
| In vivo (1) | [142] | ||||
| Direct antagonism | Predation | Bacteriophage | Psa | In vivo studies (1) | [27] |
| Psm | In vivo studies (1) | [134] | |||
| Pspo | Field trial (1) | [25] | |||
| Pss | In vivo studies (1) | [134] | |||
| Pst | Phage-based product available (1) | [135] | |||
| Mixed-path antagonism | Antibiotics | Bacteria Bacillus amyloliquefaciens D747; B. subtilis QST713 | Psa | Bacteria-based product available (2) | [21,136] |
| Pss | In vivo studies (2) | [137] | |||
| Pst | Bacteria-based product available (1) Bacillus subtilis QST713 | [21] | |||
| Undetermined | Bacteria | Pc | In vitro (1) | [132] | |
| Pss | Field trial (1) | [139] | |||
| Indirect antagonism | Competition | Bacteria Pseudomonas fluorescens A506 | Pst | Bacteria-based product available (1) | [140] |
| Induction of host resistance | Fungi | Psga | In vivo (1) | [122] | |
| Pst | In vivo (1) | [141] | |||
| In planta (1) | [132] | ||||
| In planta (1) | [142] |
| Year | Milestone | Reference |
|---|---|---|
| 1915–1917 | Phage discovery | [179] |
| 1924 | First isolation of phages that infect phytopathogenic bacteria | [169] |
| 1935 | First field test demonstrating the effectiveness of phages to treat seeds infected with Stewart’s wilt | [172] |
| 1943 | Discovery of streptomycin and the beginning of the golden age of discovery and development of antibiotics (1940–1990) | [180] |
| 1979 | First report of streptomycin resistance in a phytopathogenic bacterium 1 | [181] |
| 1984 | First report of resistance to copper in a phytopathogenic bacteria 2 | [182] |
| 2004 | Banning of the agricultural use of streptomycin in the European Union | [183] |
| 2005 | The first commercial pesticide containing bacteriophage (AgriPhage ™) against Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria is registered in the USEPA | [135] |
| 2014 | Isolation and characterization of the first specific bacteriophages against P. syringae pv. actinidiae with potential application in biocontrol | [24,184] |
| 2016 | Isolation, characterization, and evaluation in field tests of the first specific bacteriophages against P. syringae pv. porri | [25] |
| 2020 | Publication of the first study demonstrating the ability of a phage cocktail to reduce the P. syringae pv. actinidiae load on kiwi trees in vivo. | [27] |
| 2020 | Isolation, characterization, and in vivo evaluation of the first specific bacteriophages against P. syringae pv. syringae and pv. morsprunorum | [134] |
| Target Pathogen/Host | Assay Type | Principal Results | Reference |
|---|---|---|---|
| Psa/kiwi | Effect of the phage is evaluated by observing necrotic areas in kiwi plants with symptoms of Psa under greenhouse conditions (in vivo assay). Detailed phage characterization was carried out previously. | φPSA2 is effective in preventing Psa replication inside plants, and capable of reducing the number and size of lesions produced by the bacteria. The phage is also capable of killing Pseudomonas when present on the leaf surface. | [200] |
| Pss/lemon | Phages are isolated from soil samples, irrigation water and symptomatic lemons infected with P. syringae pv. syringae. Bioassays in lemons measure the percentage of necrotic tissue (in vivo assay). HR (12), TEM, ST, OSGC (MOI:0.01) and GS. | In the bioassays, SoKa reduced the symptoms of infection, but could not prevent it. | [201] |
| Psa/kiwi | Hairong and ZY21 are isolated from Psa-infected symptomatic plant tissue by the soft agar plaque method (in vitro assay). HR (31), TEM and GS. | Hairong and ZY21 have relative phylogenetic closeness to two nickie-like phages (psageB1 and nickie) based on major capsid protein sequences. | [202] |
| Pst/pepper | Bacteriophages are isolated from peppers that exhibit symptoms of Pst infection. In A. thaliana, Pst or the mixture of the bacteria with the phages is inoculated (in vivo assay). HR (8), TEM, AC, KCA (MOI:0.01), ST and GS. | In vivo, the co-inoculation of Eir4 and Eir9 requires a low MOI to obtain effective phage propagation and Pst inactivation. Even so, the separate treatment, in comparison to the plants with only Pst, resulted in leaves yellowing less, and showing an almost normal growth. | [203] |
| Psa/kiwi | The phages were isolated in a kiwi orchard from canker branches or soil suspension. The lytic activity of the phage cocktail was determined, and individual phages were Psa inoculated in red-fleshed kiwifruit seedlings (in vivo assay). TM, OCGC, ST, KCA (MOI:1) and RFLP. | The phage cocktail in the infected plant generated an increase in phage viral particles during the first 12 h; however, the determined phages had a significant increase at 72 h, thus verifying the superior effect of the phage cocktail. | [204] |
| Pss/green bean | Pf-10 phage is isolated from tissue infected with Pss of green bean. HR (7), TEM, ST, AC, KC (MOI:0.1), OSGC, RLFP and GS. | Pf-10 genome is a linear dsDNA that contains 49 genes. Presents a variety of endolysins and putative holins. | [205] |
| Psa/kiwi | Phage was isolated from soil samples of “hongyang” kiwi crops. The efficacy of PHB09 is evaluated on leaf discs of kiwi plants (in vivo assays). HR (6), TEM, OSGC (MOI:0.001), ST and GS. | In kiwi leaves with Psa, a decrease in the bacterial load is observed and the symptoms do not occur. | [206] |
| Psa/kiwi and Pph/bean | The isolated phages were obtained from plant, soil and wastewater samples close to plants infected with Psa and Pph that presented symptoms. HR (32), TEM, ST and GS. | The phages exhibited selective killing of pathogenic Pseudomonas strains in in vitro assays; however, psageB1 lysed three non-pathogenic strains. | [207] |
| Pss/cherry | Isolation and in vitro determination of lytic activity using the spot inoculation method against P. syringae pathovars. The effects of bacteriophages against Pss were determined in micro propagated cherry plantlets in vivo and under growth chamber conditions. | Results of in vivo assays performed in cherry plantlets demonstrated that at 10 days post inoculation, 4 out of 6 phage treatments (F1226, F137, F358, F369) successfully reduced more than 50% of the disease incidence caused by the high-virulence Pss strain BY5L316. | [208] |
| Psa/kiwi | Isolation and in vitro determination of lytic activity using the spot inoculation method. HR (29), TEM, OSGC (MOI:0.01), ST and GS. | PN09 showed lytic activity against the 29 Psa biovar 3 strains tested. PN09 showed specificity for Psa and did not lyse other bacterial species tested. | [191] |
| Psa/kiwi | Control efficacy of PPPL-1 phage alone and in combination with KHUφ34 and KHUφ38 against bacterial canker was tested in vivo in kiwifruit plants under greenhouse conditions. | Results showed that the disease control efficacy of PPPL-1 treatment was statistically similar to that of the phage cocktail (mix of three phages) treatment or an agrochemical containing streptomycin and oxytetracycline antibiotics as active ingredients. | [198] |
| Pss/unspecified host | The bacteriophage was isolated from irrigation water on a farm where tomatoes were grown. In vitro determination of lytic activity using the soft agar plaque method. HR (17), TEM and GS. | Host range analysis showed that 64.7% of the bacterial strains investigated were susceptible to the phage Phobos, including P. syringae pathovars syringae and tomato. Sequence analysis of the predicted proteins encoded by the Phobos genome showed no homology to known virulence factors, antibiotic resistance factors, or potential immunoreactive allergens. | [26] |
| Pspo/leek | The overall performance of a cocktail containing both phages was assessed in a seed bioassay at MOI:10. BR. Detailed phage characterization was carried out previously. | A combination of KIL3b and KIL5 phages reduced the bacterial concentration 100-fold in seed bioassay. In vitro Pspo resistance against phage infection developed quite rapidly; however, the virulence of those mutants is possibly reduced. | [209] |
| Pae/horse chestnut tree | For phage isolation, soil and leaf samples of healthy and diseased trees were used, and in vitro determination of lytic activity was carried out using the soft agar plaque method. Co-evolution experiments were also performed. HR (22), TEM, RAPD, BR and KCA (MOI:0,1). | Most phages were able to infect all the tested P. syringae pv. aesculi (2250, 6617, 6619, 6620, 6623, 6631), alongside another Pseudomonas (P. syringae pv. lachrymans, P. syringae pv. tomato, P. marginalis and pv. marginalis). In the best case, a reduction of approximately 65% in the bacterial growth was observed at 24 h in the KCA. | [22] |
| Ps/unspecified host | Phages targeting P. syringae GAW0113 were isolated from organic waste samples. HR (13), EOP, TEM and GS. | All three phages were found to infect different strains of P. syringae covering several phylogroups. Three phages were shown to have a narrow host range, infecting 3 out of 13 P. syringae strains. | [210] |
| Psa/kiwi | Phages (PN05 and PN09) were isolated from water samples. A phage combined with varying concentrations of carvacrol was added to a Psa inoculum at an MOI: 1 for the different in vitro experimental setups. KCA (MOI: 0,1, 1, 10 and 100) was performed to characterize phages. | The combined treatment of phages and carvacrol (2.0 mg/mL) showed a higher efficacy (in relation to phage therapy or carvacrol alone), reducing (by 5.87 log CFU/mL) and preventing Psa regrowth for more than 40 h. | [191] |
| Psa/kiwi | Phage φ6 (DSM 21518) was tested against two biovar 3 strains (Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10). The inactivation of Psa was assessed in vitro using liquid culture medium, and ex vivo using artificially contaminated kiwifruit leaves. AC, OSGC and KCA (MOI:1) | In the in vitro experiments, phage φ6 was effective against both tested strains (maximum reduction of 2.2 and 1.9 CFU/mL for Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10, respectively). In the ex vivo tests, the decrease was lower (maximum reduction 1.1 log and 1.8 CFU/mL for Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10, respectively). | [199] |
| Pss and Psm/cherry trees | Phages were isolated from the soil, leaf, and bark of cherry trees. In vitro determination of lytic activity was carried out the soft agar plaque method. In vivo assays were performed in bean plants and cherry trees using leaf or twig inoculation with Pss and Psm. In both cases, a MOI:0.01 was used. HR (22), KCA, TEM, RAPD-PCR, GS, ST and BR. | In bean leaves, the best results were obtained with individual phages MR6 and MR7, which reduced the bacterial population (Pss) by 50%. The bioassays performed in cherry leaves showed that phage MR16 reduced the bacterial population to almost zero, and phage cocktails reduced the Pss bacterial population by 50%. In cherry twig inoculation assays, all phages, both individually and in phage cocktails, reduced the bacterial population. The best results were obtained in the case of phage MR8, which reduced the growth of all three bacteria by 60%. | [134] |
| Psa/kiwi | The phages were isolated from soil and water samples using different strains of Psa biovar 3 obtained from Chilean kiwifruit orchards as the host. Ex vivo assays were performed using kiwifruit leaf discs. Moreover, in vivo experiments were performed with two-year old kiwifruit plants cultivated in greenhouse conditions. A MOI:10 was used in the different performed bioassays. HR (18), KCA, TEM, RFLP, ST, GS and BR. | Under laboratory conditions, with kiwifruit leaf samples, the results showed that a cocktail of phages CHF1, CHF7, CHF19, and CHF21 reduced the bacterial load below the detection limit (20 UFC/mL), even 24 h post inoculation. In addition, the treatment with the phage cocktail was able to protect kiwifruit leaf discs from the damage produced by Psa. In the in vivo experiments, the phage cocktail was able to reduce the Psa load by more than 75%, in comparison with the untreated plants. Moreover, the damage index decreased from 2.3 (without phage treatment) to 1.3 (treated with phage cocktail). | [27] |
| Pss/unspecified host | In vitro characterization of bacteriophage φ6 (DSM 21518) lytic activity against bacterial strains of P. syringae pv. syringae and other bacterial strains of interest. HR (25), ST, OSGC.AC, KCA (MOI:1 and MOI:100) and BR. | The host range analysis revealed that the phage, besides its host (P. syringae pv. syringae), also infects the P. syringae pv. actinidiae CRA-FRU 12.54 and CRA-FRU 14.10 strains, not infecting strains from the other tested species. An MOI 1 (maximum reduction of 3.9 log CFU/mL) was more effective than MOI 100 (maximum reduction of 2.6 log CFU/mL) in deactivating the bacterium. | [78] |
| Psa/kiwi | PPPL-1 was isolated from soil of a kiwifruit orchard. The lytic activity of PPPL-1 was determined in vitro against P. syringae pv. actinidiae strains and strains from other pathovars, including aptata, syringae, tomato, glycinea, phaseolicola, pisi and tabaci, among others. HR (53), KCA (MOI:0.01), ST and GS. | PPPL-1 showed specificity for P. syringae species and was effective against 16 of the 18 tested Psa strains. PPPL-1 can maintain its lytic activity against Psa strain KBE9 stably for at least 80 h. | [198] |
| Pspo/leek | Phages were isolated from soil samples from the same fields from which the P. syringae pv. porri strains were taken. In vitro assessment of the phages’ lytic activity against Pspo strains was carried out according to the soft agar overlay plate technique. In vivo bioassays and field trials were performed. The activity of phages was tested in vivo (MOI: 100) by injecting phage and bacterial suspensions into leek leaves. HR (46), TEM, KCA, AC, ST, BR and GS. | None of the phages infected all the P. syringae pv. porri strains tested, but the combined host range of the phages covered all 41 Pspo isolates tested. In vivo bioassays showed that the phages KIL1, KIL2, KIL3, and KIL3b are able to reproduce inside the plant tissue, and lead to a significant reduction in the lesion length when coinjected with the bacterial host. However, the effect of phages KIL1, KIL2, and KIL3 varied between the assays. | [25] |
| Psa/kiwi | Bacteriophages against P. syringae pv. actinidiae were isolated from soils collected from kiwifruit orchards. HR (31), TEM, KCA (MOI: 0.01), DGREA and ST. | Bacteriophage KHUφ44 was the only phage effective against all 18 Psa strains tested, but it had only limited effects on two of them. The combined host range of the phages covers all 18 Psa strains tested. Most of the bacteriophages were also effective against other P. syringae pathovars (tabaci, tomato and phaseolicola), and none showed effect on other bacteria. The lytic activity of bacteriophages KHUφ34, KHUφ38 and KHUφ44 was sustained in vitro until 80 h. | [197] |
| Different bacteria genera and species, including P. syringae spp. | Isolation from sewages samples. The main objective was finding polyvalent phages and a method to obtain those phages. TEM, HR (7), AC, OSGC and KCA (MOI:10). | Phages with multiples host tropism were obtained. Lytic phages were capable of interspecies or inter-order infectivity without a significant reduction in plating efficiency. Phage PX1 delayed the onset of exponential growth for each host by 3 h and reduced the maximum viable bacterial density (CFU reaching stationary phase) by 50% for P. syringae. | [211] |
| Psa/kiwi | Bacteriophages were obtained from leaves of A. deliciosa infected by Psa, and in vitro determination of lytic activity was carried out using the soft agar plaque method. TODHR (51), TEM, LF, AC, OSGC (MOI:0.01), ST and GS. | φPSA2 is a strictly lytic phage and exhibits a broad host range, being lytic against all the 37 Psa strains tested and some other pathovars including theae, avellanae and morsprunorum. | [184] |
| Psa/kiwi | Samples for phage isolation consist of soil, water, and leaf litter collected from infected kiwifruit orchards. Lytic activity was determined using the soft agar plaque method. HR (32), TEM, ST, BR, LF, T, DGREA and GS. | The host range of individual phages was narrow, but all the Psa strains tested were infected by at least one of the isolated phages. In total, approximately 20,000 phage–host combinations were examined, and showed clear differences in the phage profiles of P. syringae pv. Actinidiae strains from distinct geographic locations. | [24] |
| Pst/tomato | 16 phages were isolated from tomato field soils and plant debris from various locations throughout Ontario. HR (106), LF, RTD, TEM and ST. | Over 70% of the Pst strains were lysed by a group of 13 PT phages in in vitro assays. Phages PT1, PT18, PT20 and PT32 showed a high degree of specificity for Pst virulent strains, and were able to infect 89, 89, 82 and 87% of the tested Pst strains, respectively, including strains from Australia, New Zealand, Europe, and the USA. | [23] |
| Psg/soybean | Phages were isolated from raw sewage obtained from four resources in Riverside and San Bernardino counties in California. Phages’ specificity for different P. syringae pathovars was determined in vitro. HR (32), OSGC, TEM and T. | The phages isolated were virulent on most of the pathovar glycinea strains. Altogether, 6 of the 7 selected phages were able to infect most of the pathovars tested, including lachrymans, morsprunorum, phaseolicola, pisi, savastanoi, tabaci and tomato. Only the phage R4-0B was specific for the pathovar glycinea. The 7 phages proved to be specific for P. syringae, being capable of infecting 30 of the 33 tested strains belonging to this species. | [212] |
| Psm/cherry trees | Isolation and in vitro characterization of a phage specific to Psm race 2. The phage’s lytic activity was assessed via RTD. The phage was used in a survey of P. morsprunorum races isolated from commercial orchards and from the cherry cultivars Napoleon and Roundel in a research station. HR (134). | The data showed that 55 of the 134 tested Pseudomonas strains were susceptible to B1 phage, all of them belonging to the morsprunorum pathovar, and 52 belonging to race 2. | [193] |
| Limitation | Strategies |
|---|---|
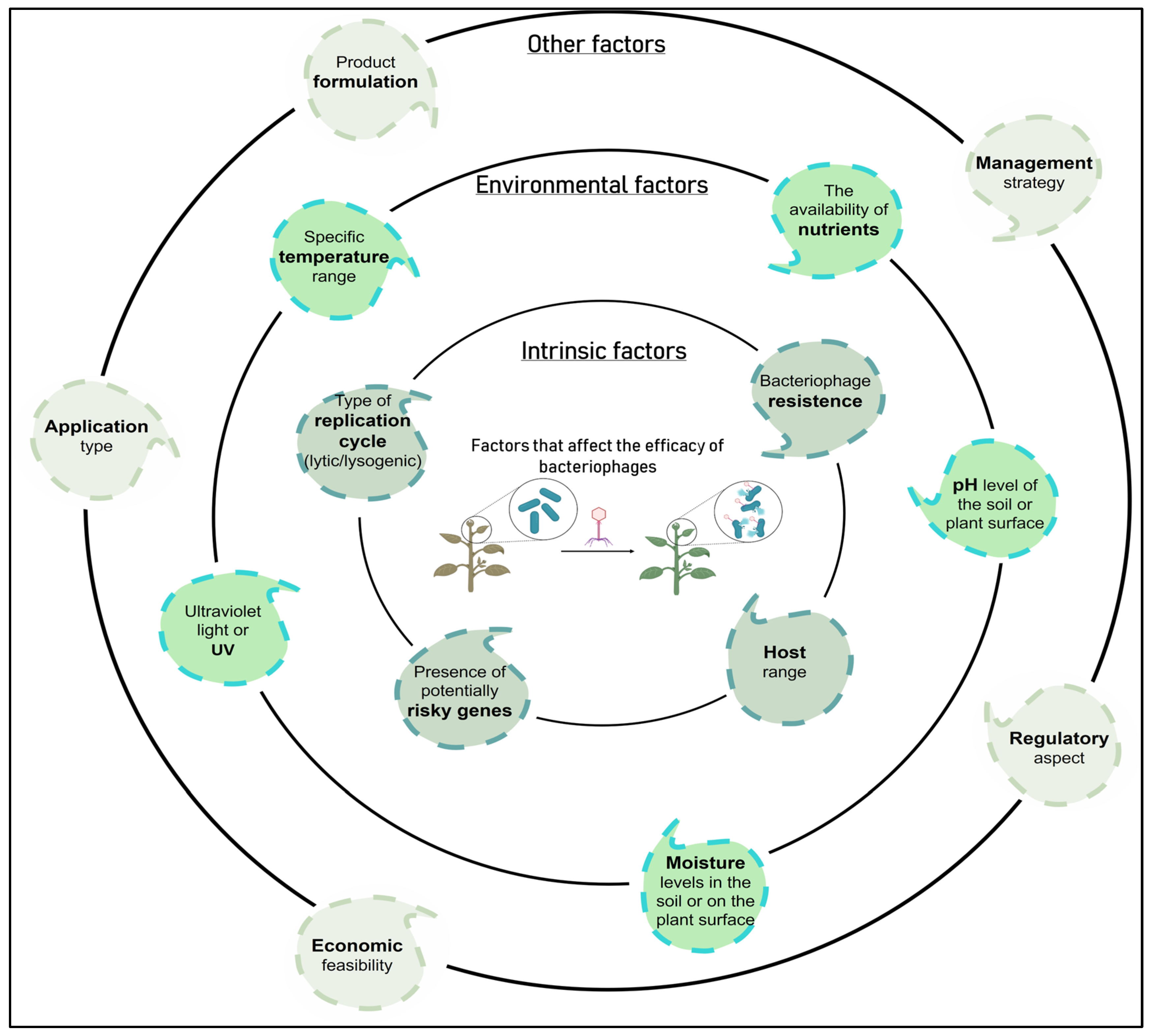
| Phage persistence in the phyllosphere and rhizosphere (formulation and mode of application) | In response to this problem, protective formulations have been investigated to minimize UV damage, although there is a great need to identify effective formulations. Based on self-replication ability phage survival can be improved in the phyllosphere and rhizosphere if they are accompanied by a viable host [2]. As an alternative, it is worth considering artificial phage evolution to increase resistance to UV-induced damage [215] Phage delivery through the soil is another approach that has been explored to improve phage persistence in the phyllosphere. There is a phage translocation pathway from the roots to the leaves of plants through the vascular system of the plant, possibly via xylem. Specifically, it has been shown that phages can translocate in tomato, rice, apple, and fire thorn plants [216,217,218]. It is suggested that if the phages can translocate systemically in the plant, then they could possibly be used therapeutically after infection by a bacterial pathogen by applying the phages to the surrounding soil of a plant instead of foliar spray [2]. |
| Potential alteration in the phage replication cycle (lytic to lysogenic) | To avoid this problem, ideally a phage for biocontrol applications should be exclusively lytic. Preferably, phages that produce transparent plaques should be chosen to reduce the isolation of temperate phages since the latter can carry out the unwanted lysogenic conversion [2,79]. Lysogens contain a prophage and are typically resistant to reinfection by the same phage, which results in turbid plaques via superinfection immunity [24]. Currently, there are validated protocols to assess whether a phage is lysogenic or if it is capable of transferring genes between bacteria (transduction test) [24], allowing to rule out those that present a risk for their use as BCAs. Additionally, it is necessary to analyze the genome of the phages to be used, discarding those with genes encoding for bacterial virulence factors or antimicrobial resistance genes, among others [27]. |
| Phage resistant bacteria | To avoid the problem of the high frequency of bacteria resistant to phage treatment, a combination of phages with different infection mechanisms can be used, reducing the probability of the appearance of resistance [80]. Increased diversity within the known phages targeting P. syringae strains also allow for development of more complex phage cocktails [210]. It should be considered that even when resistance develops, it can lead to a great cost of fitness that entails a deterioration in virulence or a reduction in the growth rate, thus reducing the severity of the disease [80]. Furthermore, in 1989, a patented process was developed to prevent the emergence of phage-resistant mutants [219]. |
| Low efficacy and consistency of control compared to conventional treatments. | Studies have shown that the timing of bacteriophage application is essential to extend the persistence of high populations in the vicinity of the host bacteria to promote biological control [18]. A strategy for a phytosanitary program should include phage applications throughout the season to reduce the population of pre-existing pathogens or avoid its proliferation [16]. Another key factor for the effectiveness of the use of phages is the time of day they are applied since ultraviolet light from the sun is capable of inactivating viruses. A possible strategy consists of night applications on the leaves, managing to increase the persistence of the phages in the phyllosphere and thus their bactericidal action [2]. The use of formulations with phages could complement other strategies for disease management, as part of an integrated phytosanitary program for pest management, increasing effectiveness and sustainability. These strategies would include hypersensitivity and systemic resistance response activators, copper-based agrochemicals, or antibiotics such as streptomycin. In this sense, it would be possible to minimize the probability of selection for resistance to these components or to phages [24]. |
| Challenges | Description |
|---|---|
| Efficacy | One of the primary challenges of biological control is ensuring its effectiveness in controlling pests and diseases. The success of biological control agents depends on several factors, including the target pest or disease, the specific biological control agent used, and the environmental conditions. Some biological control agents may not be as effective as chemical pesticides, and their efficacy can vary depending on the circumstances. It is crucial to identify and develop biological control agents that are highly specific to the target pest or disease to maximize their efficacy. |
| Compatibility | Biological control agents are living organisms, and their interactions with the target pest, the crop, and the environment can be complex. Ensuring compatibility between the biological control agents and the agricultural system is crucial. Factors such as temperature, humidity, and pesticide use can influence the survival and efficacy of biological control agents. It is necessary to carefully assess and optimize the conditions under which biological control agents are deployed to achieve the desired outcomes. |
| Cost-effectiveness | Biological control can sometimes be more expensive than chemical pesticides. Developing and mass-producing biological control agents can involve substantial costs, and their application may require specialized equipment or additional labor. Additionally, biological control often requires a longer period to achieve control compared to chemical pesticides. Farmers need to consider the cost-effectiveness of biological control in relation to their specific crop, pest, and economic conditions. |
| Regulatory challenges | The use of biological control agents in agriculture is subject to regulations to ensure their safety for humans, non-target organisms, and the environment. These regulations may vary across countries and regions. Obtaining necessary approvals and meeting regulatory requirements can be time-consuming and costly. It is essential to navigate the regulatory landscape and comply with the necessary guidelines to use biocontrol agents legally and responsibly. |
| Knowledge and expertise | Implementing biological control strategies effectively requires knowledge and expertise. Farmers need to understand the biology, behavior, and application methods of biological control agents. They must also be able to monitor pest populations, assess the impact of biological control, and make informed decisions regarding their use. Providing training and support to farmers to enhance their understanding and skills in biocontrol is crucial for successful implementation. |
| Trade-offs | There can be trade-offs associated with biological control in agriculture. For example, certain biological control agents may have a narrower range of effectiveness compared to chemical pesticides, meaning they may only target specific diseases. This specificity can be advantageous in reducing non-target effects, but it may require the use of multiple biological control agents for different diseases, thus increasing complexity and costs. Additionally, biological control agents may not provide immediate control, and may require more consistent monitoring and application compared to chemical pesticides. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova, P.; Rivera-González, J.P.; Rojas-Martínez, V.; Fiore, N.; Bastías, R.; Zamorano, A.; Vera, F.; Barrueto, J.; Díaz, B.; Ilabaca-Díaz, C.; et al. Phytopathogenic Pseudomonas syringae as a Threat to Agriculture: Perspectives of a Promising Biological Control Using Bacteriophages and Microorganisms. Horticulturae 2023, 9, 712. https://doi.org/10.3390/horticulturae9060712
Córdova P, Rivera-González JP, Rojas-Martínez V, Fiore N, Bastías R, Zamorano A, Vera F, Barrueto J, Díaz B, Ilabaca-Díaz C, et al. Phytopathogenic Pseudomonas syringae as a Threat to Agriculture: Perspectives of a Promising Biological Control Using Bacteriophages and Microorganisms. Horticulturae. 2023; 9(6):712. https://doi.org/10.3390/horticulturae9060712
Chicago/Turabian StyleCórdova, Pamela, Juan Pablo Rivera-González, Victoria Rojas-Martínez, Nicola Fiore, Roberto Bastías, Alan Zamorano, Francisca Vera, Jaime Barrueto, Belén Díaz, Carolina Ilabaca-Díaz, and et al. 2023. "Phytopathogenic Pseudomonas syringae as a Threat to Agriculture: Perspectives of a Promising Biological Control Using Bacteriophages and Microorganisms" Horticulturae 9, no. 6: 712. https://doi.org/10.3390/horticulturae9060712





